Antimicrobial Effects of L-Chg10-Teixobactin against Enterococcus faecalis In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Inoculum
2.2. Preparation of Drugs
2.3. Minimum Inhibitory Concentration (MIC)
2.4. Minimum Bactericidal Concentration (MBC)
2.5. Minimum Biofilm Inhibitory Concentration (MBIC)
2.6. Minimum Biofilm Eradication Concentration (MBEC)
2.7. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC)
3.2. Minimum Bactericidal Concentration (MBC)
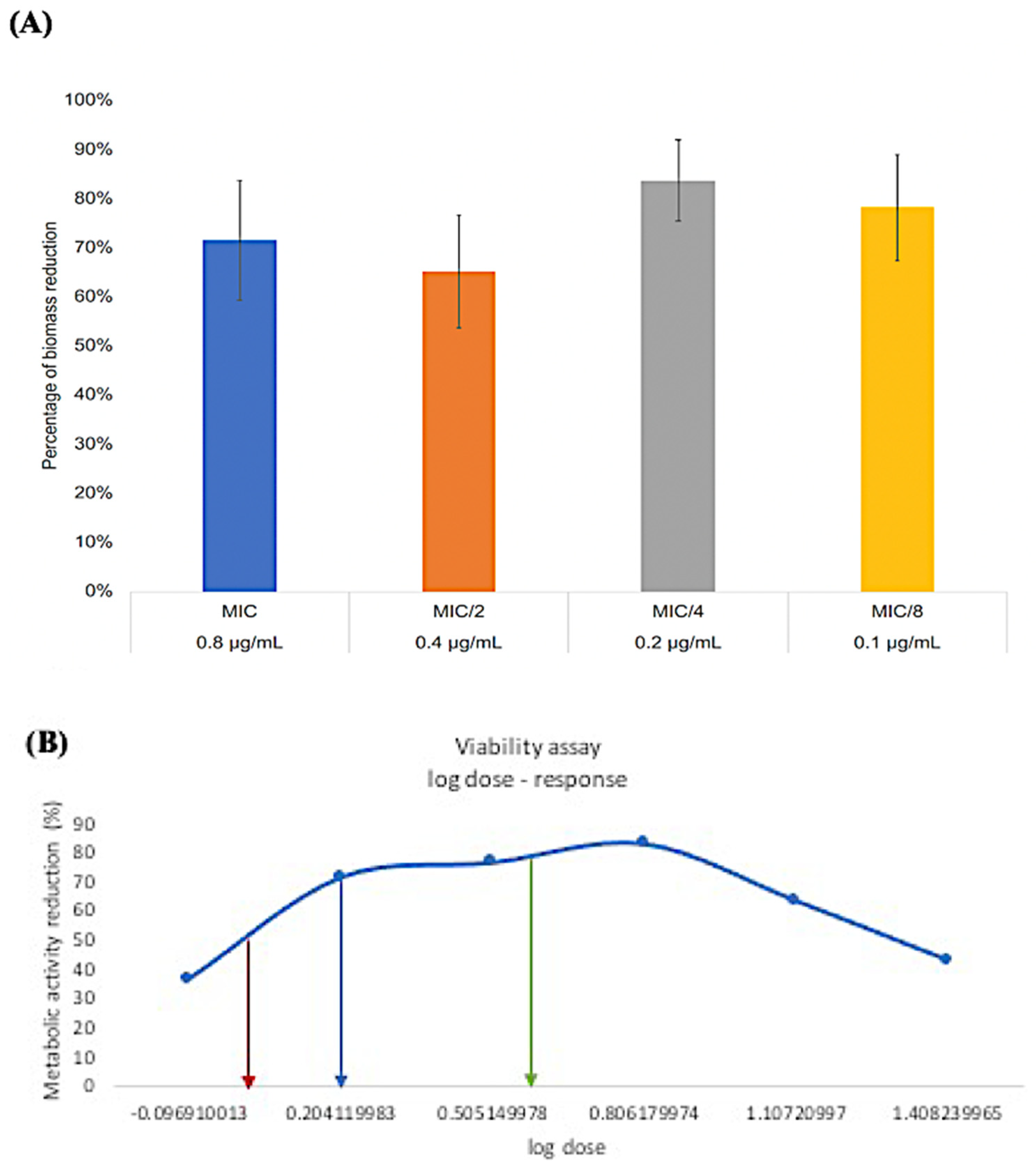
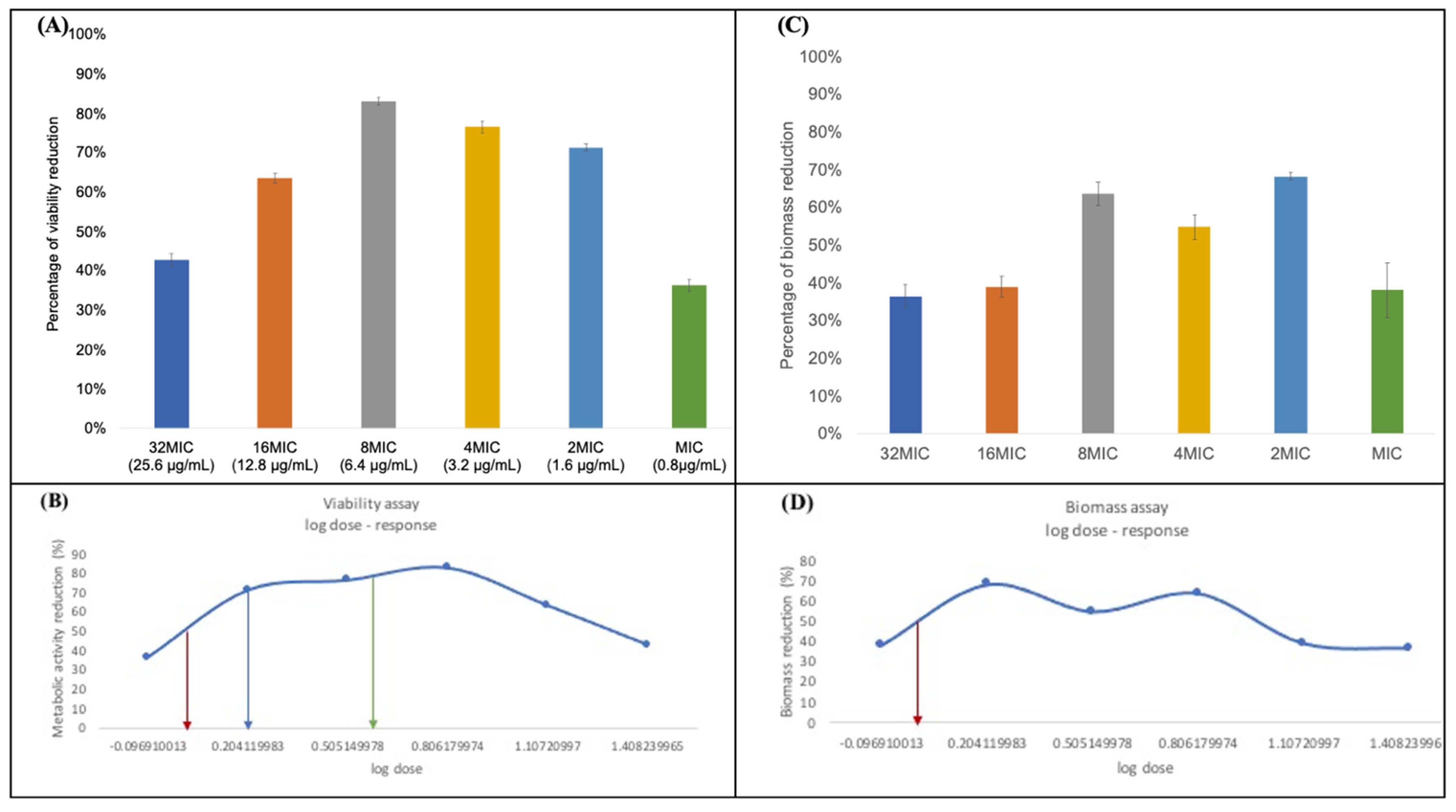
3.3. Minimum Biofilm Inhibitory Concentration (MBIC)
3.4. Minimum Biofilm Eradication Concentration (MBEC)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Wang, Q.; Zhang, C.; Cheung, G.S.; Shen, Y. Prevalence, phenotype, and genotype of Enterococcus faecalis isolated from saliva and root canals in patients with persistent apical periodontitis. J. Endod. 2010, 36, 1950–1955. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molander, A.; Reit, C.; Dahlen, G.; Kvist, T. Microbiological status of root-filled teeth with apical periodontitis. Int. Endod. J. 1998, 31, 1–7. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 231–252. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Ricucci, D.; Hülsmann, M. Causes and management of post-treatment apical periodontitis. Br. Dent. J. 2014, 216, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Villalta-Briones, N.; Baca, P.; Bravo, M.; Solana, C.; Aguado-Pérez, B.; Ruiz-Linares, M.; Arias-Moliz, M.T. A laboratory study of root canal and isthmus disinfection in extracted teeth using various activation methods with a mixture of sodium hypochlorite and etidronic acid. Int. Endod. J. 2021, 54, 268–278. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Kawashima, N.; Wadachi, R.; Suda, H.; Yeng, T.; Parashos, P. Root canal medicaments. Int. Dent. J. 2009, 59, 5–11. [Google Scholar]
