Evaluation of Salipiger thiooxidans and Exiguobacterium aestuarii from the Saemangeum Reservoir as Potential Probiotics for Pacific White Shrimp (Litopenaeus vannamei)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
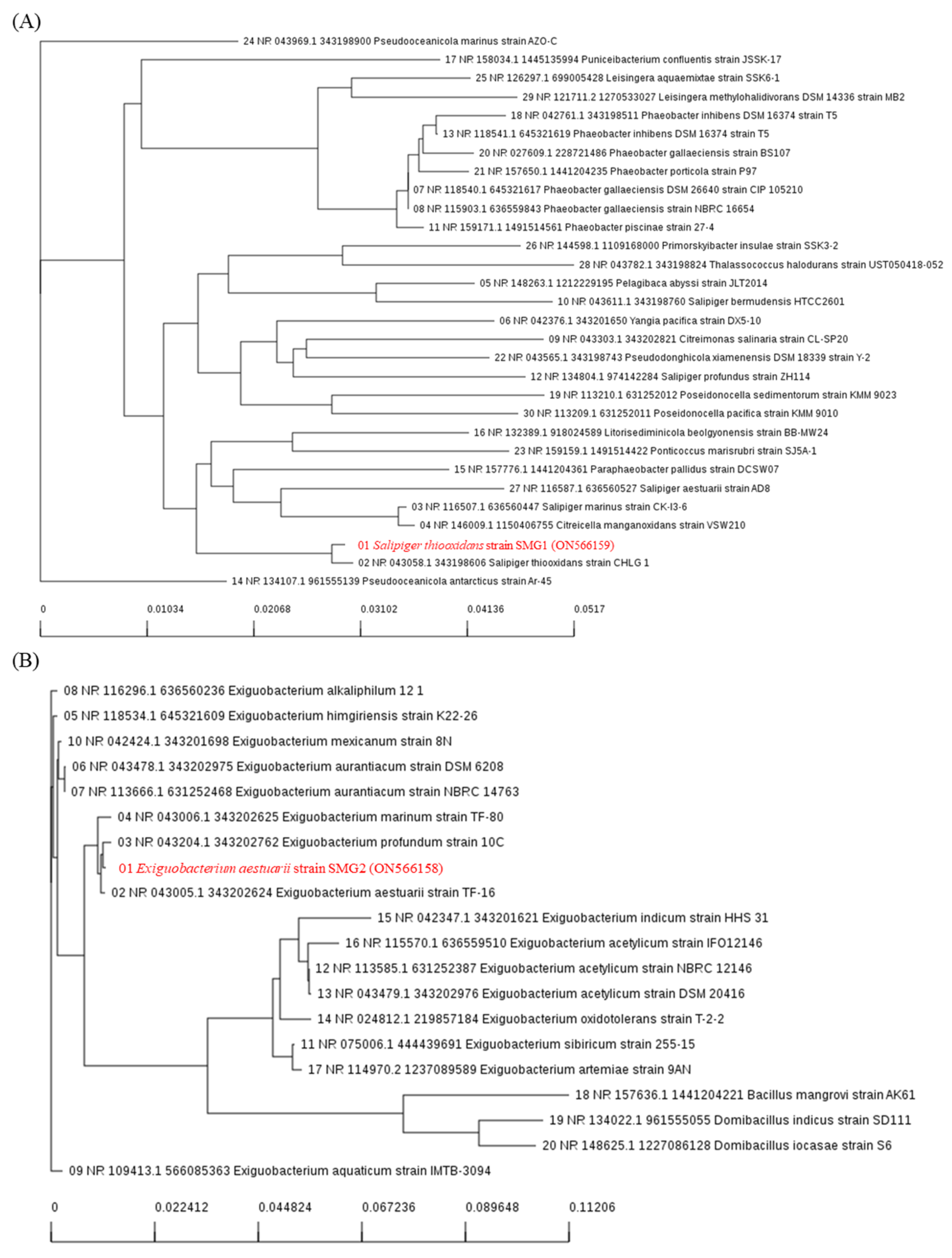
2.2. Extraction of Microorganisms from Saemangeum Reservoir
2.3. Shrimp and Experimental Condition
2.4. Growth Performance and Sample Collection
2.5. Proximate Composition Analysis
2.6. Amino Acid Analysis
2.7. Innate Immunity Analysis
2.8. Water Quality Analysis
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Whole-Body Composition
3.2. Innate Immune Response
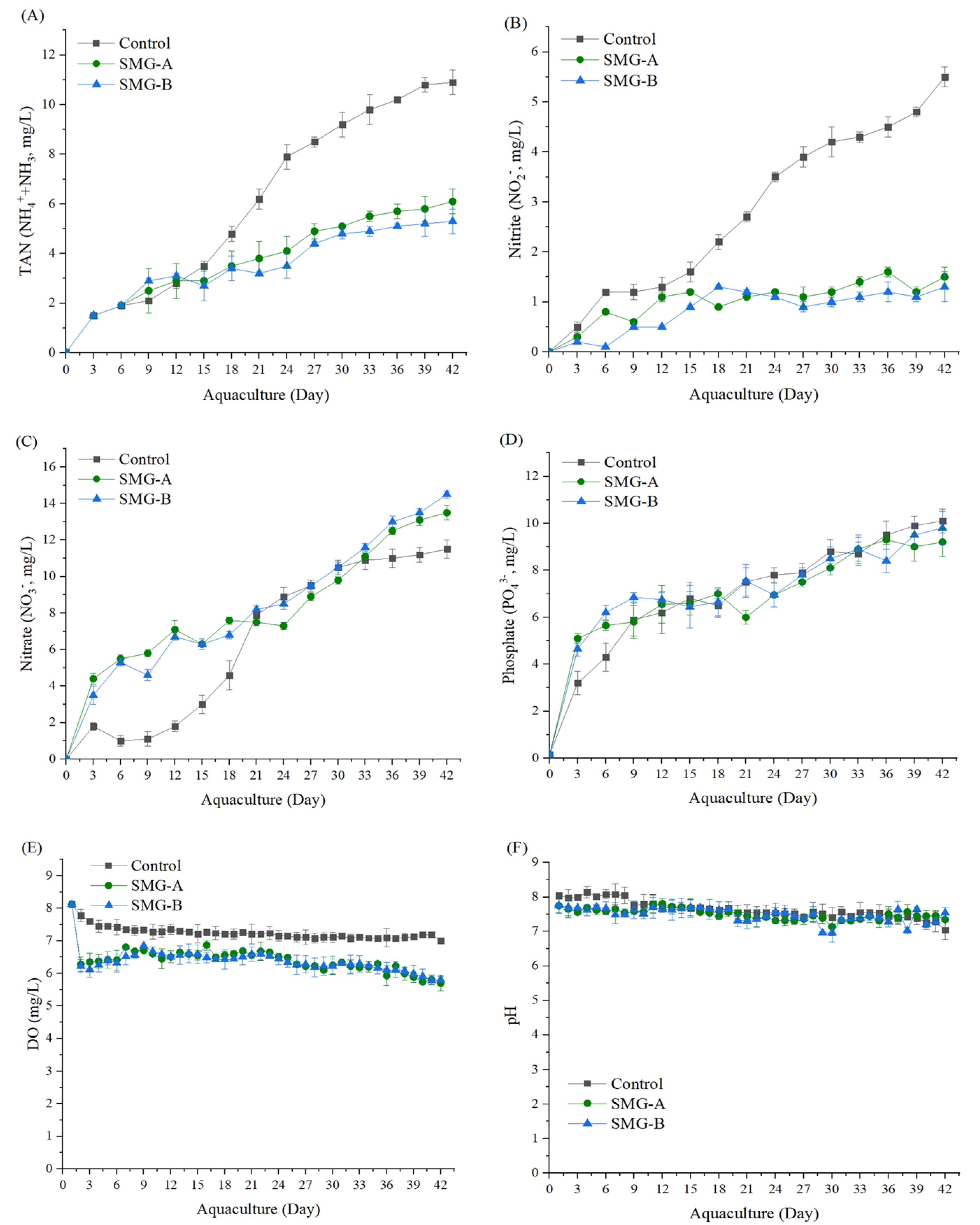
3.3. Water Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nissa, M.U.; Pinto, N.; Parkar, H.; Goswami, M.; Srivastava, S. Proteomics in fisheries and aquaculture: An approach for food security. Food Control 2021, 127, 108125. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; Rojas-Luna, T.; Cunningham, D.P. Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J. Invertebr. Pathol. 2007, 96, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Sanders, M.E.; Gaskins, H.R.; Gibson, G.R.; Mercenier, A.; Rastall, R.; Roberfroid, M.; Rowland, I.; Cherbut, C.; Klaenhammer, T.R. New scientific paradigms for probiotics and prebiotics. J. Clin. Gastroenterol. 2013, 37, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenzie, V.J.; Kueneman, J.G.; Harris, R.N. Probiotics as a tool for disease mitigation in wildlife: Insights from food production and medicine. Ann. N. Y. Acad. Sci. 2018, 1429, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, O.; Zemlyanva, M.; Vialkova, E. Investigation of probiotic agent influence on sewage quality and active sludge properties. IOP Conf. Ser. Mater. Sci. Eng. 2018, 451, 012209. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Zuo, Z.H.; Shang, B.J.; Shao, Y.C.; Li, W.Y.; Sun, J.S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China—a review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- Irianto, A.; Austin, B. Probiotics in aquaculture. J. Fish Dis. 2002, 25, 633–642. [Google Scholar] [CrossRef]
- Chauhan, A.; Singh, R. Probiotics in aquaculture: A promising emerging alternative approach. Symbiosis 2019, 77, 99–113. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Ángeles Esteban, M.; Dadar, M.; Dawood, M.A.; Faggio, C. Host-associated probiotics: A key factor in sustainable aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
