Abstract
Bacillus velezensis is a widely used biocontrol agent closely related to B. amyloliquefaciens, and the two species cannot be distinguished by universal primers that are currently available. The study aimed to establish a rapid, specific detection approach for B. velezensis. Many unique gene sequences of B. velezensis were selected through whole genome sequence alignment of B. velezensis strains and were used to design a series of forward and reverse primers, which were then screened by PCR and qPCR using different Bacillus samples as templates. The colonization ability of B. velezensis ZF2 in different soils and different soil environmental conditions was measured by qPCR and a 10-fold dilution plating assay. A specific primer pair targeting the sequence of the D3N19_RS13500 gene of B. velezensis ZF2 was screened and could successfully distinguish B. velezensis from B. amyloliquefaciens. A rapid specific real-time qPCR detection system for B. velezensis was established. B. velezensis ZF2 had a very strong colonization ability in desert soil, and the optimal soil pH was 7–8. Moreover, the colonization ability of strain ZF2 was significantly enhanced when organic matter from different nitrogen sources was added to the substrate. This study will provide assistance for rapid specificity detection and biocontrol application of B. velezensis strains.
1. Introduction
Bacillus velezensis is a Gram-positive, rod-shaped, motile, spore-forming, aerobic bacterium that has been used as a biocontrol agent for many plant diseases [1]. B. velezensis was described in 2005 and first reported as a heterotypic synonym of B. amyloliquefaciens based on DNA–DNA relatedness values [2]. Later, B. velezensis and B. amyloliquefaciens were distinguished based on core genome sequences [3] or phylogenetic analysis of polygenes; however, the two species cannot be distinguished by universal primers that are currently available. Previous studies showed that the specific genes trpE, yecA and tetB were used to specifically detect three closely related B. amyloliquefaciens strains, UCMB5033, UCMB5036 and UCMB5113, respectively [4]. The primer pair designed based on the unique gene ukfpg of B. amyloliquefaciens TF28 was able to distinguish strain TF28 from other Bacillus strains [5]. In addition, a colorimetric assay easily distinguished B. subtilis from Escherichia coli and Staphylococcus aureus [6]. However, very few studies have been used to specifically detect B. velezensis strains or distinguish B. velezensis from B. amyloliquefaciens. Therefore, a rapid and specific identification method for B. velezensis is necessary for the detection of specific strains in the environment.
B. velezensis is widely distributed in various environments, and an increasing number of B. velezensis strains have been used as biocontrol agents for plant disease. For example, B. velezensis FZB42T was reported to produce many kinds of lipopeptides and showed direct suppression of many plant pathogens [7]. B. velezensis SQR9 could stimulate resident rhizosphere-beneficial microorganisms and protect plants against diseases [8]. B. velezensis QST713 showed broad-spectrum antimicrobial or antibacterial activities and has been used as an antagonist against green mold disease [9]. B. velezensis ZF2 has been reported as a potential biocontrol agent that shows a broad spectrum of antagonistic activities against many plant pathogens and exhibits strong inhibitory activity against Corynespora leaf spot disease in cucumber [10]. However, taking plant-beneficial microorganisms from lab to agricultural application is a great challenge, as exogenous inoculum is usually eliminated in soils due to competition from indigenous microbes or complicated soil environments [11,12]. Research on these biocontrol agents indicated that the biocontrol efficacy of the microbes to control plant diseases was related to their ability to survive and maintain an abundant population in rhizosphere soil [13]. Therefore, it is important to detect the viability of inoculum in soil. The current detection method is a 10-fold dilution plating test, which is time-consuming and laborious. Therefore, a rapid detection system for B. velezensis is essential for determining the colonization ability of strains in biocontrol applications.
It is worth noting that many B. velezensis strains do not show excellent control effects for plant disease in field applications, although these strains show broad-spectrum antagonistic activities against phytopathogens. One possible explanation is that antagonistic bacteria cannot rapidly colonize the rhizosphere and soil because of the complicated environmental conditions [14]. It is important to explore the colonization ability of B. velezensis under different environmental conditions for rational biocontrol application of strains.
In this study, we screened a pair of primers that could distinguish B. velezensis from B. amyloliquefaciens. A new, rapid and specific detection system was established that facilitates rapid detection of the B. velezensis genus. Moreover, we measured the colonization ability of B. velezensis ZF2 in different soils and different soil environmental conditions, including pH and nutrient elements. Our study will promote the application and detection of B. velezensis in biocontrol applications.
2. Materials and Methods
2.1. Strains, Culture Conditions and DNA Extraction
The bacterial reference strains used in this study are listed in Table 1. All of the test strains used in the study were cultivated in Luria broth medium (LB) or nutrient broth (NB) at 28 °C with shaking for 24 h. Genomic DNA was extracted from the cultured cells (OD600 = 0.8) using a TIANamp Bacteria DNA kit (Tiangen Biotech (Beijing) Co., Ltd., Beijing, China).

Table 1.
Reference strains used in this study.