- Vera, J.; Siqueira, J.F., Jr.; Ricucci, D.; Loghin, S.; Fernández, N.; Flores, B.; Cruz, A.G. One-versus two-visit endodontic treatment of teeth with apical periodontitis: A histobacteriologic study. J. Endod. 2012, 38, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Gould, K.; Şen, B.H.; Jonasson, P.; Cotti, E.; Mazzoni, A.; Sunay, H.; Tjäderhane, L.; Dummer, P.M.H. European Society of Endod.ontology position statement: The use of antibiotics in endodontics. Int. Endod. J. 2018, 51, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, M.E.F.; Neves, M.A.S.; Diogenes, A.; Mdala, I.; Guilherme, B.P.; Siqueira, J.F., Jr.; Rôças, I.N. Infection Control in Teeth with Apical Periodontitis Using a Triple Antibiotic Solution or Calcium Hydroxide with Chlorhexidine: A Randomized Clinical Trial. J. Endod. 2018, 44, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Fiers, W.D.; Craighead, M.; Singh, I. Teixobactin and Its Analogues: A New Hope in Antibiotic Discovery. ACS Infect. Dis. 2017, 3, 688–690. [Google Scholar] [CrossRef]
- Piddock, L.J. Teixobactin, the first of a new class of antibiotics discovered by iChip technology? J. Antimicrob. Chemother. 2015, 70, 2679–2680. [Google Scholar] [CrossRef]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Karas, J.A.; Chen, F.; Schneider-Futschik, E.K.; Kang, Z.; Hussein, M.; Swarbrick, J.; Hoyer, D.; Giltrap, A.M.; Payne, R.J.; Li, J.; et al. Synthesis and structure-activity relationships of teixobactin. Ann. N. Y. Acad. Sci. 2020, 1459, 86–105. [Google Scholar] [CrossRef]
