Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Growth and Root Inoculation
2.2. Plant Growth
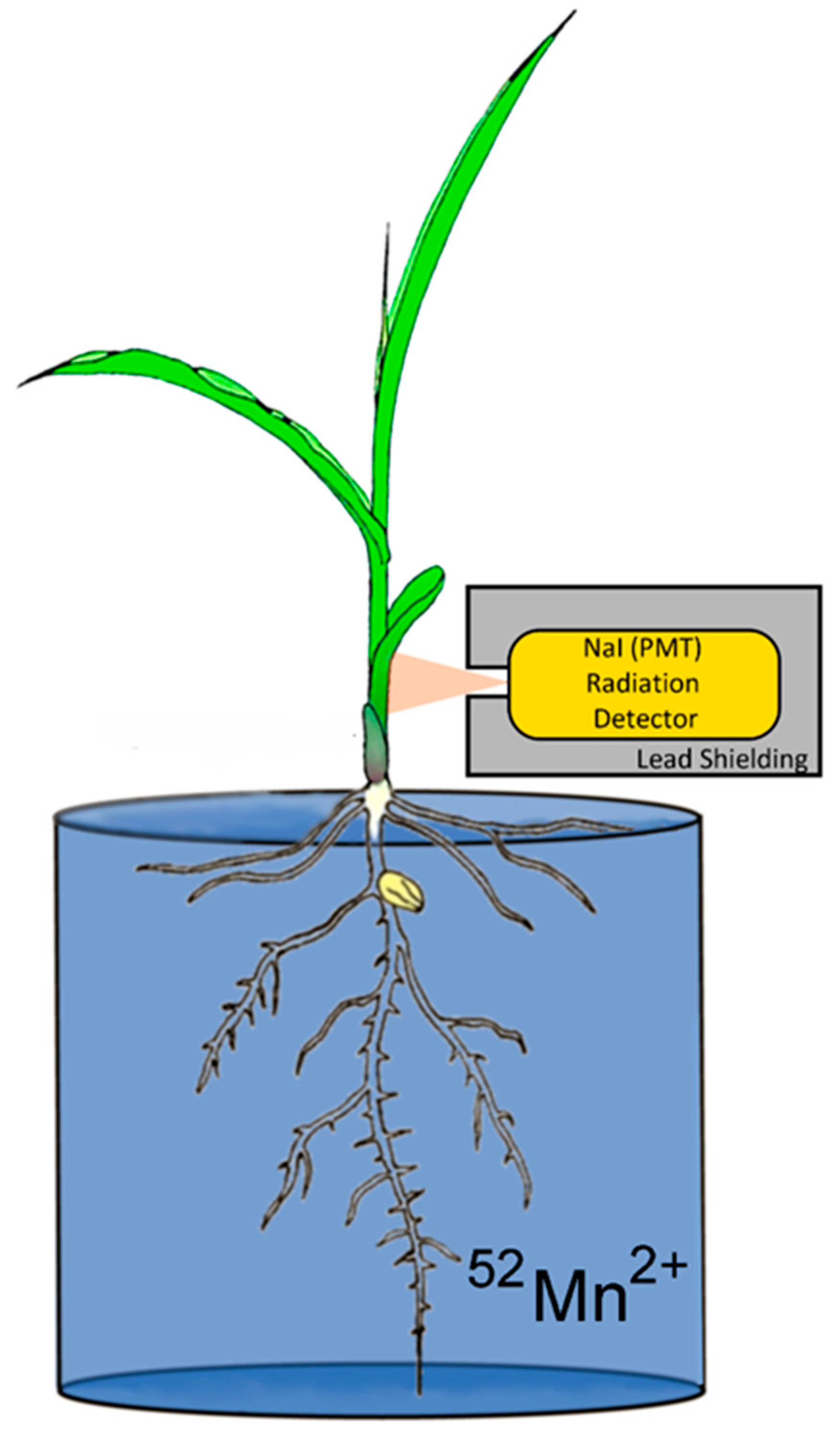
2.3. Plant 52Mn2+ Uptake Studies
2.4. Production and Administration of Radioactive 11CO2
2.5. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) of Root and Leaf Tissue for Mn2+
2.6. Leaf Chlorophyll Analysis
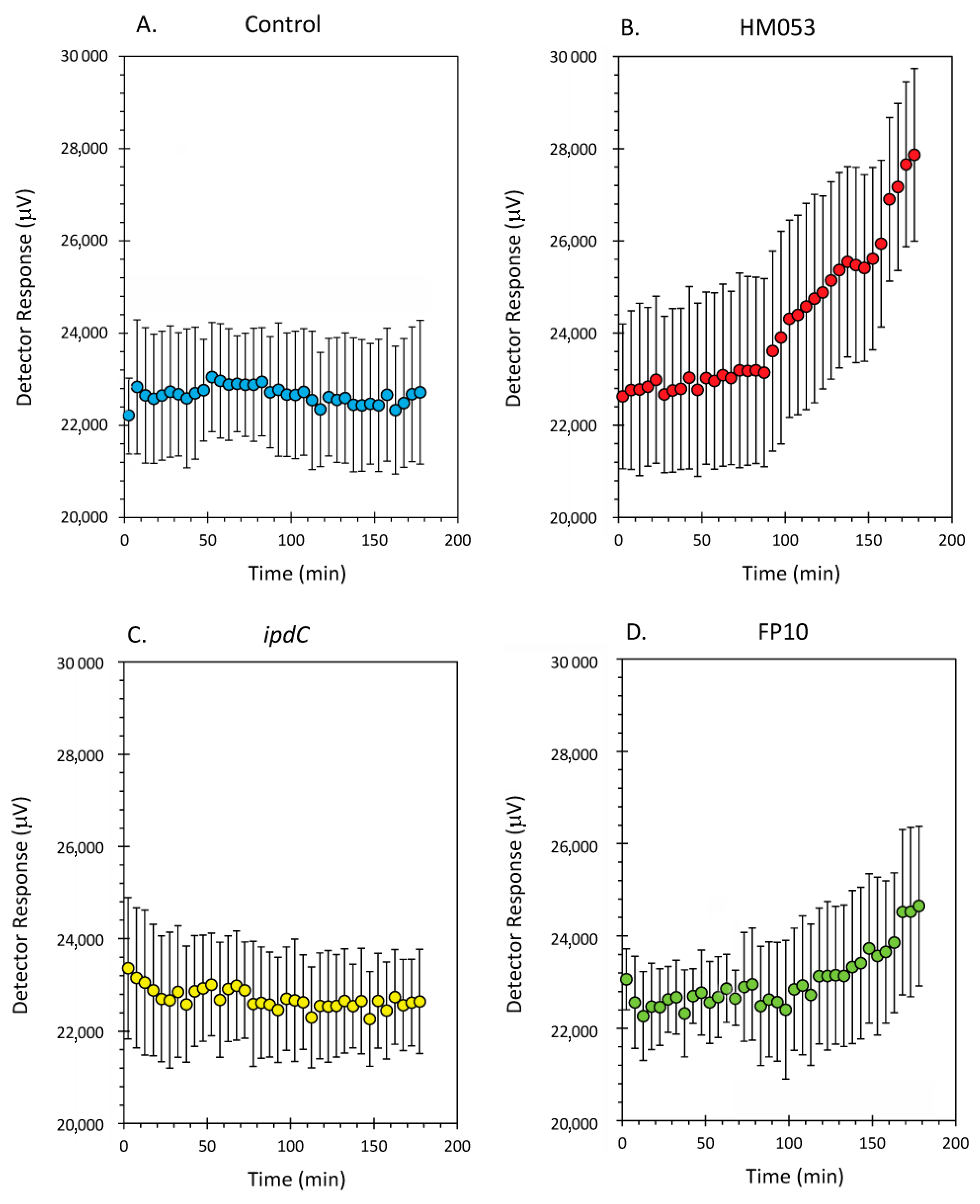
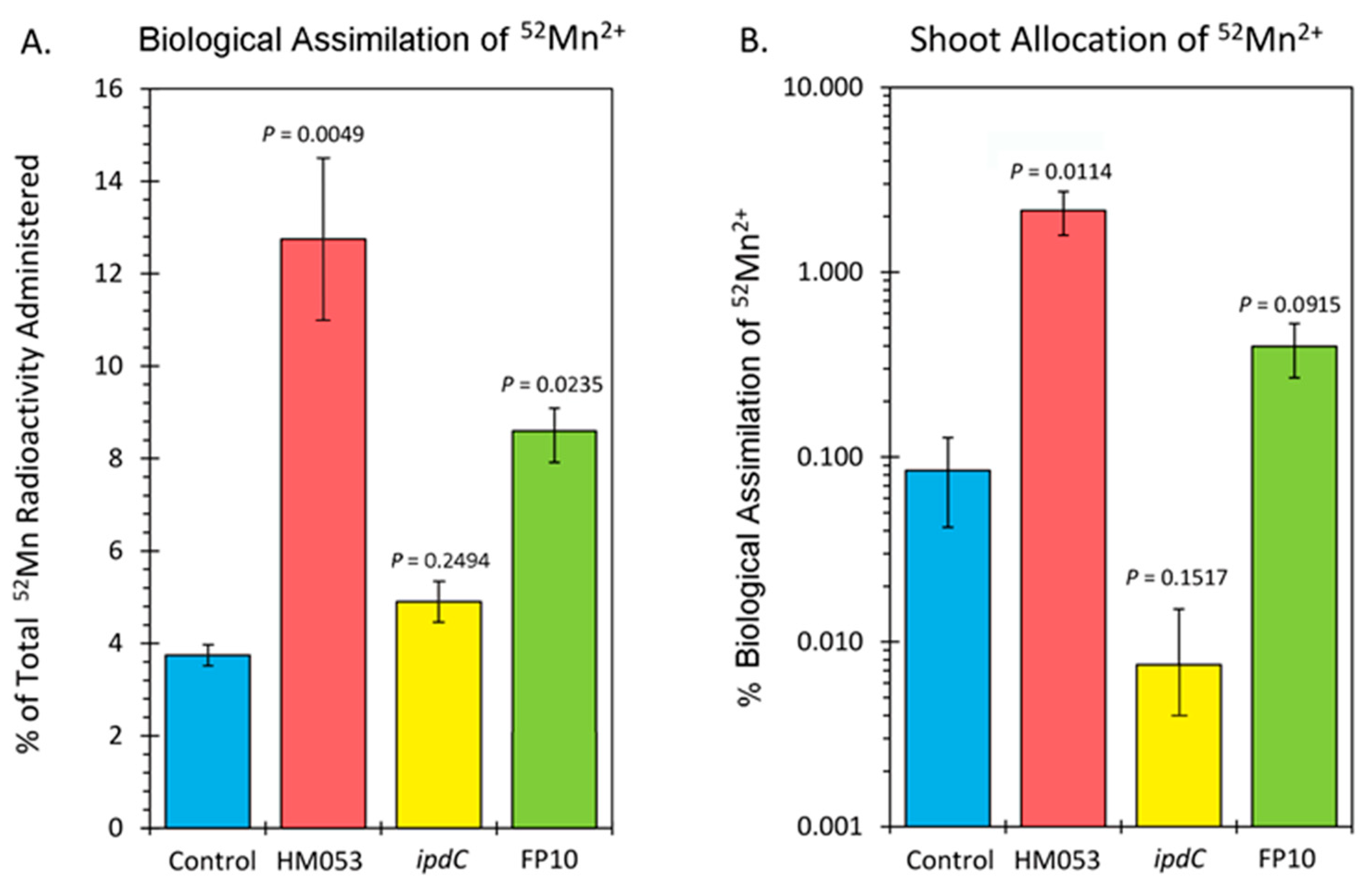
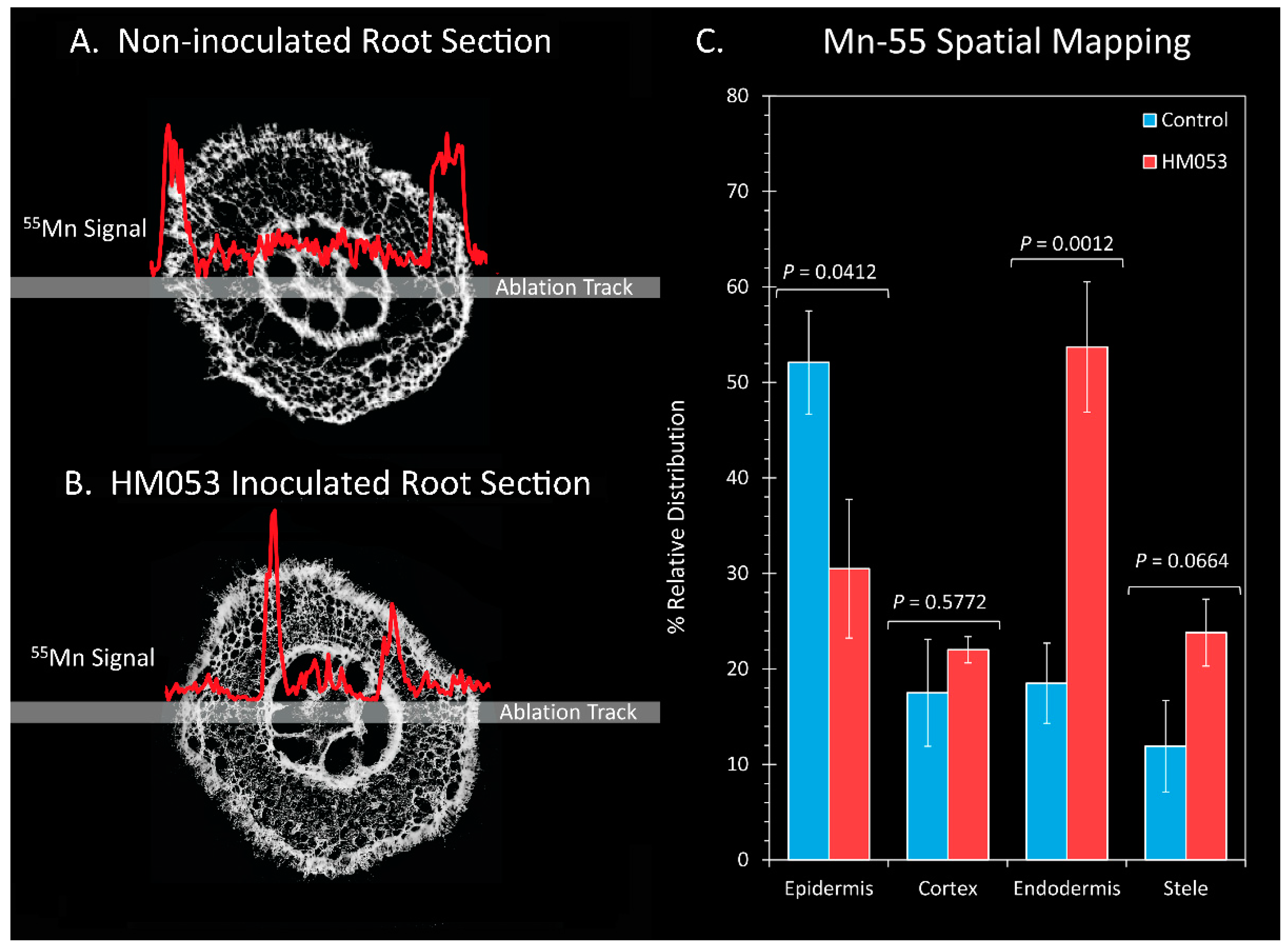
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnell, J.N. The biochemistry of manganese in plants. In Manganese in Soils and Plants; Graham, R.D., Hannam, J., Uren, N.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 125–133. [Google Scholar]
- Alejando, S.; Holler, S.; Meier, B.; Peiter, E. Manganese in plants: Acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Boardman, N.K.; David, D.J. Trace metal composition of fractions obtained by digitonin fragmentation of spinach chloroplasts. Biochem. Biophys. Res. Commun. 1964, 17, 685–689. [Google Scholar] [CrossRef]