2.2. Design and Selection of Species-Specific Primers
The sequences of the housekeeping genes gyrB, gap, rpoD, atpD, rho, 16S rRNA and other functional genes galE, metC, pdhA, and pgk of strains ZF2, FZB42, DSM 7, and 168 were obtained from the corresponding whole genome sequence (GenBank: CP032154.1, CP000560.1, FN597644.1, AL009126.3, respectively). Every gene sequence of B. velezensis ZF2 and B. velezensis FZB42 was aligned with those of the Bacillus strains DSM 7 and 168 using DNAMAN 7.0 [15]. Sequence regions unique to B. velezensis were used to design a series of forward and reverse primers. Our previous study showed that the sequence of the fliC gene (coding flagellin) from strains ZF2 and FZB42 exhibited low homology to strains DSM 7 and 168 (88% and 56%, respectively) [10], so the unique region sequence of the flic gene in B. velezensis ZF2 was also used to design a series of forward and reverse primers based on the flic sequence alignment results. In addition, to increase the possibility of primer specificity for the B. velezensis species, the whole genome sequence of B. velezensis ZF2 was compared with other B. velezensis strains and used to design a series of forward and reverse primers by Primer Premier 5 [16]. As a result, 26 pairs of primers were designed (Table S1) and synthesized by Biomed Biotech (Beijing, China) Co., Ltd. and were screened by PCR using diverse DNA templates of different Bacillus strains.
2.3. Primer Specificity Verification and Real-Time qPCR Assays
All primers were screened by PCR using different bacterial genomic DNA samples, including B. velezensis ZF2, B. velezensis ZF128, B. velezensis FZB42T [7], B. amyloliquefaciens DSM 7T [17] and B. subtilis ZF168T [18]. The primer with the best specificity for B. velezensis was tested against diverse bacterial genomic DNA templates, including B. velezensis ZF2, B. velezensis ZF128, B. velezensis ZF145, B. velezensis LS69 [19], B. velezensis SQR9 [8], B. velezensis FZB42T, B. amyloliquefaciens 75, B. amyloliquefaciens DSM 7T, B. subtilis ZF161, B. subtilis 168T, B. safensis ZF438, P. polymyxa ZF129 [20], P. peoriae ZF390, Rahnella aceris ZF458, R. aquatilis ZF7 [21], Lysobacter enzymogenes CX03, Pectobacterium brasiliense [22], Pseudomonas amygdali pv. lachrymans [23] and Xanthomonas campestris pv. campestris 8004 [24] (Table 1).
Selecting the best primer pair, the specific PCR conditions were optimized, including template DNA (10 ng of genomic DNA) and the annealing temperature (52.0–57.0 °C with approximately 1.0 °C increments). Amplifications were performed in a 20 μL reaction mixture including 10 μL of 2 × Taq DNA polymerase mix (Biomed Biotech (Beijing, China) Co., Ltd.), 1 μL of the genomic DNA template, 1 μL of each primer (10 μM) and 7 μL sterile water. Negative controls were included for each PCR assay. The PCR procedure consisted of one cycle of 3 min at 94 °C and 30 cycles of 30 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C, and a final extension step was run for 5 min at 72 °C. After amplification, 10 μL of each PCR product was analyzed through electrophoresis in a 1% agarose gel in 1× Tris-acetate-EDTA (TAE) buffer, stained with ethidium bromide and visualized using an ultraviolet transilluminator.
After PCR validation, the screened primers were verified by qPCR. All qPCR assays were performed on an Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) [25] with a MicroAmp® Optical 96-Well Reaction Plate closed with the MicroAmp® Optical 8-Cap Strip (Applied Biosystems). The reaction was performed in a final volume of 20 μL containing 1 μL of the genomic DNA template, 10 μL of 2 × SYBR®Green PCR Mastermix (TIANamp), 0.4 μL ROX, 0.5 μL of each primer (10 μM) and 7.6 μL sterile water. The following thermal program was applied: a single cycle of DNA polymerase activation for 10 min at 98 °C followed by 40 amplification cycles of 15 s at 98 °C (denaturing step) and 32 s at 60 °C (annealing and extension step). The melting curve and CT value were used to assess the specific amplification and amplification efficiency.
2.4. Standard Curve Determination and Sensitivity Test
After determining the optimal specific primer, the corresponding purification amplification fragment was cloned into the pMD18-T vector by heat shock transformation and copied into Escherichia coli DH5α according to a previously reported method [26]. Plasmid DNA containing the target gene was extracted from cultured DH5α cells using a TIANamp plasmid DNA kit (Tiangen Biotech (Beijing, China) Co., Ltd.). Standard curves were generated using 10-fold serial dilutions (concentration from 108 fg·μL−1 to 1 fg·μL−1) of the plasmid containing the fragment copy of the optimal targeted gene as described by a previous study [27]. The abundance of B. velezensis was determined using SYBR Green assays with the selected primers. Each assay was performed in triplicate, and a linear relationship equation was established between the plasmid DNA concentration and the CT value. The copy number was calculated with the equation N = CM, where N is the sample copy number, C is the concentration of DNA, and M is the mean mass of the genomic DNA [28]. The minimum detection limit was calculated according to the linear relationship equation.
