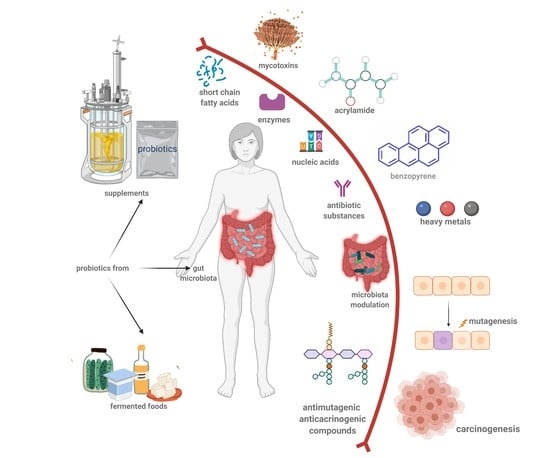
Biodetoxification and Protective Properties of Probiotics
Abstract
:1. Introduction
2. Probiotics in Human Health and Microbiota Modulation
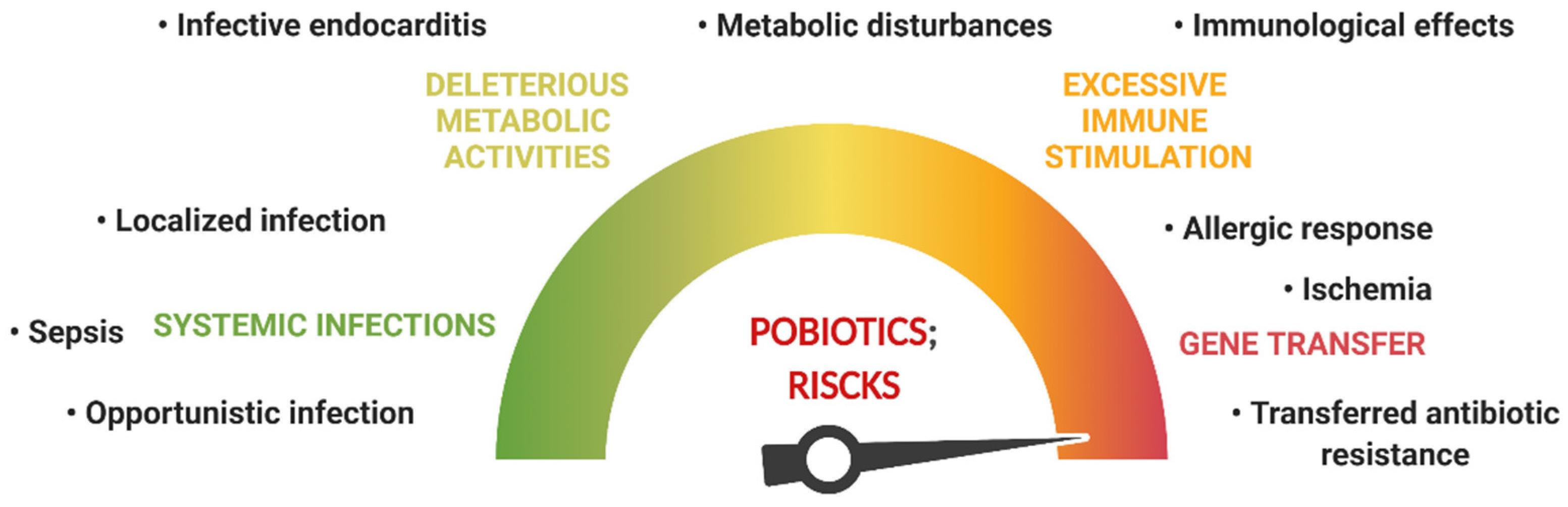
3. Probiotic Safety Issues
4. Food Contaminants and Their Impact on Human Health
4.1. Chemical Contaminants and Their Impact on Human Health
4.1.1. Heavy Metals
4.1.2. Acrylamide
4.1.3. Polycyclic Aromatic Hydrocarbons—Benzo[a]Pyrene
4.2. Biological Contaminants and Their Impact on Human Health
Fungi—Molds and Yeasts and Their Mycotoxins
5. Biodetoxification Activity of Probiotics
5.1. Lactobacillus (LAB) Genera and Their Biodetoxification Capacity
5.2. Bifidobacteria Genera and Their Biodetoxification Capacity
5.3. Probiotic Yeasts and Their Biodetoxification Capacity
5.4. Other Probiotics or Promising Probiotic Candidates and Their Biodetoxification Capacity
6. Probiotic Antimutagenic Activity
7. Anti-Carcinogenic Effect of Probiotics
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhvi, N.; Gupta, V.; Gaur, M.; Sharma, V.; Puri, A.; Singh, Y.; Dubey, G.P.; Lal, R. Interplay of Human Gut Microbiome in Health and Wellness. Indian J. Microbiol. 2019, 60, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, V.; Mokarram, R.R.; Khiabani, M.S.; Askari, F.; Ahmadi, E.; Hassanzadeh, A.m.; Aghazadeh, S.b.; Asgharzadeh, M.; Kafil, H.S. Molecular Identification of Lactobacillus Acidophilus as a Probiotic Potential from Traditional Doogh Samples and Evaluation of Their Antimicrobial Activity against Some Pathogenic Bacteria. Biomed. Res. 2017, 28, 1458–1463. [Google Scholar]
- Hashemi, S.M.B.; Gholamhosseinpour, A. Fermentation of table cream by Lactobacillus plantarum strains: Effect on fungal growth, aflatoxin M1 and ochratoxin A. Int. J. Food Sci. Technol. 2019, 54, 347–353. [Google Scholar] [CrossRef]
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Megeed, R.M. Probiotics: A Promising Generation of Heavy Metal Detoxification. Biol. Trace Element Res. 2020, 199, 2406–2413. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [Green Version]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferraù, F.; Libra, M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Rodrigues, C.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Prabu, S.M.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Lee, E.-S.; Song, E.-J.; Nam, Y.-D.; Lee, S.-Y. Probiotics in human health and disease: From nutribiotics to pharmabiotics. J. Microbiol. 2018, 56, 773–782. [Google Scholar] [CrossRef]
- Min, M.; Bunt, C.R.; Mason, S.L.; Hussain, M.A. Non-dairy probiotic food products: An emerging group of functional foods. Crit. Rev. Food Sci. Nutr. 2018, 59, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [Green Version]
- Pop, O.L.; Socaci, S. Chapter 10—Pro and Prebiotics foods that modulate human health. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 283–313. [Google Scholar]
- Coman, V.; Vodnar, D.C. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Exp. Gerontol. 2020, 141, 111095. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, Y.-G.; Jin, Y.-Y.; Jayabalan, R.; Yang, S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. Nutr. 2017, 58, 2743–2767. [Google Scholar] [CrossRef]
- Azad, A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Alitheen, N.B.M.; Yeap, S.K.; Mutalib, N.E.A.; Rahim, R.A.; Yusoff, K. Postbiotic metabolites produced by Lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Sood, U.; Gupta, V.; Singh, M.; Scaria, J.; Lal, R. Recent Advancements in the Development of Modern Probiotics for Restoring Human Gut Microbiome Dysbiosis. Indian J. Microbiol. 2019, 60, 12–25. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, T.-L.; Tsai, Y.-L.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Ahtesh, F.B.; Stojanovska, L.; Apostolopoulos, V. Anti-hypertensive peptides released from milk proteins by probiotics. Maturitas 2018, 115, 103–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.; Jain, T.; Bose, S.; Bhosale, V. Importance of Probiotics in Human Health. In Functional Food and Human Health; Rani, V., Yadav, U.C.S., Eds.; Springer: Singapore, 2018; pp. 539–554. [Google Scholar]