- Sabu, E.A.; Gonsalves, M.J.; Sreepada, R.A.; Shivaramu, M.S.; Ramaiah, N. Evaluation of the physiological bacterial groups in a tropical biosecured, zero-exchange system growing whiteleg shrimp, Litopenaeus vannamei. Microb. Ecol. 2021, 81, 335–346. [Google Scholar] [CrossRef]
- Chiu, S.T.; Chu, T.W.; Simangunsong, T.; Ballantyne, R.; Chiu, C.S.; Liu, C.H. Probiotic, Lactobacillus pentosus BD6 boost the growth and health status of white shrimp, Litopenaeus vannamei via oral administration. Fish Shellfish Immunol. 2021, 117, 124–135. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Ferreira, G.S.; Bolivar, N.C.; Pereira, S.A.; Guertler, C.; do Nascimento Vieira, F.; Mouriño, J.L.P.; Seiffert, W.Q. Microbial biofloc as source of probiotic bacteria for the culture of Litopenaeus vannamei. Aquaculture 2015, 448, 273–279. [Google Scholar] [CrossRef]
- Ulloa Walker, D.A.; Morales Suazo, M.C.; Emerenciano, M.G.C. Biofloc technology: Principles focused on potential species and the case study of Chilean river shrimp Cryphiops caementarius. Rev. Aquac. 2020, 12, 1759–1782. [Google Scholar] [CrossRef]
- Schveitzer, R.; Arantes, R.; Costódio, P.F.S.; do Espírito Santo, C.M.; Arana, L.V.; Seiffert, W.Q.; Andreatta, E.R. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquac. Eng. 2013, 56, 59–70. [Google Scholar] [CrossRef]
- Ekasari, J.; Azhar, M.H.; Surawidjaja, E.H.; Nuryati, S.; De Schryver, P.; Bossier, P. Immune response and disease resistance of shrimp fed biofloc grown on different carbon sources. Fish Shellfish Immunol. 2014, 41, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Pan, L.Q. Dietary protein level and C/N ratio manipulation in zero-exchange culture of Litopenaeus vannamei: Evaluation of inorganic nitrogen control, biofloc composition and shrimp performance. Aquac. Res. 2014, 45, 1842–1851. [Google Scholar] [CrossRef]
- Rajkumar, M.; Pandey, P.K.; Aravind, R.; Vennila, A.; Bharti, V.; Purushothaman, C.S. Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (Boone, 1931). Aquac. Res. 2016, 47, 3432–3444. [Google Scholar] [CrossRef]
- Xu, W.J.; Morris, T.C.; Samocha, T.M. Effects of C/N ratio on biofloc development, water quality, and performance of Litopenaeus vannamei juveniles in a biofloc-based, high-density, zero-exchange, outdoor tank system. Aquaculture 2016, 453, 169–175. [Google Scholar] [CrossRef]