2.5. Colonization Ability of B. velezensis ZF2 in Different Soils
B. velezensis ZF2 was marked as rifampicin resistant according to a previously reported method with modifications [29]. Rifampicin-resistant mutants of strain ZF2 were obtained by transferring colonies to LB medium containing increasing concentrations (1, 5, 10, 20, 30, 40, 50 ng·μL−1) of rifampicin. After that, equal suspensions of rifampicin-resistant strain ZF2rif+ (OD600 = 1.0) were mixed in different soils at equivalent weights (including black soil, red soil, yellow brown soil, brown soil and desert soil), and the colonization ability of the strain in different soils was determined using a standard 10-fold dilution plating assay as previously described [30]. For quantification of the ZF2 density, three aliquots (100 mL) per dilution were spread on LA agar medium with rifampicin (50 ng·μL−1), and the plates were incubated at 28 °C for 2 days prior to colony counting. The soil samples were detected weekly after mixing with the strain ZF2 suspension (1, 7, 14, 21, 28, and 35 days), and each sample was tested three times. In addition, the genomic DNA of diverse soil samples in different periods was extracted using a TIANamp Bacteria DNA kit (Tiangen Biotech (Beijing, China) Co., Ltd.), and the corresponding natural soils were used as negative control. Then, the colonization ability of ZF2 in different soils was detected using the rapid specific detection system established above.
2.6. Determination of Physicochemical Properties of the Different Soils
The physicochemical properties, including pH, organic matter, total N, ammonium N, nitrate N, total P, available P, total K and available K, of the five kinds of soils were measured at the China National Rice Research Institute.
2.7. Colonization Ability of B. velezensis ZF2 under Different Environmental Conditions (pH and Nutrient Elements)
To understand the effect of different environmental conditions on the colonization ability of B. velezensis ZF2rif+, the nursery substrate with a mixed-strain ZF2 suspension (OD600 = 1.0) was placed in plastic boxes and incubated at different pH values (4, 5, 6, 7, 8, 9 and 10). The boxes were placed in an incubator to maintain stable environmental conditions. The colonization ability of strain ZF2rif+ in different substrate environmental conditions was detected using the rapid, specific real-time qPCR detection system and the standard 10-fold dilution plating assay every week, and each sample was tested three times.
Similarly, the effect of different nutrient elements on the colonization ability of B. velezensis ZF2rif+ was tested in the same way. Corn flour, yeast extract, peanut flour, peptone, soybean flour and soluble starch were selected as nitrogen sources; maltose, sucrose, fructose, dextrin, and molasses were selected as carbon sources; MgCl2, CaCl2, FeSO4, KCl and NaCl were selected as the inorganic compounds. The colonization ability of strain ZF2rif+ in substrate samples mixed with different nutrient elements was detected using the rapid, specific real-time qPCR detection system and the standard 10-fold dilution plating assay every week, and each sample was tested three times.
3. Results
3.1. Establishment of Specific Detection System
3.1.1. Design and Selection of Specific Primers
Through multiple sequence alignment, the sequence identities of the four genes 16S rRNA, gap, rpoD and rho between ZF2 and other B. amyloliquefaciens or B. subtilis strains were over 98%, while the sequence identities of the genes galE, gyrB, pdhA, pgk, fliC and metC among the four strains were under 95%. Primers targeting the unique region sequences of the galE, gyrB, pdhA, pgk, fliC and metC genes from B. velezensis ZF2 were designed. Another 12 pairs of primers were designed based on the unique gene sequence of B. velezensis (Table S1). After PCR screening, no primers targeting the housekeeping genes or flic gene could distinguish B. velezensis from other Bacillus species. As expected, primer pairs based on the unique gene sequence of B. velezensis were evaluated for their ability to distinguish B. velezensis from the closely related species B. amyloliquefaciens and B. subtilis. One pair of primers targeting the sequence of gene D3N19_RS13500 (Figure 1) yielded amplification products of the predicted size (192 bp) only in the B. velezensis template except for the strain FZB42T but not in the B. amyloliquefaciens and B. subtilis templates (Figure 2). The targeting gene could be retrieved in many B. velezensis strains in the NCBI database, and the sequence identities were over 94% (Table 2). Therefore, the pair of primers amplifying the D3N19_RS13500 gene fragments was selected for subsequent verification.

Figure 1.
Specific primer of gene D3N19_RS13500 existed in many B. velezensis strains. The red fonts indicate the primer sequence. The black fonts indicate the corresponding gene sequence in different B. velezensis strains (including ZF2, K01, GY65, LDO2, LG37, LS69, JTYP2 and ZF145).
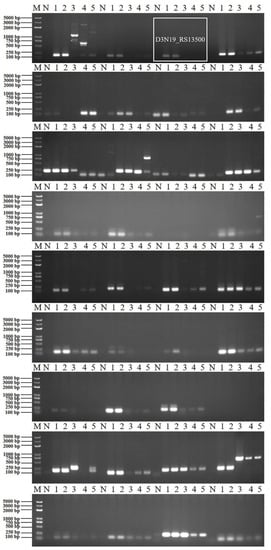
Figure 2.
Specific primers targeting B. velezensis screening in different Bacillus strains. M, DNA marker (from large to small was 5000 bp, 3000 bp, 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, 100 bp); N, negative control; 1, amplification template of B. velezensis ZF2 genomic DNA; 2, amplification template of B. velezensis ZF128 genomic DNA; 3, amplification template of B. velezensis FZB42T genomic DNA; 4, amplification template of B. amyloliquefaciens DSM 7T genomic DNA; 5, amplification template of B. subtilis 161T genomic DNA.

Table 2.
Search results for D3N19_RS13500 gene in NCBI database.