- Amin, N.; Boccardi, V.; Taghizadeh, M.; Jafarnejad, S. Probiotics and bone disorders: The role of RANKL/RANK/OPG pathway. Aging (Milan, Italy) 2019, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med J. [SQUMJ] 2020, 20, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, S.; Silva, J.; Teixeira, P. The role of lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2013, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2019, 65, 245–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedin, C.; Whelan, K.; Lindsay, J.O. Evidence for the use of probiotics and prebiotics in inflammatory bowel disease: A review of clinical trials. Proc. Nutr. Soc. 2007, 66, 307–315. [Google Scholar] [CrossRef]
- Islam, S.U. Clinical Uses of Probiotics. Medicine 2016, 95, e2658. [Google Scholar] [CrossRef]
- Barouei, J.; Moussavi, M.; Hodgson, D. Perinatal maternal probiotic intervention impacts immune responses and ileal mucin gene expression in a rat model of irritable bowel syndrome. Benef. Microbes 2015, 6, 83–95. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Sheu, B.-S. Probiotics-Containing Yogurts Suppress Helicobacter pylori Load and Modify Immune Response and Intestinal Microbiota in the Helicobacter pylori-Infected Children. Helicobacter 2012, 17, 297–304. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control 2019, 108, 106872. [Google Scholar] [CrossRef]
- Zuo, F.; Chen, S.; Marcotte, H. Engineer probiotic bifidobacteria for food and biomedical applications - Current status and future prospective. Biotechnol. Adv. 2020, 45, 107654. [Google Scholar] [CrossRef] [PubMed]
- FAO. Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002. [Google Scholar]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, S.; Ding, S.; Shen, J.; Zhu, K. Toxins and mobile antimicrobial resistance genes in Bacillus probiotics constitute a potential risk for One Health. J. Hazard. Mater. 2019, 382, 121266. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Ouwehand, A.; Reid, G.; Salminen, S.; Cabana, M.D.; Paraskevakos, G.; Leyer, G. Probiotic use in at-risk populations. J. Am. Pharm. Assoc. 2016, 56, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Sotoudegan, F.; Daniali, M.; Hassani, S.; Nikfar, S.; Abdollahi, M. Reappraisal of probiotics’ safety in human. Food Chem. Toxicol. 2019, 129, 22–29. [Google Scholar] [CrossRef]
- Kirk, M.D.; Angulo, F.J.; Havelaar, A.H.; Black, R.E. Diarrhoeal disease in children due to contaminated food. Bull. World Health Organ. 2017, 95, 233–234. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Średnicka, P.; Juszczuk-Kubiak, E.; Wójcicki, M.; Akimowicz, M.; Roszko, M. Probiotics as a biological detoxification tool of food chemical contamination: A review. Food Chem. Toxicol. 2021, 153, 112306. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Chen, C.-Y.; Lu, T.-H.; Liao, C.-M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mater. 2018, 366, 703–713. [Google Scholar] [CrossRef]
- Yao, Y.; Long, M. The biological detoxification of deoxynivalenol: A review. Food Chem. Toxicol. 2020, 145, 111649. [Google Scholar] [CrossRef] [PubMed]
- Madreseh, S.; Ghaisari, H.R.; Hosseinzadeh, S. Effect of Lyophilized, Encapsulated Lactobacillus fermentum and Lactulose Feeding on Growth Performance, Heavy Metals, and Trace Element Residues in Rainbow Trout (Oncorhynchus mykiss) Tissues. Probiotics Antimicrob. Proteins 2018, 11, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Majlesi, M.; Shekarforoush, S.S.; Ghaisari, H.R.; Nazifi, S.; Sajedianfard, J.; Eskandari, M.H. Effect of Probiotic Bacillus Coagulans and Lactobacillus Plantarum on Alleviation of Mercury Toxicity in Rat. Probiotics Antimicrob. Proteins 2017, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-J.; Lim, J.-M.; Gu, S.; Lee, W.-K.; Oh, E. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb(II) toxicity. J. Microbiol. 2017, 55, 296–303. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Panwar, R.; Ram, C. Efficacy of indigenous probiotic Lactobacillus strains to reduce cadmium bioaccessibility - An in vitro digestion model. Environ. Sci. Pollut. Res. 2016, 24, 1241–1250. [Google Scholar] [CrossRef]
- Khorshidian, N.; Yousefi, M.; Shadnoush, M.; Siadat, S.D.; Mohammadi, M.; Mortazavian, A.M. Using probiotics for mitigation of acrylamide in food products: A mini review. Curr. Opin. Food Sci. 2020, 32, 67–75. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Chapter Five—Recent Insights in Acrylamide as Carcinogen in Foodstuffs. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 163–193. [Google Scholar]