- Kumar, V.S.; Pandey, P.K.; Anand, T.; Bhuvaneswari, G.R.; Dhinakaran, A.; Kumar, S. Biofloc improves water, effluent quality and growth parameters of Penaeus vannamei in an intensive culture system. J. Environ. Manag. 2018, 215, 206–215. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nguyen, P.N.; Bossier, P. Water quality, animal performance, nutrient budgets and microbial community in the biofloc-based polyculture system of white shrimp, Litopenaeus vannamei and gray mullet, Mugil cephalus. Aquaculture 2020, 515, 734610. [Google Scholar] [CrossRef]
- Holanda, M.; Santana, G.; Furtado, P.; Rodrigues, R.V.; Cerqueira, V.R.; Sampaio, L.A.; Wasielesky, W., Jr.; Poersch, L.H. Evidence of total suspended solids control by Mugil liza reared in an integrated system with pacific white shrimp Litopenaeus vannamei using biofloc technology. Aquac. Rep. 2020, 18, 100479. [Google Scholar] [CrossRef]
- Khoa, T.N.D.; Tao, C.T.; Van Khanh, L.; Hai, T.N. Super-intensive culture of white leg shrimp (Litopenaeus vannamei) in outdoor biofloc systems with different sunlight exposure levels: Emphasis on commercial applications. Aquaculture 2020, 524, 735277. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Y.; Su, H.; Hu, X.; Yang, K.; Wen, G.; Cao, Y. Characteristics of ammonia removal and nitrifying microbial communities in a hybrid biofloc-ras for intensive Litopenaeus vannamei culture: A pilot-scale study. Water 2020, 12, 3000. [Google Scholar] [CrossRef]
- Hussain, A.S.; Mohammad, D.A.; Sallam, W.S.; Shoukry, N.M.; Davis, D.A. Effects of culturing the Pacific white shrimp Penaeus vannamei in “biofloc” vs. “synbiotic” systems on the growth and immune system. Aquaculture 2021, 542, 736905. [Google Scholar] [CrossRef]
- Pinho, S.M.; Emerenciano, M.G.C. Sensorial attributes and growth performance of whiteleg shrimp (Litopenaeus vannamei) cultured in biofloc technology with varying water salinity and dietary protein content. Aquaculture 2021, 540, 736727. [Google Scholar] [CrossRef]
- Knipe, H.; Temperton, B.; Lange, A.; Bass, D.; Tyler, C.R. Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev. Aquac. 2021, 13, 324–352. [Google Scholar] [CrossRef]
- Nimrat, S.; Suksawat, S.; Boonthai, T.; Vuthiphandchai, V. Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet. Microbiol. 2012, 159, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, Y.; Jiang, F.; Hu, Z.; Zheng, Y. Performance of Platymonas and microbial community analysis under different C/N ratio in biofloc technology aquaculture system. J. Water Process. Eng. 2021, 43, 102257. [Google Scholar] [CrossRef]