3.1.2. Verification of Primer Specificity and Optimization of PCR Reaction Conditions
To verify the specificity of the D3N19_RS13500 primer, diverse bacterial genomic DNA was used in PCR and real-time qPCR detection. The results showed that only the primer targeting D3N19_RS13500 gave amplification products of the predicted size (192 bp) in B. velezensis strains except for FZB42T and SQR9, but not in other bacterial strains (Figure 3A). To improve the amplification efficiency, the annealing temperature was optimized by performing gradient PCR. A unique clear target product was obtained at annealing temperatures ranging from 54–57 °C (Figure 3B). As expected, a fluorescence signal appeared when using the genomic DNA of B. velezensis as the template in the real-time qPCR (Figure 3C).
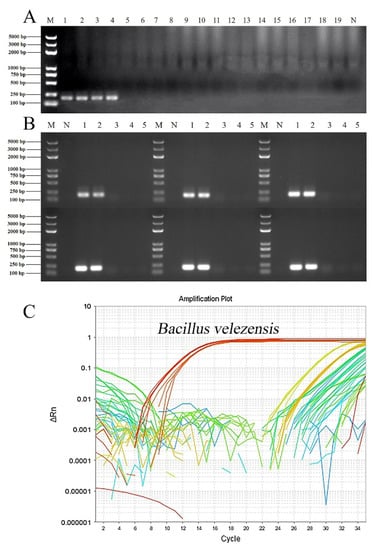
Figure 3.
Verification of specific primers targeting B. velezensis among different bacterial strains. (A), PCR verification. M, DNA marker (from large to small was 5000 bp, 3000 bp, 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, 100 bp); N, negative control; 1, amplification template of B. velezensis ZF2 genomic DNA; 2, amplification template of B. velezensis ZF128 genomic DNA; 3, amplification template of B. velezensis ZF145 genomic DNA; 4, amplification template of B. velezensis LS69 genomic DNA; 5, amplification template of B. velezensis SQR9 genomic DNA; 6, amplification template of B. velezensis FZB42T genomic DNA; 7, amplification template of B. amyloliquefaciens ZF75 genomic DNA; 8, amplification template of B. amyloliquefaciens DSM 7T genomic DNA; 9, amplification template of B. subtilis ZF161 genomic DNA; 10, amplification template of B. subtilis 168T genomic DNA; 11, amplification template of B. safensis ZF438 genomic DNA; 12, amplification template of P. polymyxa ZF129 genomic DNA; 13, amplification template of P. peoriae ZF390 genomic DNA; 14, amplification template of R. aceris ZF458 genomic DNA; 15, amplification template of R. aquatilis ZF7 genomic DNA; 16, amplification template of L. enzymogenes CX03, genomic DNA; 17, amplification template of Pectobacterium brasiliense genomic DNA; 18, amplification template of Pseudomonas amygdali pv. lachrymans genomic DNA; 19, amplification template of Xanthomonas campestris pv. campestris 8004 genomic DNA. (B), Screening of the optimal PCR annealing temperature of the specific primer. The temperature gradient was 52 °C, 53 °C, 54 °C, 55 °C, 55 °C, 56 °C, and 57 °C. M, DNA marker (from large to small was 5000 bp, 3000 bp, 2000 bp, 1000 bp, 750 bp, 500 bp, 250 bp, 100 bp); N, negative control; 1, amplification template of B. velezensis ZF2 genomic DNA; 2, amplification template of B. velezensis ZF128 genomic DNA; 3, amplification template of B. velezensis FZB42T genomic DNA; 4, amplification template of B. amyloliquefaciens DSM 7T genomic DNA; 5, amplification template of B. subtilis 161T genomic DNA. (C), Specific primers targeting B. velezensis verification among different bacterial strains by qPCR. Curves in different colors represent different amplifications using genomic DNA of different strains as templates.
3.1.3. Standard Curve Determination and Detection Limit
Different amplification curves were generated using different concentrations of genomic DNA as templates (Figure 4A). Standard curves of real-time PCR displayed dynamic ranges on 8 log DNA dilutions (Figure 4B). All of the qPCR assays were performed in a linear manner with the linear equation y = −3.875x + 30.713 (y represents the CT value, x represents the lg DNA concentration), and the R2 value was 0.9972. The results from the dynamic range analyses allowed the determination of PCR efficiency, as the E value was 81%. The R2 and E values of the developed SYBR® Green qPCR complied with the acceptance limits. According to the linear equation, the minimum detection concentration and the minimum detection gene copies were calculated as 0.1 fg·μL−1 and 474 copies·μL−1, respectively.
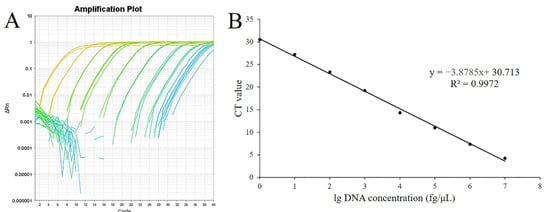
Figure 4.
The standard curve for the rapid detection system of B. velezensis ZF2. (A), Amplification plot of real-time qPCR using different concentrations of plasmid DNA containing the target gene of the B. velezensis ZF2 (D3N19_RS13500) fragment. (B), Standard curves based on different concentrations of plasmid DNA and the corresponding amplified CT value. X represents the lg DNA concentration, and y represents the CT value.