- Bartkiene, E.; Jakobsone, I.; Juodeikiene, G.; Vidmantiene, D.; Pugajeva, I.; Bartkevics, V. Study on the reduction of acrylamide in mixed rye bread by fermentation with bacteriocin-like inhibitory substances producing lactic acid bacteria in combination with Aspergillus niger glucoamylase. Food Control 2013, 30, 35–40. [Google Scholar] [CrossRef]
- Cuevas-González, P.; Aguilar-Toalá, J.; García, H.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Protective Effect of the Intracellular Content from Potential Probiotic Bacteria against Oxidative Damage Induced by Acrylamide in Human Erythrocytes. Probiotics Antimicrob. Proteins 2020, 12, 1459–1470. [Google Scholar] [CrossRef]
- Fu, L.; Ning, Y.; Zhao, H.; Fan, J.; Zhang, B. The In Vitro Adsorption Ability of Lactobacillus acidophilus NCFM to Benzo(a)pyrene in PM2.5. J. Toxicol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Shoukat, S.; Liu, Y.; Rehman, A.; Zhang, B. Screening of Bifidobacterium strains with assignment of functional groups to bind with benzo[a]pyrene under food stress factors. J. Chromatogr. B 2019, 1114–1115, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Trovato, L.; Volti, G.L.; Oliveri, S.; Blandino, G. In vitro inhibitory activity of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 alone or in combination against bacterial and Candida reference strains and clinical isolates. Heliyon 2019, 5, e02891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Taheur, F.; Mansour, C.; Kouidhi, B.; Chaieb, K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 2019, 166, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kosgey, J.C.; Jia, L.; Fang, Y.; Yang, J.; Gao, L.; Wang, J.; Nyamao, R.; Cheteu, M.; Tong, D.; Wekesa, V.; et al. Probiotics as antifungal agents: Experimental confirmation and future prospects. J. Microbiol. Methods 2019, 162, 28–37. [Google Scholar] [CrossRef]
- Liu, F.; Malaphan, W.; Xing, F.; Yu, B. Biodetoxification of fungal mycotoxins zearalenone by engineered probiotic bacterium Lactobacillus reuteri with surface-displayed lactonohydrolase. Appl. Microbiol. Biotechnol. 2019, 103, 8813–8824. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, X.; Yuan, L.; Li, J. Complicated interactions between bio-adsorbents and mycotoxins during mycotoxin adsorption: Current research and future prospects. Trends Food Sci. Technol. 2019, 96, 127–134. [Google Scholar] [CrossRef]
- Alu’Datt, M.H.; Rababah, T.; Sakandar, H.A.; Imran, M.; Mustafa, N.; Alhamad, M.N.; Mhaidat, N.; Kubow, S.; Tranchant, C.; Al-Tawaha, A.R.; et al. Fermented food-derived bioactive compounds with anticarcinogenic properties: Fermented royal jelly as a novel source for compounds with health benefits. In Anticancer Plants: Properties and Application; Springer: Singapore, 2018; pp. 141–165. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Lee, S.; Lee, J.; Ha, J.; Choi, Y.; Yoon, Y.; Choi, K.-H. Invited review: Microbe-mediated aflatoxin decontamination of dairy products and feeds. J. Dairy Sci. 2017, 100, 871–880. [Google Scholar] [CrossRef]
- Wochner, K.F.; Moreira, M.C.C.; Kalschne, D.L.; Colla, E.; Drunkler, D.A. Detoxification of Aflatoxin B 1 and M 1 by Lactobacillus acidophilus and prebiotics in whole cow’s milk. J. Food Saf. 2019, 39, e12670. [Google Scholar] [CrossRef]
- Tajik, H.; Sayadi, M. Effects of probiotic bacteria of Lactobacillus acidophilus and Lactobacillus casei on aflatoxin B1 detoxification within a simulated gastrointestinal tract model. Toxin Rev. 2020, 41, 92–99. [Google Scholar] [CrossRef]
- Wu, J.-C.; Tsai, M.-L.; Lai, C.-S.; Lo, C.-Y.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Polymethoxyflavones prevent benzo[a ]pyrene/dextran sodium sulfate-induced colorectal carcinogenesis through modulating xenobiotic metabolism and ameliorate autophagic defect in ICR mice. Int. J. Cancer 2017, 142, 1689–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halttunen, T.; Finell, M.; Salminen, S. Arsenic removal by native and chemically modified lactic acid bacteria. Int. J. Food Microbiol. 2007, 120, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente, M.J.; Vivó, M.D.L.; Puig, S.; Zúñiga, M.; Monedero, V.; Devesa, V.; Vélez, D. In vitro evaluation of the efficacy of lactobacilli and yeasts in reducing bioavailability of inorganic arsenic. LWT 2020, 126, 109272. [Google Scholar] [CrossRef]