- Manan, H.; Rosland, N.A.; Mat Deris, Z.; Che Hashim, N.F.; Kasan, N.A.; Ikhwanuddin, M.; Suloma, A.; Fauzan, F. 16S rRNA sequences of Exiguobacterium spp. bacteria dominant in a biofloc pond cultured with whiteleg shrimp, Penaeus vannamei. Aquac. Res. 2022, 53, 2029–2041. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, H.; Han, H.S.; Hur, J.W. Evaluation of Bacillus albus SMG-1 and B. safensis SMG-2 isolated from Saemangeum Lake as probiotics for aquaculture of white shrimp (Litopenaeus vannamei). Aquac. Rep. 2021, 20, 100743. [Google Scholar] [CrossRef]
- Tepaamorndech, S.; Chantarasakha, K.; Kingcha, Y.; Chaiyapechara, S.; Phromson, M.; Sriariyanun, M.; Kirschke, C.P.; Huang, L.; Visessanguan, W. Effects of Bacillus aryabhattai TBRC8450 on vibriosis resistance and immune enhancement in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 4–13. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Kannan, S.V.; Das, R.R.; Kumar, R.S.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C: N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish Immunol. 2018, 81, 329–337. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martínez-Porchas, M.; Arvayo, M.A.; Villalpando-Canchola, E.; Gollas-Galván, T.; Porchas-Cornejo, M.A. Immunophysiological response of Pacific white shrimp exposed to a probiotic mixture of Proteobacteria and Firmicutes in farm conditions. N. Am. J. Aquac. 2016, 78, 193–202. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Q. Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Mar. Biol. Res. 2010, 6, 327–332. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chi, C.C.; Liu, C.H. The growth and apparent digestibility of white shrimp, Litopenaeus vannamei, are increased with the probiotic, Bacillus subtilis. Aquac. Res. 2019, 50, 1475–1481. [Google Scholar] [CrossRef]
- Dinoto, A.; Handayani, R.; Saputra, S. The 16S rRNA analysis of proteolytic bacteria isolated from recirculating aquaculture system. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012019. [Google Scholar] [CrossRef]
- Fox, J.M.; Lawrence, A.L.; Li-Chan, E. Dietary requirement for lysine by juvenile Penaeus vannamei using intact and free amino acid sources. Aquaculture 1995, 131, 279–290. [Google Scholar] [CrossRef]