3.2. Detection of the Colonization Ability of B. velezensis ZF2
3.2.1. Colonization Ability of B. velezensis ZF2 in Different Soils
The colonization ability of strain ZF2 in different soils was measured by a real-time qPCR rapid detection system. The results showed that strain ZF2 had a maximum gene copy number in desert soil (approximately 108 copy numbers in 1 g soil) (Table 3). To verify the results of qPCR detection, the colonization ability of B. velezensis ZF2 in diverse soils was tested by a 10-fold dilution plating assay. As expected, the maximum number of ZF2 colonies was isolated from the desert soil (over 108 cfu·g−1 in 35 days) (Table 3), followed by black soil, yellow brown soil, brown soil and red soil. These results revealed that the ZF2 strain exhibited the strongest colonization ability in desert soil (from Ningxia Province), which was consistent with the qPCR detection results.

Table 3.
Real-time PCR and plating assay detection of strain ZF2 in different soil types.
3.2.2. Determination of Soil Physicochemical Properties
To understand the different colonization abilities of ZF2 in different soils, the physicochemical properties of diverse soils were measured. The desert soil had a higher pH value (8.40) than other types of soils and had higher nitrate N (43.75 mg·kg−1) and ammonium N (24.02 mg·kg−1) than other types of soils (Table 4). These data indicated that the colonization abilities of strain ZF2 in different soils may be associated with the soil physicochemical properties, especially the pH, ammonium N and nitrate N.

Table 4.
The physicochemical properties of different types of soils.
3.2.3. Colonization Ability of B. velezensis ZF2 under Different Environmental Conditions (pH)
The results showed that soil pH was associated with the colonization ability of strain ZF2. Strain ZF2 showed the strongest colonization ability when the soil pH value was 7 to 8 (the number of colonies and gene copies were approximately equal to 107·g−1 substrate), while the number of ZF2 colonies and gene copies were less than 107·g−1 substrate when the pH value was over 8 or below 7 (Table 5). These results indicated that neutral or weakly alkaline soil conditions may be favorable for the colonization of B. velezensis ZF2.

Table 5.
The colonization abilities of strain ZF2 in different pH.
3.2.4. Colonization Ability of B. velezensis ZF2 under Different Nutrient Additions (Carbon Source, Nitrogen Source, Inorganic Compounds)
Furthermore, the effect of different nutrient additions on the colonization ability of strain ZF2 in the nursery substrate was measured in the same way. The results indicated that adding different carbon sources or inorganic compounds did not enhance the colonization ability of the ZF2 strain, as the number of ZF2 colonies remained the same as that in the control or less than that in the control. Interestingly, the number of ZF2 colonies and gene copies were 105−106 when different nitrogen sources were added to the nursery substrate, while the number of colonies and gene copies were only 104 in the control nursery substrate at 35 days (Table 6). These results indicated that adding a nitrogen source to the soil significantly enhanced the colonization ability of strain ZF2.

Table 6.
The colonization abilities of strain ZF2 under different nutrient additions.
4. Discussion
Bacillus velezensis is widely distributed in soil, water, and plants, and has been used for controlling plant disease due to its direct or indirect growth improvement effect on many plants [31,32]. B. velezensis ZF2, isolated from the stem of cucumber, has been reported to have broad-spectrum antagonistic activities and a significant ability to control Corynespora leaf spot disease [10]. In this study, a rapid, specific real-time qPCR detection system for B. velezensis based on the unique gene sequence of strain ZF2 was established, and its application in measuring the colonization ability of strain ZF2 was studied.
Housekeeping genes, including 16S rRNA, gyrB, gap, and rpoD, are widely used for the identification and classification of bacteria [33], and real-time quantitative polymerase chain reaction is often used for the rapid detection of bacteria [34]. However, a single gene usually cannot distinguish a species from other closely related species. A primer pair targeting the 16S rRNA gene of B. pumilus failed to distinguish B. megaterium, B. circulans and Paenibacillus mucilaginosus. It was reported that the gyrB gene has a relatively higher discrimination ability than the 16S rRNA gene, and it was used for the specific identification of P. mucilaginosus [35]. However, a primer pair targeting the gyrB gene of B. velezensis ZF2 failed to distinguish B. velezensis from B. amyloliquefaciens and B. subtilis. Moreover, primer pairs targeting other housekeeping genes could not distinguish B. velezensis from B. amyloliquefaciens and B. subtilis due to the close relationships among B. velezensis, B. amyloliquefaciens and B. subtilis [3].
In recent years, many non-conserved genes have been used to identify bacterial species. For instance, the phosphoenolpyruvate/sugar phosphotransferase system I gene and the adenylosuccinate synthetase gene were reported to discriminate B. anthracis from B. cereus [36]. In this study, primer pairs targeting the fliC gene (which showed low homology among B. velezensis, B. amyloliquefaciens and B. subtilis) of strain ZF2 were designed for the rapid identification of B. velezensis [10]. However, these primer pairs could not distinguish B. velezensis from B. amyloliquefaciens and B. subtilis. Satisfactorily, a primer pair targeting the gene D3N19_RS13500 of strain ZF2 showed specific amplification only from genomic DNA of B. velezensis strains. Interestingly, the 192 bp product was not amplified when using B. velezensis FZB42T and SQR9 as templates in the PCR screening test. This phenomenon might be related to the taxonomic position of the two strains, based on the fact that B. velezensis FZB42T and SQR9 were originally classified as B. amyloliquefaciens [37,38]. In addition, there was substantial genomic diversity in bacteria, even in strains of the same genus, so, many genera were divided into different subgroups. As the largest group of Pseudomonas, Pseudomonas fluorescens was divided further into eight or nine subgroups [39]. To explain this interesting phenomenon, more in-depth studies are needed, and it would be necessary to design a separate primer to detect these exclusive strains. However, the gene could be retrieved in most B. velezensis strains and its sequence had high homology. These results indicated that the selected primer pair had the ability to distinguish most B. velezensis strains from B. amyloliquefaciens strains and B. subtilis strains; however, in special cases, its specificity needs to be further verified. To our knowledge, this is the first report to distinguish B. velezensis from B. amyloliquefaciens and B. subtilis through a single gene. A rapid and specific detection system is essential to detect the colonization ability of B. velezensis strains in biocontrol applications.