- Yachandra, V.K.; Sauer, K.; Klein, M.P. Manganese cluster in photosynthesis: Where plants oxidize water to dioxygen. Chem. Rev. 1996, 96, 2927–2950. [Google Scholar] [CrossRef]
- Henriques, F.S. Reduction in chloroplast number accounts for the decrease in photosynthetic capacity of Mn-deficient pecan leaves. Plant Sci. 2004, 166, 1051–1055. [Google Scholar] [CrossRef]
- Anderson, J.M.; Pyliotis, A.N. Studies with manganese-deficient spinach chloroplasts. Biochim. Biophys. Acta 1969, 189, 280–293. [Google Scholar] [CrossRef]
- Homann, P. Studies on the manganese of chloroplast suspensions. Plant Physiol. 1967, 42, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possingham, J.V.; Spencer, D. Manganese as a functional component of chloroplasts. Aust. J. Biol. Sci. 1962, 15, 58–68. [Google Scholar]
- Spencer, D.; Possingham, J.V. The effects of Mn deficiency on photophosphorylation and the oxygen—Evolving system in spinach chloroplasts. Biochim. Biophys. Acta 1961, 52, 379–381. [Google Scholar] [CrossRef]
- Ohki, K. Manganese deficiency and toxicity effects on photosynthesis, chlorophyll, and transformation in wheat. Crop Sci. 1985, 25, 187–191. [Google Scholar] [CrossRef]