- Liu, F.J.; Liu, Y.J.; Tian, L.X.; Chen, W.D.; Yang, H.J.; Du, Z.Y. Quantitative dietary leucine requirement of juvenile Pacific white shrimp, Litopenaeus vannamei (Boone) reared in low-salinity water. Aquac. Nutr. 2014, 20, 332–340. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.J.; Liu, Y.J.; Tian, L.X. Dietary phenylalanine requirement of the juvenile Pacific white shrimp Litopenaeus vannamei (Boone) reared in low-salinity water. J. Shellfish Res. 2019, 38, 35–41. [Google Scholar] [CrossRef]
- Fernandes, S.; Kerkar, S.; Leitao, J.; Mishra, A. Probiotic role of salt pan bacteria in enhancing the growth of whiteleg shrimp, Litopenaeus vannamei. Probiotics Antimicrob. Proteins 2019, 11, 1309–1323. [Google Scholar] [CrossRef]
- Xie, S.W.; Tian, L.X.; Li, Y.M.; Zhou, W.; Zeng, S.L.; Yang, H.J.; Liu, Y.J. Effect of proline supplementation on anti-oxidative capacity, immune response and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2015, 448, 105–111. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Jeter, J.M.; Drulis, J.M.; Nelson, S.E.; Haschke, F.; Steenhout, P.; Brown, C.; Maire, J.C.; Hager, C. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: Infant growth and health. Monatsschrift Kinderheilkd. 2003, 151, S65–S71. [Google Scholar] [CrossRef]
- Jinendiran, S.; Nathan, A.A.; Ramesh, D.; Vaseeharan, B.; Sivakumar, N. Modulation of innate immunity, expression of cytokine genes and disease resistance against Aeromonas hydrophila infection in goldfish (Carassius auratus) by dietary supplementation with Exiguobacterium acetylicum S01. Fish Shellfish Immunol. 2019, 84, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sadat Hoseini Madani, N.; Adorian, T.J.; Ghafari Farsani, H.; Hoseinifar, S.H. The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquac. Res. 2018, 49, 1926–1933. [Google Scholar] [CrossRef]
- Liu, K.F.; Chiu, C.H.; Shiu, Y.L.; Cheng, W.; Liu, C.H. Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish Shellfish Immunol. 2010, 28, 837–844. [Google Scholar] [CrossRef]
- Adel, M.; Yeganeh, S.; Dawood, M.A.O.; Safari, R.; Radhakrishnan, S. Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 1401–1409. [Google Scholar] [CrossRef]
- Yang, C.; Kong, J.; Wang, Q.; Liu, Q.; Tian, Y.; Luo, K. Heterosis of haemolymph analytes of two geographic populations in Chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2007, 23, 62–70. [Google Scholar] [CrossRef]
- Yan, F.J.; Tian, X.L.; Dong, S.L.; Fang, Z.H.; Yang, G. Growth performance, immune response, and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus fed a supplementary diet of the potential probiotic Paracoccus marcusii DB11. Aquaculture 2014, 420, 105–111. [Google Scholar] [CrossRef]