Bacillus was reported to be a biocontrol agent with the best application potential due to its stability, broad-spectrum antagonism and environmental friendliness. However, the prevention and control effect of Bacillus for controlling plant disease is affected by many factors. It was reported that soil bacterial diversity could be strongly affected by pH, soil type, latitude, vegetation, moisture, temperature, and nutrient availability [40]. Among these, soil pH was the best predictor of both soil bacterial diversity and richness, whereas soil type strongly influenced soil bacterial composition [41,42]. Although the general patterns underlying variations in biodiversity have been observed, the influence of these factors on the viability of microorganisms remains unclear.
Recently, many beneficial microbes have been used as biological control agents to control plant diseases and were reported as a potential way to decrease the negative effect of chemicals on the environment [43]. Successful colonization of biocontrol agents in the rhizosphere soil is a prerequisite for disease control [44]. Soil is a complex environmental matrix, and the soil physicochemical properties have a great influence on the colonization of biocontrol agents [45]. In this study, the colonization ability of B. velezensis ZF2 in different types of soils was measured by real-time qPCR detection and a 10-fold dilution plating assay. The results showed that different types of soils had a strong influence on the colonization ability of strain ZF2. B. velezensis ZF2 exhibited the strongest colonization ability in desert soil compared with other soils. However, the colonies and gene copies of strain ZF2 decreased in all test soils over time, which indicated that B. velezensis ZF2 was degraded in different soils due to the indigenous soil environment. Indeed, many previous studies have reported that the inocula failed to propagate and decreased significantly after inoculation in exogenous soils [46,47,48]. The reason might be that the inocula were inhibited or eliminated by the indigenous microbes and/or local soil conditions [13]. Interestingly, evaluation of the physicochemical properties showed that desert soil had a higher pH value, higher nitrate N and ammonium N than other soils, although the total N in desert soil was very low. Previous studies showed that soil with a higher pH had an estimated bacterial richness 60% higher than that of more acidic soil [41]. As expected, our research found that B. velezensis ZF2 had a strong colonization ability in neutral or weakly alkaline soil conditions, which indicated that pH was closely related to the colonization ability of Bacillus. Furthermore, adding a nitrogen source to the soil could significantly enhance the colonization ability of strain ZF2, which revealed that available nitrogen could promote the colonization ability of Bacillus in soil.
In addition, a 10-fold dilution plating assay showed that strain ZF2 had a strong colonization ability in red soil at the beginning; however, qPCR detection showed the opposite result: a low gene copy number of strain ZF2 emerged in red soil. The reason for this phenomenon may be that red soil has strong adsorption and is not suitable for the extraction of soil genomic DNA. Colonization experiments were conducted under controlled environmental conditions in growth chambers rather than in the field. The advantages of using controlled environmental conditions are obvious in that they enable the assessment of specific factors, and thus, the conditions would not fluctuate and interact with other factors.
5. Conclusions
In this study, a pair of specific primers for B. velezensis targeting the gene D3N19_RS13500 of strain ZF2 was designed and could distinguish B. velezensis from the closely related B. amyloliquefaciens and B. subtilis species. Real-time polymerase chain reaction assays for the rapid detection of B. velezensis were developed, and the minimum number of detected gene copies was 474 copies·μL−1. According to the rapid detection method and 10-fold dilution plating assay, strain ZF2 had the strongest colonization ability in desert soil. In addition, this study showed that neutral or weakly alkaline soil conditions might be suitable for the colonization of B. velezensis, and adding a nitrogen source to the soil was proven to enhance the colonization ability of B. velezensis. Our study provides convenience for rapid detection and biocontrol applications of Bacillus strains in agricultural fields.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10061216/s1, Table S1: Primers designed in this study.
Author Contributions
S.X., L.L. and B.L. conceived and designed the experiments. S.X. performed the experiments and analyzed the data. S.X. and L.L. wrote the manuscript. L.L., X.X., Y.S. and A.C. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed with the support of the China Agriculture Research System of MOF and MARA, the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-IVFCAAS), and the Key Laboratory of Horticultural Crops Genetic Improvement, Ministry of Agriculture in China (IVF2020).
Data Availability Statement
The sequence of the D3N19_RS13500 gene of B. velezensis ZF2 can be obtained in the complete genome sequence of strain ZF2. The complete genome sequence of Bacillus velezensis ZF2 has been deposited in NCBI under the GenBank accession number CP032154.1.
Acknowledgments
We would like to acknowledge all of the researchers who provided the different Bacillus strains used in the study for genome data analysis.
Conflicts of Interest
All authors declare that they have no potential conflict of interest.