- Hamad, G.M.; Taha, T.H.; E Hafez, E.; Ali, S.H.; A El Sohaimy, S. Supplementation of Cerelac baby food with yeast–probiotic cocktail strains induces high potential for aflatoxin detoxification both in vitro and in vivo in mother and baby albino rats. J. Sci. Food Agric. 2017, 98, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jin, D.; Yu, S.; Evivie, S.E.; Muhammad, Z.; Huo, G.; Liu, F. In Vitro and In vivo Evaluation of Lactobacillus delbrueckii subsp. bulgaricus KLDS1.0207 for the Alleviative Effect on Lead Toxicity. Nutrients 2017, 9, 845. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, B.N.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Reduction of acrylamide in whole-wheat bread by combining lactobacilli and yeast fermentation. Food Addit. Contam. Part A 2017, 34, 1904–1914. [Google Scholar] [CrossRef]
- Śliżewska, K.; Cukrowska, B.; Smulikowska, S.; Cielecka-Kuszyk, J. The Effect of Probiotic Supplementation on Performance and the Histopathological Changes in Liver and Kidneys in Broiler Chickens Fed Diets with Aflatoxin B1. Toxins 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Martínez, B.; Rodríguez, A.; Kulakauskas, S.; Chapot-Chartier, M.-P. Cell wall homeostasis in lactic acid bacteria: Threats and defences. FEMS Microbiol. Rev. 2020, 44, 538–564. [Google Scholar] [CrossRef]
- Chalova, V.I.; Lingbeck, J.M.; Kwon, Y.M.; Ricke, S.C. Extracellular antimutagenic activities of selected probiotic Bifidobacterium and Lactobacillus spp. as a function of growth phase. J. Environ. Sci. Health Part B 2008, 43, 193–198. [Google Scholar] [CrossRef]
- He, X.; Zhao, L.; Zhang, B.; Zhao, H. [Removal of benzo(a)pyrene by Lactobacillus strains under simulated starch conditions]. Acta Microbiol. Sin. 2016, 56, 814–823. [Google Scholar]
- Zhao, H.; Zhou, F.; Qi, Y.; Dziugan, P.; Bai, F.; Walczak, P.; Zhang, B. Screening of Lactobacillus strains for their ability to bind Benzo(a)pyrene and the mechanism of the process. Food Chem. Toxicol. 2013, 59, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Sohrabvandi, S.; Mortazavian, A.M.; Mohammadi, R.; Rezaei-Tavirani, M. Reduction of aflatoxin in fermented milks during production and storage. Toxin Rev. 2012, 31, 44–53. [Google Scholar] [CrossRef]
- Sarlak, Z.; Rouhi, M.; Mohammadi, R.; Khaksar, R.; Mortazavian, A.M.; Sohrabvandi, S.; Garavand, F. Probiotic biological strategies to decontaminate aflatoxin M1 in a traditional Iranian fermented milk drink (Doogh). Food Control 2017, 71, 152–159. [Google Scholar] [CrossRef]
- Bahmani, S.; Azarpira, N.; Moazamian, E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk. J. Gastroenterol. 2019, 30, 835–842. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Pei-Ren, L.; Chuiyu, R.; Cheng-Chun, C.; Ya-Hui, T. Antimutagenic activity of several probiotic bifidobacteria against Benzo[a]pyrene. J. Biosci. Bioeng. 2002, 94, 148–153. [Google Scholar] [CrossRef]
- Xu, M.; Fu, L.; Zhang, J.; Wang, T.; Fan, J.; Zhu, B.; Dziugan, P.; Zhang, B.; Zhao, H. Potential of Inactivated Bifidobacterium Strain in Attenuating Benzo(A)Pyrene Exposure-Induced Damage in Colon Epithelial Cells In Vitro. Toxics 2020, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Valdez, R.M.A.; Ximenez-Fyvie, L.A.; Caiaffa, K.S.; dos Santos, V.R.; Cervantes, R.M.G.; Almaguer-Flores, A.; Duque, C. Antagonist effect of probiotic bifidobacteria on biofilms of pathogens associated with periodontal disease. Microb. Pathog. 2020, 150, 104657. [Google Scholar] [CrossRef]
- Lili, Z.; Junyan, W.; Hongfei, Z.; Baoqing, Z.; Bolin, Z. Detoxification of cancerogenic compounds by lactic acid bacteria strains. Crit. Rev. Food Sci. Nutr. 2017, 58, 2727–2742. [Google Scholar] [CrossRef]
- Cecchini, F.; Morassut, M.; Saiz, J.-C.; Garcia-Moruno, E. Anthocyanins enhance yeast’s adsorption of Ochratoxin A during the alcoholic fermentation. Eur. Food Res. Technol. 2018, 245, 309–314. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Wu, C.; Peng, B. Physical adsorption of patulin by Saccharomyces cerevisiae during fermentation. J. Food Sci. Technol. 2019, 56, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Magnoli, A.; Pereyra, M.G.; Cavaglieri, L. Probiotic bacteria and yeasts adsorb aflatoxin M1 in milk and degrade it to less toxic AFM1-metabolites. Toxicon 2019, 172, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jakopović, Ž.; Čiča, K.H.; Mrvčić, J.; Pucić, I.; Čanak, I.; Frece, J.; Pleadin, J.; Stanzer, D.; Zjalic, S.; Markov, K. Properties and Fermentation Activity of Industrial Yeasts Saccharomyces cerevisiae, S. uvarum, Candida utilis and Kluyveromyces marxianus Exposed to AFB1, OTA and ZEA. Food Technol. Biotechnol. 2018, 56, 208–217. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.; Liu, B.; Wang, Z.; Yuan, Y.; Yue, T. Effect of Yeast Cell Morphology, Cell Wall Physical Structure and Chemical Composition on Patulin Adsorption. PLoS ONE 2015, 10, e0136045. [Google Scholar] [CrossRef] [Green Version]