- Cheng, T.C. Immunodeficiency diseases in marine mollusks: Measurements of some variables. J. Aquat. Anim. Health 1989, 1, 209–216. [Google Scholar] [CrossRef]
- Blasco, J.; Puppo, J.; Sarasquete, M.C. Acid and alkaline phosphatase activities in the clam Ruditapes philippinarum. Mar. Biol. 1993, 115, 113–118. [Google Scholar] [CrossRef]
- Xie, J.J.; Liu, Q.Q.; Liao, S.; Fang, H.H.; Yin, P.; Xie, S.W.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 90, 456–465. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Zhou, L.; Ao, S.; Tang, H.; Zhou, Y.; Chen, Q.; Gao, X.; Jiang, Q.; Zhang, X. Probiotic potential of Bacillus velezensis: Antimicrobial activity against non-O1 Vibrio cholerae and immune enhancement effects on Macrobrachium nipponense. Aquaculture 2021, 541, 736817. [Google Scholar] [CrossRef]
- Du, Y.; Wang, B.; Jiang, K.; Wang, M.; Zhou, S.; Liu, M.; Wang, L. Exploring the influence of the surface proteins on probiotic effects performed by Lactobacillus pentosus HC-2 using transcriptome analysis in Litopenaeus vannamei midgut. Fish Shellfish Immunol. 2019, 87, 853–870. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yuan, W.; Wang, S.; Guo, W.; Li, A.; Wu, Y.; Chen, X.; Ren, Z.; Zhou, Y. In vitro screening of putative probiotics and their dual beneficial effects: To white shrimp (Litopenaeus vannamei) postlarvae and to the rearing water. Aquaculture 2019, 498, 61–71. [Google Scholar] [CrossRef]
- AftabUddin, S.; Siddique, M.A.M.; Sein, A.; Dey, P.K.; Rashed-Un-Nabi, M.; Haque, M.A. First use of biofloc technology for Penaeus monodon culture in Bangladesh: Effects of stocking density on growth performance of shrimp, water quality and bacterial growth. Aquac. Rep. 2020, 18, 100518. [Google Scholar] [CrossRef]
- Kamilya, D.; Devi, W.M. Bacillus Probiotics and Bioremediation: An Aquaculture Perspective. In Bacilli in Agrobiotechnology; Islam, M.T., Rahman, M., Pandey, P., Eds.; Springer: Cham, Switzerland, 2022; pp. 335–347. [Google Scholar] [CrossRef]
- Truong, Q.P.; Phan, T.C.; Vu, H.H.; Pham, T.T.; Huynh, T.G.; Vu, N.U. Isolation of Potential Probiotic Bacillus subtilis CM3. 1 and Its Effects on the Water Quality and Growth Performance of the Whiteleg Shrimp Litopenaeus vannamei in the Mekong Delta, Vietnam. AACL Bioflux 2021, 14. Available online: www.bioflux.com.ro/docs/2021.3347–3357.pdf (accessed on 1 January 2022).