References
- Ruiz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.T.; Lee, F.L.; Tai, C.J.; Kuo, H.P. Bacillus velezensis is a later heterotypic synonym of Bacillus amyloliquefaciens. Int. J. Syst. Evol. Microbiol. 2008, 58, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, C.A.; Kim, S.J.; Kwon, S.W.; Rooney, A.P. Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 2016, 66, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.H.; Bejai, S.; Niazi, A.; Manzoor, S.; Bongcam-Rudloff, E.; Meijer, J. Studies of plant colonisation by closely related Bacillus amyloliquefaciens biocontrol agents using strain specific quantitative PCR assays. Antonie Van Leeuwenhoek 2014, 106, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, Y.; Jiang, W.; Meng, L.; Cao, X.; Hu, J.; Chen, J.; Li, J. Development of a Strain-Specific Quantification Method for Monitoring Bacillus amyloliquefaciens TF28 in the Rhizospheric Soil of Soybean. Mol. Biotechnol. 2020, 62, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Tan, F.; Xu, A.; Deng, K.; Zeng, Y.; Huang, H. UV-induced peroxidase-like activity of gold nanoclusters for differentiating pathogenic bacteria and detection of enterotoxin with colorimetric readout. Sens. Actuators 2019, 279, 289–297. [Google Scholar] [CrossRef]
- Luo, C.; Chen, Y.; Liu, X.; Wang, X.; Wang, X.; Li, X.; Zhao, Y.; Wei, L. Engineered biosynthesis of cyclic lipopeptide locillomycins in surrogate host Bacillus velezensis FZB42 and derivative strains enhance antibacterial activity. Appl. Microbiol. Biotechnol. 2019, 103, 4467–4481. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R.; et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2021, 30, 774–787. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef]
- Xu, S.; Xie, X.W.; Zhao, Y.R.; Shi, Y.X.; Chai, A.L.; Li, L.; Li, B.J. Whole-genome analysis of Bacillus velezensis ZF2, a biocontrol agent that protects cucumis sativus against corynespora leaf spot diseases. 3 Biotech 2020, 10, 186. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the Lab to the Farm: An Industrial Perspective of Plant Beneficial Microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Cheeke, T.E.; Darby, H.; Rosenstiel, T.N.; Bever, J.D.; Cruzan, M.B. Effect of Bacillus thuringiensis (Bt) maize cultivation history on arbuscular mycorrhizal fungal colonization, spore abundance and diversity, and plant growth. Agric. Ecosyst. Environ. 2014, 195, 29–35. [Google Scholar] [CrossRef]
- Lee, B.Y.; Lim, H.R.; Choi, J.Y.; Ryu, K.H. Development of Molecular Detection of Three Species of Seed-Transmissible Viruses Useful for Plant Quarantine. Plant Pathol. J. 2004, 20, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.K.; Mangalam, A.K.; Dwivedi, S.; Naik, S. Primer premier: Program for design of degenerate primers from a protein sequence. Biotechniques 1998, 24, 318–319. [Google Scholar] [CrossRef] [Green Version]
- Borriss, R.; Chen, X.H.; Rueckert, C.; Blom, J.; Becker, A.; Baumgarth, B.; Fan, B.; Pukall, R.; Schumann, P.; Spröer, C.; et al. Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: A proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int. J. Syst. Evol. Microbiol. 2011, 61, 1786–1801. [Google Scholar] [CrossRef]
- Tye, A.J.; Siu, F.K.; Leung, T.Y.; Lim, B.L. Molecular cloning and the biochemical characterization of two novel phytases from B. subtilis 168 and B. licheniformis. Appl. Microbiol. Biotechnol. 2002, 59, 190–197. [Google Scholar] [CrossRef]
- Liu, G.; Kong, Y.; Fan, Y.; Geng, C.; Peng, D.; Sun, M. Whole-genome sequencing of Bacillus velezensis LS69, a strain with a broad inhibitory spectrum against pathogenic bacteria. J. Biotechnol. 2017, 249, 20–24. [Google Scholar] [CrossRef]
- Li, J.Y.; Gao, T.T.; Wang, Q. Comparative and Functional Analyses of Two Sequenced Paenibacillus polymyxa Genomes Provides Insights into Their Potential Genes Related to Plant Growth-Promoting Features and Biocontrol Mechanisms. Front. Genet. 2020, 11, 564939. [Google Scholar] [CrossRef]
- Yuan, L.; Li, L.; Zheng, F.; Shi, Y.X.; Xie, X.W.; Chai, A.; Li, B.J. The complete genome sequence of Rahnella aquatilis ZF7 reveals potential beneficial properties and stress tolerance capabilities. Arch. Microbiol. 2019, 202, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, L.F.; Shi, Y.X.; Xie, X.W.; Chai, A.L.; Wang, Q.; Li, B.J. Comparative genomic analysis of Pectobacterium carotovorum subsp. brasiliense SX309 provides novel insights into its genetic and phenotypic features. BMC Genom. 2019, 20, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yuan, L.F.; Shi, Y.X.; Xie, X.W.; Chai, A.L.; Wang, Q.; Li, B.J. Comparative genomic analysis of Pseudomonas amygdali pv. lachrymans NM002: Insights into its potential virulence genes and putative invasion determinants. Genomics 2019, 111, 1493–1503. [Google Scholar] [CrossRef]