- Sahebghalam, H.; Sani, A.; Mehraban, M. Bio-detoxification of AFB1 in animal feeds using Saccharomyces cerevisiae. J. Innov. Food Sci. Technol. 2013, 5, 99–104. [Google Scholar]
- Di Francesco, A.; Mari, M.; Ugolini, L.; Parisi, B.; Genovese, J.; Lazzeri, L.; Baraldi, E. Reduction of acrylamide formation in fried potato chips by Aureobasidum pullulans L1 strain. Int. J. Food Microbiol. 2018, 289, 168–173. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Randive, S.A.; Jadhav, D.B.; Nadaf, N.H.; Parulekar, R.S.; Sonawane, K.D. Production of novel antimicrobial protein from Bacillus licheniformis strain JS and its application against antibiotic-resistant pathogens. J. Proteins Proteom. 2019, 10, 17–22. [Google Scholar] [CrossRef]
- Jin, P.; Tan, Z.; Wang, H.; Liu, W.; Miao, W. Antimicrobial effect of Bacillus licheniformis HN-5 bacitracin A on rice pathogen Pantoea ananatis. BioControl 2020, 66, 249–257. [Google Scholar] [CrossRef]
- Yi, P.-J.; Pai, C.-K.; Liu, J.-R. Isolation and characterization of a Bacillus licheniformis strain capable of degrading zearalenone. World J. Microbiol. Biotechnol. 2010, 27, 1035–1043. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, J. Isolation and characterization of an Enterococcus strain from Chinese sauerkraut with potential for lead removal. Eur. Food Res. Technol. 2020, 246, 2055–2064. [Google Scholar] [CrossRef]
- Mohsin, M.; Guenther, S.; Schierack, P.; Tedin, K.; Wieler, L.H. Probiotic Escherichia coli Nissle 1917 reduces growth, Shiga toxin expression, release and thus cytotoxicity of enterohemorrhagic Escherichia coli. Int. J. Med Microbiol. 2015, 305, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, R.; Chaudhari, A.; Kumar, G.N. 2-Ketogluconic acid and pyrroloquinoline quinone secreting probiotic Escherichia coli Nissle 1917 as a dietary strategy against heavy metal induced damage in rats. J. Funct. Foods 2017, 37, 541–552. [Google Scholar] [CrossRef]
- Nandy, A.; Basak, S.C. Simple numerical descriptor for quantifying effect of toxic substances on DNA sequences. J. Chem. Inf. Comput. Sci. 2000, 40, 915–919. [Google Scholar] [CrossRef]
- Weisburger, J.H.; Barnes, W.S.; Czerniak, R. Mutagens and carcinogens in food. In Diet, Nutrition, and Cancer: A Critical Evaluation; CRC Press: Boca Raton, FL, USA, 2018; pp. 115–134. [Google Scholar]
- Križková, L.; Belicová, A.; Dobias, J.; Krajčovič, J.; Ebringer, L. Selenium enhances the antimutagenic activity of probiotic bacterium Enterococcus faecium M-74. World J. Microbiol. Biotechnol. 2002, 18, 867–873. [Google Scholar] [CrossRef]
- Lim, S.-M. Antimutagenicity activity of the putative probiotic strain Lactobacillus paracasei subsp. tolerans JG22 isolated from pepper leaves Jangajji. Food Sci. Biotechnol. 2013, 23, 141–150. [Google Scholar] [CrossRef]
- Sah, B.; Vasiljevic, T.; McKechnie, S.; Donkor, O. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J. Dairy Sci. 2015, 98, 5905–5916. [Google Scholar] [CrossRef]
- Chandel, D.; Sharma, M.; Chawla, V.; Sachdeva, N.; Shukla, G. Isolation, characterization and identification of antigenotoxic and anticancerous indigenous probiotics and their prophylactic potential in experimental colon carcinogenesis. Sci. Rep. 2019, 9, 14769. [Google Scholar] [CrossRef] [Green Version]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of pineapple waste powder on probiotic growth, antioxidant and antimutagenic activities of yogurt. J. Food Sci. Technol. 2015, 53, 1698–1708. [Google Scholar] [CrossRef]
- Song, M.; Yun, B.; Moon, J.-H.; Park, D.-J.; Lim, K.; Oh, S. Characterization of Selected Lactobacillus Strains for Use as Probiotics. Korean J. Food Sci. Anim. Resour. 2015, 35, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, H.; Roser, M. Causes of Death. Our World in Data. 2018. Available online: https://ourworldindata.org/causes-of-death (accessed on 16 June 2021).