- Llario, F.; Falco, S.; Sebastiá-Frasquet, M.T.; Escrivá, J.; Rodilla, M.; Poersch, L.H. The role of bacillus amyloliquefaciens on litopenaeus vannamei during the maturation of a biofloc system. J. Mar. Sci. Eng. 2019, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Feng, L.; Dai, J.; Yang, G.; Mu, J. Characteristics of nitrogen removal and microbial community in biofilm system via combination of pretreated lignocellulosic carriers and various conventional fillers. Biodegradation 2017, 28, 337–349. [Google Scholar] [CrossRef]
| Control | SMG-A | SMG-B | |
|---|---|---|---|
| Initial body weight (g) | 0.40 ± 0.01 | 0.40 ± 0.01 | 0.40 ± 0.01 |
| Final body weight (g) | 4.67 ± 0.16 b | 4.88 ± 0.09 b | 5.48 ± 0.11 a |
| WG (%) 2 | 1068.38 ± 41.19 b | 1191.01 ± 22.54 b | 1268.92 ± 26.59 a |
| FE (%) 3 | 90.05 ± 7.43 b | 94.71 ± 2.72 ab | 108.26 ± 6.53 a |
| Survival (%) 4 | 90.83 ± 3.82 | 93.33 ± 1.44 | 91.67 ± 6.29 |
| (%) | Pacific White Shrimp 2 | Microorganisms 3 | |||
|---|---|---|---|---|---|
| Control | SMG-A | SMG-B | S. thiooxidans | E. aestuarii | |
| Moisture | 71.95 ± 0.25 | 71.46 ± 0.65 | 71.28 ± 0.59 | 0.42 ± 0.02 | 0.54 ± 0.01 |
| Crude protein | 19.87 ± 0.23 b | 19.99 ± 0.35 b | 23.11 ± 0.41 a | 71.65 ± 0.62 | 72.23 ± 0.84 |
| Crude lipid | 1.48 ± 0.11 | 1.53 ± 0.08 | 1.65 ± 0.10 | 0.61 ± 0.02 | 0.66 ± 0.05 |
| Crude ash | 3.65 ± 0.27 | 3.88 ± 0.24 | 3.75 ± 0.31 | 8.40 ± 0.18 | 8.45 ± 0.09 |
| Pacific White Shrimp 2 | Microorganisms 2 | ||||
|---|---|---|---|---|---|
| Control | SMG-A | SMG-B | S. thiooxidans | E. aestuarii | |
| Essential amino acids (EAA) 3 | |||||
| Arginine | 4.60 ± 0.08 b | 5.10 ± 0.10 a | 5.37 ± 0.33 a | 2.25 ± 0.05 | 2.71 ± 0.13 |
| Threonine | 2.88 ± 0.05 | 3.21 ± 0.03 | 2.85 ± 0.24 | 2.02 ± 0.02 | 2.01 ± 0.05 |
| Valine | 2.88 ± 0.02 | 3.18 ± 0.02 | 3.28 ± 0.23 | 2.98 ± 0.12 | 3.42 ± 0.08 |
| Isoleucine | 2.52 ± 0.07 | 2.72 ± 0.03 | 2.86 ± 0.29 | 2.24 ± 0.03 | 2.75 ± 0.06 |
| Leucine | 4.04 ± 0.12 b | 4.40 ± 0.05 ab | 4.67 ± 0.34 a | 3.21 ± 0.02 | 3.55 ± 0.08 |
| Methionine | 1.43 ± 0.02 | 1.46 ± 0.04 | 1.45 ± 0.02 | 0.49 ± 0.02 | 0.68 ± 0.03 |
| Lysine | 4.27 ± 0.01 b | 4.34 ± 0.08 b | 5.02 ± 0.37 a | 3.63 ± 0.05 | 4.12 ± 0.04 |
| Phenylalanine | 2.59 ± 0.12 b | 2.79 ± 0.04 ab | 3.00 ± 0.23 a | 2.09 ± 0.11 | 2.29 ± 0.14 |