- Bedini, E.; Carabellese, A.; Barone, G.; Parrilli, M. First synthesis of the beta-D-rhamnosylated trisaccharide repeating unit of the O-antigen from Xanthomonas campestris pv. campestris 8004. J. Org. Chem. 2005, 70, 8064–8070. [Google Scholar] [CrossRef] [PubMed]
- Petrauskene, O.V.; Cao, Y.; Zoder, P.; Wong, L.Y.; Ba Lachandran, P.; Furtado, M.R.; Tebbs, R.S. Evaluation of applied biosystems MicroSEQ real-time PCR system for detection of Listeria spp. in food and environmental samples. J. AOAC Int. 2012, 95, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Kumar, P.; Kumar, V.; Doley, R.; Ray, S.K. Heat shock at 37 °C with plasmid ligated at 37 °C yields more number of Escherichia coli transformants than plasmid ligated at 16 °C a possible role of ligated plasmid conformation during heat shock. Curr. Sci. 2013, 104, 747–751. [Google Scholar]
- Vandersea, M.W.; Kibler, S.R.; Holland, W.C.; Tester, P.A.; Schultz, T.F.; Faust, M.A.; Holmes, M.J.; Chinain, M.; Wayne Litaker, R. Development of semi-quantitative pcr assays for the detection and enumeration of Gambierdiscus species (gonyaulacales, dinophyceae)1. J. Phycol. 2012, 48, 902–915. [Google Scholar] [CrossRef]
- Farrelly, V.F.; Rainey, F.A.; Stackebrandt, E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl. Environ. Microbiol. 1995, 61, 2798–2801. [Google Scholar] [CrossRef] [Green Version]
- Glandorf, D.; Brand, I.; Bakker, P.; Schippers, B. Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 1992, 147, 135–142. [Google Scholar] [CrossRef]
- Wang, B.B.; Yuan, J.; Zhang, J.; Shen, Z.Z.; Zhang, M.J. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol. Fertil. Soils 2013, 49, 435–446. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Qi, G.; Mao, Z.; Hu, X.; Du, B.; Liu, K.; Ding, Y. Effects of Bacillus velezensis FKM10 for Promoting the Growth of Malus hupehensis Rehd. and Inhibiting Fusarium verticillioides. Front. Microbiol. 2020, 10, 2889. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.A.; Webster, J.A.; Straus, N. Rapid identification of bacteria on the basis of Polymerase Chain Reaction-amplified ribosomal DNA spacer polymorphism. Appl. Environ. Microbiol. 1993, 59, 945–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golpayegani, A.; Nodehi, R.N.; Rezaei, F.; Alimohammadi, M.; Douraghi, M. Real-time polymerase chain reaction assays for rapid detection and virulence evaluation of the environmental Pseudomonas aeruginosa isolates. Mol. Biol. Rep. 2019, 46, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.G.; Wang, J.F.; Zhang, X.H.; Zhang, S.S.; Hu, X.F.; Chen, J.S. A gyrB-targeted PCR for rapid identification of Paenibacillus mucilaginosus. Appl. Microbiol. Biotechnol. 2010, 87, 739–747. [Google Scholar] [CrossRef]
- Irenge, L.M.; Durant, J.F.; Tomaso, H.; Pilo, P.; Olsen, J.S.; Ramisse, V.; Mahillon, J.; Gala, J.-L. Development and validation of a real-time quantitative PCR assay for rapid identification of Bacillus anthracis in environmental samples. Appl. Microbiol. Biotechnol. 2010, 88, 1179–1192. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Borriss, R. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Hesse, C.; Schulz, F.; Bull, C.T.; Shaffer, B.T.; Yan, Q.; Shapiro, N.; Hassan, K.A.; Varghese, N.; Elbourne, L.D.H.; Paulsen, I.T.; et al. Genome-based evolutionary history of Pseudomonas spp. Environ. Microbiol. 2018, 20, 2142–2159. [Google Scholar] [CrossRef]
- Xun, W.; Li, W.; Xiong, W.; Ren, Y.; Liu, Y.; Miao, Y.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat. Commun. 2019, 10, 3833. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Rio, T.G.D.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciancio, A.; Pieterse, C.M.; Mercado-Blanco, J. Editorial: Harnessing Useful Rhizosphere Microorganisms for Pathogen and Pest Biocontrol. Front. Microbiol. 2016, 7, 1620. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas biocontrol agents of soilborne pathogens: Looking back over 30 years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial commumities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Bashan, Y.; Puente, M.E.; Rodriguez-Mendoza, M.N.; Toledo, G.; Holguin, G.; Ferrera-Cerrato, R.; Pedrin, S. Survival of Azospirillum brasilense in the Bulk Soil and Rhizosphere of 23 Soil Types. Appl. Environ. Microbiol. 1995, 61, 1938–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadhave, K.R.; Devlin, P.F.; Ebertz, A.; Ross, A.; Gange, A.C. Soil Inoculation with Bacillus spp. Modifies Root Endophytic Bacterial Diversity, Evenness, and Community Composition in a Context-Specific Manner. Microb. Ecol. 2018, 76, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Mallon, C.A.; Le Roux, X.; van Doorn, G.S.; Dini-Andreote, F.; Poly, F.; Salles, J.F. The impact of failure: Unsuccessful bacterial invasions steer the soil microbial community away from the invader’s niche. ISME J. 2018, 12, 728–741. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).