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic Effect of Saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiotics Antimicrob. Proteins 2020, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Pan, D.; Yang, Y.; Jiang, X.; Zhang, J.; Zeng, X.; Wu, Z.; Sun, Y.; Guo, Y. Effect of Lactobacillus acidophilus CICC 6074 S-Layer Protein on Colon Cancer HT-29 Cell Proliferation and Apoptosis. J. Agric. Food Chem. 2020, 68, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Dianatpour, A.; Ghafouri-Fard, S. The Role of Probiotics in Cancer Treatment: Emphasis on their In vivo and In Vitro Anti-metastatic Effects. Int. J. Mol. Cell. Med. 2017, 6, 66–76. [Google Scholar] [CrossRef]
- Panebianco, C.; Latiano, T.; Pazienza, V. Microbiota Manipulation by Probiotics Administration as Emerging Tool in Cancer Prevention and Therapy. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Pescuma, M.; Gomez-Gomez, B.; Perez-Corona, T.; Font, G.; Madrid, Y.; Mozzi, F. Food prospects of selenium enriched-Lactobacillus acidophilus CRL 636 and Lactobacillus reuteri CRL 1101. J. Funct. Foods 2017, 35, 466–473. [Google Scholar] [CrossRef]
- Rajoka, M.S.R.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 2018, 9, 2705–2715. [Google Scholar] [CrossRef]
- Utz, V.E.M.; Visñuk, D.P.; Perdigón, G.; Leblanc, A.d.M.d. Milk fermented by Lactobacillus casei CRL431 administered as an immune adjuvant in models of breast cancer and metastasis under chemotherapy. Appl. Microbiol. Biotechnol. 2020, 105, 327–340. [Google Scholar] [CrossRef]
- Silveira, D.S.C.; Veronez, L.C.; Lopes-Júnior, L.C.; Anatriello, E.; Brunaldi, M.O.; Pereira-Da-Silva, G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J. Gastroenterol. 2020, 26, 6782–6794. [Google Scholar] [CrossRef]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer Apc Min/+ mouse model. Appl. Microbiol. Biotechnol. 2016, 101, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, M.; Nicoletti, M.; Ratti, M.; Tomasello, G.; Lonati, V.; Ghilardi, M.; Parati, M.; Borgonovo, K.; Cabiddu, M.; Petrelli, F. Lactobacillus Kefiri LKF01 (Kefibios®) for Prevention of Diarrhoea in Cancer Patients Treated with Chemotherapy: A Prospective Study. Nutrients 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ge, X.; Yang, L.; Chen, X.; Xu, Q.; Rui, X.; Fan, X.; Feng, L.; Zhang, Q.; Dong, M.; et al. Anticancer potential of an exopolysaccharide from Lactobacillus helveticus MB2-1 on human colon cancer HT-29 cells via apoptosis induction. Food Funct. 2020, 11, 10170–10181. [Google Scholar] [CrossRef]
- Luo, M.; Hu, M.; Feng, X.; XiaoLi, W.; Dong, D.; Wang, W. Preventive effect of Lactobacillus reuteri on melanoma. Biomed. Pharmacother. 2020, 126, 109929. [Google Scholar] [CrossRef]
- Rahimi, A.M.; Nabavizadeh, F.; Ashabi, G.; Halimi, S.; Rahimpour, M.; Vahedian, J.; Panahi, M. Probiotic Lactobacillus rhamnosus Supplementation Improved Capecitabine Protective Effect against Gastric Cancer Growth in Male BALB/c Mice. Nutr. Cancer 2020, 73, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Suzutani, T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian J. Hepato-Gastroenterol. 2018, 8, 11–17. [Google Scholar] [CrossRef]
- Jaskulski, I.B.; Uecker, J.; Bordini, F.; Moura, F.; Gonçalves, T.; Chaves, N.G.; Camargo, F.; Grecco, F.B.; Fiorentini, M.; da Silva, W.P.; et al. In vivo action of Lactococcus lactis subsp. lactis isolate (R7) with probiotic potential in the stabilization of cancer cells in the colorectal epithelium. Process Biochem. 2019, 91, 165–171. [Google Scholar] [CrossRef]


| Lactobacillus Strain/mix | Cell Count CFU/mL | Contaminant | Food/Environment | Contaminant Level | Biodetox. Mechanism | Detox. Rate | Ref. |
|---|---|---|---|---|---|---|---|
| L. acidophilus ATCC 4356 L. casei ATCC 39392 | 109 | AFB1 |
| 5 µg /mL |
| 14–70% | [65] |
| L. acidophilus ATCC 9224, 4356, CECT 4529, CECT 4179 L. acidophilus CNRZ 55, 217L. brevis ATCC 14869 L. brevis DSMZ 1268 L. casei CECT 5275 L. crispatus M247, DSMZ 20584 L. plantarum WCFS1 L. rhamnosus ATCC 53103 | OD10 | As |
| 30 mg/kg |
| 1–6% | [69] |
| L. acidophilus La-5 | 108 | AFB1 AFM1 |
| 1 µg /mL 100 µg /mL |
| 13.53–35.53% 17.65–71.52% | [64] |
| L. acidophilus EMCC 1324 | 1, 5, 7 × 109 | AFB1, AFB2, AFG1, AFG2 |
| 50 µg /mL |
| 95.59% | [70] |
| L. acidophilus (isolated from traditional dough) | 106 | S. aureus ATCC 25923, Si. dysenteriae |
| 106 CFU/mL |
| 12.1 mm 20.9 mm 14.7 mm | [2] |
| L. acidophilus NCFM | 108 9 10 | B[a]P |
| 1.0 μg/mL |
| 45–60% | [53] |
| L. brevis LN871494 L. kefiri MH107106 | 108 | A. flavus A. carbonarius |
| 106 spores/mL |
| 20–50% | [57] |
| L. bulgaricus KLDS1.0207 | 1010 | Pb |
| 50 mg/kg/day |