| Histidine | 2.77 ± 0.04 b | 3.18 ± 0.06 a | 3.54 ± 0.12 a | 1.98 ± 0.06 | 2.08 ± 0.11 |
| Non-essential amino acids (NEAA) 3 | |||||
| Serine | 2.35 ± 0.01 | 2.67 ± 0.04 | 2.63 ± 0.07 | 1.65 ± 0.04 | 1.58 ± 0.09 |
| Glutamic acid | 9.46 ± 0.03 b | 10.55 ± 0.16 a | 10.53 ± 0.06 a | 6.53 ± 0.22 | 7.52 ± 0.18 |
| Proline | 10.42 ± 0.01 a | 6.36 ± 0.15 b | 4.98 ± 0.79 c | 1.88 ± 0.05 | 1.00 ± 0.04 |
| Glycine | 4.40 ± 0.01 c | 4.89 ± 0.07 b | 5.38 ± 0.16 a | 2.45 ± 0.09 | 2.55 ± 0.14 |
| Alanine | 2.33 ± 0.01 | 2.53 ± 0.13 | 2.56 ± 0.09 | 4.13 ± 0.46 | 4.26 ± 0.22 |
| Tyrosine | 1.94 ± 0.18 | 1.97 ± 0.06 | 2.16 ± 0.17 | 1.17 ± 0.13 | 1.50 ± 0.14 |
| Aspartic acid | 7.87 ± 0.07 | 7.90 ± 0.03 | 7.89 ± 0.51 | 4.17 ± 0.22 | 3.25 ± 0.07 |
| Cysteine | 1.51 ± 0.02 | 1.43 ± 0.03 | 1.56 ± 0.01 | 0.53 ± 0.08 | 0.50 ± 0.05 |
| Control | SMG-A | SMG-B | |
|---|---|---|---|
| Lysozyme 1 | 0.128 ± 0.006 b | 0.141 ± 0.017 b | 0.206 ± 0.011 a |
| ACP 2 | 11.95 ± 0.23 b | 12.38 ± 0.48 b | 20.35 ± 0.30 a |
| ALP 3 | 2.08 ± 0.08 b | 2.19 ± 0.13 b | 4.32 ± 0.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Jeon, H.; Bai, S.C.; Hur, J.-W.; Han, H.-S. Evaluation of Salipiger thiooxidans and Exiguobacterium aestuarii from the Saemangeum Reservoir as Potential Probiotics for Pacific White Shrimp (Litopenaeus vannamei). Microorganisms 2022, 10, 1113. https://doi.org/10.3390/microorganisms10061113
Kim S, Jeon H, Bai SC, Hur J-W, Han H-S. Evaluation of Salipiger thiooxidans and Exiguobacterium aestuarii from the Saemangeum Reservoir as Potential Probiotics for Pacific White Shrimp (Litopenaeus vannamei). Microorganisms. 2022; 10(6):1113. https://doi.org/10.3390/microorganisms10061113
Chicago/Turabian StyleKim, Soohwan, Hyuncheol Jeon, Sungchul Charles Bai, Jun-Wook Hur, and Hyon-Sob Han. 2022. "Evaluation of Salipiger thiooxidans and Exiguobacterium aestuarii from the Saemangeum Reservoir as Potential Probiotics for Pacific White Shrimp (Litopenaeus vannamei)" Microorganisms 10, no. 6: 1113. https://doi.org/10.3390/microorganisms10061113
APA StyleKim, S., Jeon, H., Bai, S. C., Hur, J.-W., & Han, H.-S. (2022). Evaluation of Salipiger thiooxidans and Exiguobacterium aestuarii from the Saemangeum Reservoir as Potential Probiotics for Pacific White Shrimp (Litopenaeus vannamei). Microorganisms, 10(6), 1113. https://doi.org/10.3390/microorganisms10061113