- Gonzalez, A.; Steffen, K.L.; Lynch, J.P. Light and excess manganese: Implications of oxidative stress in common bean. Plant Physiol. 1998, 118, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Gerretsen, F.C. Manganese in relation to photosynthesis. III. Uptake of oxygen by illuminated crude chloroplasts suspensions. Plant Soil 1950, 2, 323–342. [Google Scholar] [CrossRef]
- Clairmont, K.B.; Hagar, W.G.; Davis, E.A. Manganese toxicity to chlorophyll synthesis in tobacco callus. Plant Physiol. 1986, 80, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Thekker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sanna, B.K.; Yadav, S.K.; Singh, D.P.; Singh, H.B. Rhizobacteria mediated induced systemic tolerance in plants: Prospects for abiotic stress management. In Bacteria in Agrobiology: Stress Management; Springer: Berlin/Heidelberg, Germany, 2012; pp. 225–238. [Google Scholar]
- Peréz-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Pankievicz, V.C.S.; do Amaral, F.P.; Santos, K.F.D.N.; Agtuca, B.; Xu, Y.; Schueller, M.J.; Arisi, A.C.M.; Steffens, M.B.R.; de Souza, E.M.; Pedrosa, F.O.; et al. Robust biological nitrogen fixation in a model grass-bacterial association. Plant J. 2015, 81, 907–919. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth—Promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Housh, A.B.; Powell, G.; Scott, S.; Anstaett, A.; Gerheart, A.; Benoit, M.; Guthrie, J.M.; Higgins, B.; Wilder, S.L.; Schueller, M.J.; et al. Mutant Azospirillum brasilense bacterium elicits beneficial physiological and metabolic responses in Zea mays that contribute to increased host iron assimilation. ISME J. 2021, 15, 1505–1522. [Google Scholar] [CrossRef]
- Okon, Y.; Labandera-Gonzalez, C.A. Agronomic applications of Azospirillum: An evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 1994, 26, 1591–1601. [Google Scholar] [CrossRef]
- Döbbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Vanderleyden, J.; Dutto, P.; Labandera-Gonzalez, C.; Caballero-Mellado, J.; Aguirre, J.F.; Kapulnik, Y.; et al. Responses of agro-nomically important crops to inoculation with Azospirillum. Funct. Plant Biol. 2001, 28, 871–879. [Google Scholar] [CrossRef]
- Hungria, M.; Campo, R.J.; de Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and Azospirillum lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Zeffa, D.M.; Perini, L., Jr.; Silva, M.B.; de Sousa, N.V.; Scapim, C.A.; Martinez de Oliveira, A.L.; Teixeira do Amaral, A., Jr.; Goncalves, L.S.A. Azospirillum brasilense promotes increases in growth and nitrogen use efficiency of maize genotypes. PLoS ONE 2019, 14, e0215332. [Google Scholar] [CrossRef] [Green Version]
- Reinhold, B.; Hurek, T.; Niemann, E.-G.; Fendrik, I. Close association of Azospirillum and diazotrophic rods with different root zones of Kallar grass. Appl. Environ. Microbiol. 1986, 52, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housh, A.B.; Benoit, M.; Wilder, S.L.; Scott, S.; Powell, G.; Schueller, M.J.; Ferrieri, R.A. Plant growth promoting bacteria can impact zinc uptake in Zea mays: An examination of the mechanisms of action using functional mutants of Azospirillum brasilense. Microorganisms 2021, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Machado, H.B.; Funayama, S.; Rigo, L.U.; Pedrosa, F.O. Excretion of ammonium by Azospirillum brasilense mutants resistant to ethylenediamine. Can. J. Microbiol. 1991, 37, 549–553. [Google Scholar] [CrossRef]
- Schenberger Santos, A.R.; Etto, R.M.; Furmam, R.W.; de Freitas, D.L.; d’Eça Nogueira Santos, K.F.; de Souza, E.M.; Pedrosa, F.O.; Ayub, R.A.; Steffens, M.B.R.; Galvão, C.W. Labeled Azospirillum brasilense wild type and excretion-ammonium strains in association with barley roots. Plant Physiol. Biochem. 2017, 118, 422–426. [Google Scholar] [CrossRef]
- Pedrosa, F.O.; Yates, M.G. Regulation of nitrogen fixation (nif) genes of Azospirillum brasilense by nifA and ntr (gln) type gene products (Azospirillum brasilense; Nif- mutants; nifA; ntrC.; MoFe protein). FEMS Microbiol. Lett. 1984, 23, 95–101. [Google Scholar] [CrossRef]
- Näsvall, J.; Knöppel, A.; Andersson, D.I. Duplication-Insertion Recombineering: A fast and scar-free method for efficient transfer of multiple mutations in bacteria. Nucleic Acids Res. 2017, 45, e33. [Google Scholar] [CrossRef] [Green Version]
- De Souza, E.M.; Federal University of Paraná, Curitiba, Brazil. Private communication, 2019.