| ↑ Pb excretion | [71] |
| L. reuteri CGMCC 1.3264 | 108 | ZEN |
| 5 mg/L |
| 100% | [59] |
| L. fermentum 1744 ATCC 14931 | 109 | Heavy metals Pb, Zn, Ni, Cd |
| - |
| na | [45] |
| L. delbrueckii subsp. bulgaricus DSM 20,081 L. sakei subsp. sakei DSM 20,017 L. rhamnosus DSM 20,021 L. plantarum subsp. plantarum PTCC 1896 | 107 | Acrylamide |
| 47.6 µg /kg |
| 85.5% | [72] |
| L. paracasei LOCK 1091 L. pentosus LOCK 1094 L. plantarum LOCK 0860 L. reuteri LOCK 1092 L. rhamnosus LOCK 1091 | 4.5 × 1010 | AFB1 |
| 1 mg/kg 5 mg/kg |
| 41–68% | [73] |
| L. plantarum Bacillus coagulans | 109 | Hg |
| 20 μg/mL of mercuric chloride |
| >50% | [46] |
| L. plantarum PTCC 1058, LP3, AF1, LU5 | 1.6 × 105 | A. flavus PTCC 5004 A. parasiticus PTCC 5018 A. nidulans PTCC 5014 A. ochraceus PTCC 5060 |
|
| 27.6 ± 0.9 mm | [3] | |
| L. plantarum PTCC 1058, LP3, AF1, LU5 | 1.6 × 105 | AFM1 OTA |
| 0.5 µg /kg 0.5 µg /kg |
| 26–52% 32–58% | [3] |
| Bifidobacteria Strain/Mix | Cell Count CFU/mL | Contaminant | Food/Environment | Contaminant Level | Biodetox. Mechanism | Detox. Rate | Ref. |
|---|---|---|---|---|---|---|---|
| B. animalis subsp. Lactis BI-04 | 5 × 108 | B[a]P |
| 0.5 µg /mL |
| 95% | [83] |
| B. animalis subsp. Lactis B. longum subsp. Infantis ATCC 15697 B. longum subsp. Longum ATCC 15707 | 108 | Fusobacterium nucleatum ATCC 25585 Porphyromonas gingivalis 33277 Streptococcus oralis |
| 106 CFU/mL |
| 64.9%; 54% | [84] |
| B. animalis subsp. Lactis BI-04, 1.2226, and HN019 B. bifidum Bb-02 B. breve 1.2213 and BD-01 B. longum subsp. Infantis Bi26 and BY12, B. longum subsp. Longum 1.2186 | 5 × 108 | B[a]P |
| 100 μg/mL |
| 78% | [55] |
| B. bifidum | 1; 5; 7 × 109 | AFB1, AFB2, AFG1, AFG2 |
| 50 µg /mL |
| 95.59% | [70] |
| B. bifidum DDBA | 1 × 1011 | ZEN |
| 2.5 μg/mL |
| 98% | [42] |
| B. bifidum NRRL B-41410 | 2 × 106 | AFM1 |
| 50 µg /mL |
| 45.17% | [85] |
| Probiotic Yeasts Strain/mix | Cell Count CFU/mL | Contaminant | Food/Environment | Contaminant Level | Biodetox. Mechanism | Detox. Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Aureobasidum pullulans L1 | 108 | acrylamide |
| 1600 μg/kg in the control |
| 83% | [92] |
| Saccharomyces cerevisiae CCTCC 93161 | 1.5 × 106 | PAT |
| 500 μg/L |
| 53.97% (6 h fermentation) 85.88% (24 h fermentation) | [87] |
| S. cerevisiae S10c S. cerevisiae S6u | 106 | OTA |
| 2 µg/kg |
| 29% white win 45.4–49.5% red win with extra anthocyanins | [86] |
| S. cerevisiae LOCK 0119 | 4 × 106 | AFB1 |
| 1 mg/kg 5 mg/kg |
| 41–68% | [73] |
| Kyokai 6 S. cerevisiae BY4743, VRB, Ultralevura, YPS128, UWOPS03–461.4 | OD4 | As |
| 30 mg/kg |
| 1–6% | [69] |
| S. cerevisiae ATCC 64712 Kluyveromyces lactis CBS 2359 | 1; 5; 7 × 109 | AFB1, AFB2, AFG1, AFG2 |
| 50 µg /mL |
| 95.59% | [70] |
| S. cerevisiae RC016 S. boulardii RC009 Kluveromyces marxianus VM003 | 107 | AFM1 |
| 10 ng/mL |
| 19, 25, 36% 100, 46, 100% | [88] |
| Other Probiotic/Probiotic Candidates Strain /Mix | Cell Count CFU/mL | Contaminant | Food/Environment | Contaminant Level | Biodetox. Mechanism | Detox. Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Bacillus licheniformis strain JS (antimicrobial peptides) | 50, 70, 100 μL | B.cereus Shigella dysenteriae |
| nm |
| 21 mm B. cereus 14 mm S. dysenteriae | [93] |
| B. licheniformis HN-5 | 107–108 | Pantoea ananatis |
| 10 / 40 µg/mL |
| 48.49 ± 0.15%/75.26 ± 0.15% | [94] |
| B. licheniformis CK1 | Unknown | ZEN |
| 2.75 μg/mL |
| 98% | [95] |
| Enterococcus strain E. faecium DUTYH_16120012 | 0.5 OD600 | Pb |
| 50 mg/L |
| 80.58 ± 1.65% | [96] |
| Escherichia coli Nissle 1917 (EcN) | 2 × 103 | Escherichia coli |
| 2 × 103 |
| 49.6% and 67.8% at 4 and 24 h of cultivation 2 and 5.4 fold at 4 and 24 h of cultivation | [97] |
| E. coli Nissle 1917 (EcN-2, EcN-22, EcN-23) | 109 | Cd Hg Pb |
| 1.6 ± 0.24 μg/mL |
| 80% ↑ survival rate | [98] |
| Streptomyces cacaoi subsp. Asoensis K234 | Unknown | AFB1 |
| 1 μg/mL |
| 88.34 ± 15.62 | [4] |
| Streptococcus thermophiles | 106 | AFM1 |
| 50 μg/mL |
| 58.5% | [4] |
| Pediococcus acidilactici KTU05-7, KTU05-8 | 9.2 | Acrylamide |
| - |
| 38.33% | [51] |
| P. acidilactici RC005 P. pentosaceus RC006 | 107 | AFM1 |
| 10 ng/mL |
| 34, 26% 33, 71% | [88] |
| P. pentosaceus TMU457 | 1010-15 | AFB1 |
| 5 µg /mL |
| 75.06 ± 1.60% | [4] |
| P. pentosaceus LN828199 P. pentosaceus LN871493 | 108 | A. flavus A. carbonarius |
| 106 spores/mL |
| 20–50% | [57] |
| Probiotic Strain | Study Type | Cancer Type/Cell Lines/Carcinogen | Way of Action/Findings | Conditions | Ref. |
|---|---|---|---|---|---|
| L. acidophilus CICC 6074 S-layer protein | In vitro | HT-29 human CRC cells | ↓ proliferation, chromatin condensation, nuclear fragmentation, induce apoptosis | 25, 50, and 100 mg/L S-layer protein | [110] |