- Graves, S.A.; Hernandez, R.; Fonslet, J.; England, C.G.; Valdovinos, H.F.; Ellison, P.A.; Barnhart, T.E.; Elema, D.R.; Theuer, C.P.; Cai, W.; et al. Novel Preparation Methods of Mn-52 for ImmunoPET Imaging. Bioconjug. Chem. 2015, 26, 2118–2124. [Google Scholar] [CrossRef] [Green Version]
- Ferrieri, R.A.; Wolf, A.P. The chemistry of positron-emitting nucleogenic (hot) atoms with regard to preparation of labeled compounds of practical utility. Radiochim. Acta. 1983, 34, 69–83. [Google Scholar] [CrossRef]
- Ferrieri, R.A. Production and application of synthetic precursors labeled with carbon-11 and fluorine-18. In Handbook of Radiopharmaceuticals: Radiochemistry and Applications; Welch, M.J., Redvanly, C.S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2003. [Google Scholar]
- Ferrieri, R.A.; Gray, D.W.; Babst, B.A.; Schueller, M.J.; Schlyer, D.J.; Thorpe, M.R.; Orians, C.M.; Lerdau, M. Use of carbon-11 in Populus shows that exogenous jasmonic acid increases biosynthesis of isoprene from recently fixed carbon. Plant Cell Environ. 2005, 25, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Wilder, S.L.; Scott, S.; Waller, S.; Powell, A.; Benoit, M.; Guthrie, J.M.; Schueller, M.J.; Awale, P.; McSteen, P.; Matthes, M.S.; et al. Carbon-11 Radiotracing Reveals Physiological and Metabolic Responses of Maize Grown under Different Regimes of Boron Treatment. Plants 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K. Managing the manganese: Molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Li, H.; Liu, Y.; Zhu, L.; Guo, J.; Liu, X.; Fan, Y.; Chen, J.; Chen, R. Overexpression of ZmIRT1 and ZmZIP3 Enhances Iron and Zinc Accumulation in Transgenic Arabidopsis. PLoS ONE 2015, 10, e0136647. [Google Scholar] [CrossRef]
- Castaings, L.; Alcon, C.; Kosuth, T.; Correia, D.; Curie, C. Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1. Plant J. 2021, 106, 1328–1337. [Google Scholar] [CrossRef]
- Quintana, J.; Bernal, M.; Scholle, M.; Heike Holländer-Czytko, H.; Nguyen, N.T.; Piotrowski, M.; Mendoza-Cözatl, D.G.; Haydon, M.J.; Krämer, U. Root-to-shoot iron partitioning in Arabidopsis requires IRON-REGULATED TRANSPORTER1 (IRT1) protein but not its iron (II) transport function. Plant J. 2022, 109, 992–1013. [Google Scholar] [CrossRef]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal transporters NRAMP1 and IRT1 team up to take up iron under sufficient metal provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [Green Version]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, E450–E458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Z.; Bai, J.; Cui, H.; Gong, C. A missing link in radial ion transport: Ion transporters in the endodermis. Front. Plant Sci. 2019, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M. The endodermis as a checkpoint for nutrients. New Phytol. 2017, 213, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Pauluzzi, G.; Bailey-Serres, J. Flexible ion barrier. Cell 2016, 164, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Housh, A.B.; Powell, G.; Benoit, M.; Anstaett, A.; Gerheart, A.; Wilder, S.L.; Schueller, M.J.; Ferrieri, R.A. Azospirillum brasilense (HM053) boosts corn yield and ferritin content. Agron. J. 2020, 10, 394–405. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Housh, A.B.; Waller, S.; Sopko, S.; Powell, A.; Benoit, M.; Wilder, S.L.; Guthrie, J.; Schueller, M.J.; Ferrieri, R.A. Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis. Microorganisms 2022, 10, 1290. https://doi.org/10.3390/microorganisms10071290
Housh AB, Waller S, Sopko S, Powell A, Benoit M, Wilder SL, Guthrie J, Schueller MJ, Ferrieri RA. Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis. Microorganisms. 2022; 10(7):1290. https://doi.org/10.3390/microorganisms10071290
Chicago/Turabian StyleHoush, Alexandra B., Spenser Waller, Stephanie Sopko, Avery Powell, Mary Benoit, Stacy L. Wilder, James Guthrie, Michael J. Schueller, and Richard A. Ferrieri. 2022. "Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis" Microorganisms 10, no. 7: 1290. https://doi.org/10.3390/microorganisms10071290
APA StyleHoush, A. B., Waller, S., Sopko, S., Powell, A., Benoit, M., Wilder, S. L., Guthrie, J., Schueller, M. J., & Ferrieri, R. A. (2022). Azospirillum brasilense Bacteria Promotes Mn2+ Uptake in Maize with Benefits to Leaf Photosynthesis. Microorganisms, 10(7), 1290. https://doi.org/10.3390/microorganisms10071290