| L. acidophilus CRL 636 + L. reuteri CRL 1101 + selenium | In vitro | - | Preventive effect ↑ intracellular SeCys and SeMet | 5 mg Se/L as selenite | [114] |
| L. casei SR1, L. casei SR2, L. paracasei SR4 isolated from human breast milk | In vivo | HeLa cervix cancer cells | Sustain apoptosis by ↑ the expression of apoptotic genes BAX, BAD, caspase3, caspase8, and caspase9 ↓ expression of BCl-2 gene, ↓ proliferation | 1.0 × 107 to 1.0 × 108 CFU/mL | [115] |
| L. casei CRL431 | In vivo In vivo | 4T1 breast cancer cells BALB/c mice | Improve the capecitabine’s toxicity on 4T1 cells ↓ capecitabine side effects ↓ intestinal mucositis and mortality ↓ decreased IL-6 ↑ immune response | 1 × 109 CFU/mL 36-day experimental protocol | [116] |
| L. debrueckii spp. bulgaricus LB-G040 | In vivo | Colitis-associated cancer C57BL/6 mice | modulate inflammatory responses, inhibit tumor growth, ↓clinical signs of intestinal inflammation | 3 times/week 1 × 109 CFU by gavage | [117] |
| L. fermentum NCIMB 5221 + Lactobacillus acidophilus ATCC 314 | In vitro | CaCo2 adenoma cells | ↓ proliferation induce apoptosis | L. fermentum 0.5 × 1010 CFU L. acidophilus 0.5 × 1010 CFU | [118] |
| L. kefiri LKF01 Kefibios ® | In vivo | 76 patients with any solid tumor under therapy | ↓ chemotherapy, radiotherapy, and immunotherapy side effects—diarrhea | 5 drops/day (109 CFU) | [119] |
| L. helveticus MB2-1 exopolysaccharides | In vitro | HT-29 CRC human cells | Induce apoptosis antiproliferative activity ↑ intracellular reactive oxygen species ↑ pro-apoptotic Bax and mitochondrial cytochrome c ↓ anti-apoptotic Bcl-2 | 0, 100, 200, 400 and 600 μg/mL exopolysaccharides | [120] |
| L. plantarum I-UL4, TL1, RS5, RI11, RG11, and RG14 isolated from Malaysian food | In vitro | Cancer cells: MCF-7 breast CRC, HT-29 HeLa cervical Hep-G2 liver HL60, K562 leukemia | Antiproliferative and apoptotic effects on MCF-7 strain-specific and cell type-dependent cytotoxic effects no toxic effect or hemolysis on normal cells | L. plantarum added in conc. 0.47–30% (v/v) | [20] |
| L. reuteri FLRE5K1 | In vivo | Melanoma cell line B16-F10 injected in 8-week-old female BALB/C mice | ↓ melanoma occurrence ↑ survival rate | 109 CFU/mL/day, 7 days prior to and after melanoma injection | [121] |
| L. rhamnosus GC | In vivo | Gastric cancer-induced in male NMRI inbred albino mice | ↓ tumor volume, size ↑ white blood cells no. ↑ level of Bax/Bcl-2 ratio improvement capecitabine chemotherapy | 1 × 108 CFU/100 µL saline/day | [122] |
| B. bifidum (isolated from infants’ feaces) | In vitro | SW742 human colon cancer cell line | Necrosis of the tumor cells | Probiotic growth in aerobic conditions, 1 × 105 CFU application on cell | [80] |
| B. longum BB536-y and fructooligosaccharides | In vivo | Human colorectal cancer | Preventive action ↑ amount of SCFA ↓ Bacteroides fragilis enterotoxin production ↓ growth of putrefactive bacteria | 1/day-5days BB536-y and BB536-y and FOS | [123] |
| B. lactis Bl-04 + Lactobacillus acidophilus NCFM | In vivo | CRC | Therapeutic action by microbiota modulation, ↑ butyrate-producing bacteria (Faecalibacterium and Clostridiales spp.) | 2/day 1.4 × 1010 CFU B. lactis, 7 × 109 CFU L. acidophilus | [109] |
| Saccharomyces cerevisiae PTCC 5052 –heat-killed | In vitro | CRC SW480 cell line | antiproliferative effect pro-apoptotic effect via Akt/NF-kB signaling pathway | 1 × 106 cell/mL heat-killed cells | [108] |
| E. coli Nissle 1917 | In vivo | SMMC-7721 cancer cell injected into BALB/c nude mice | ↓ tumor growth ↑ treatment response | 5 × 106 CFUs/100 μL | [98] |
| Lactococcus lactis subsp. lactis isolate (R7) | In vivo | Wistar rats Induced CRC | Anticancerigenic action ↓ intestinal morphological changes | 1 mL bacterial suspension (108 CFU/mL) 1/day, 6 weeks. by gavage | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, O.L.; Suharoschi, R.; Gabbianelli, R. Biodetoxification and Protective Properties of Probiotics. Microorganisms 2022, 10, 1278. https://doi.org/10.3390/microorganisms10071278
Pop OL, Suharoschi R, Gabbianelli R. Biodetoxification and Protective Properties of Probiotics. Microorganisms. 2022; 10(7):1278. https://doi.org/10.3390/microorganisms10071278
Chicago/Turabian StylePop, Oana Lelia, Ramona Suharoschi, and Rosita Gabbianelli. 2022. "Biodetoxification and Protective Properties of Probiotics" Microorganisms 10, no. 7: 1278. https://doi.org/10.3390/microorganisms10071278
APA StylePop, O. L., Suharoschi, R., & Gabbianelli, R. (2022). Biodetoxification and Protective Properties of Probiotics. Microorganisms, 10(7), 1278. https://doi.org/10.3390/microorganisms10071278