- Jin, K.; Sam, I.H.; Po, K.H.L.; Lin, D.A.; Ghazvini Zadeh, E.H.; Chen, S.; Yuan, Y.; Li, X. Total synthesis of teixobactin. Nat. Commun. 2016, 7, 12394. [Google Scholar] [CrossRef] [Green Version]
- Conceição, N.; de Oliveira Cda, C.; da Silva, L.E.; de Souza, L.R.; de Oliveira, A.G. Ampicillin susceptibility can predict in vitro susceptibility of penicillin-resistant, ampicillin-susceptible Enterococcus faecalis Isolates to amoxicillin but not to imipenem and piperacillin. J. Clin. Microbiol. 2012, 50, 3729–3731. [Google Scholar] [CrossRef] [Green Version]
- Ommen, P.; Zobek, N.; Meyer, R.L. Quantification of Biofilm Biomass by Staining: Non-Toxic Safranin Can Replace the Popular Crystal Violet. J. Microbiol. Methods 2017, 141, 87–89. [Google Scholar] [CrossRef]
- Gunjal, V.B.; Thakare, R.; Chopra, S.; Reddy, D.S. Teixobactin: A Paving Stone toward a New Class of Antibiotics? J. Med. Chem. 2020, 63, 12171–12195. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhang, Y.; Ling, J.; Ma, J.; Huang, L.; Zhang, L. An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis. PLoS ONE 2014, 9, e89209. [Google Scholar] [CrossRef] [PubMed]
- Yamazhan, T.; Aydemir, S.; Tünger, A.; Serter, D.; Gökengin, D. In vitro activities of various antimicrobials against Brucella melitensis strains in the Aegean region in Turkey. Med. Princ. Pract. 2005, 14, 413–416. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- Darnell, R.L.; Knottenbelt, M.K.; Todd Rose, F.O.; Monk, I.R.; Stinear, T.P.; Cook, G.M. Genomewide Profiling of the Enterococcus faecalis Transcriptional Response to Teixobactin Reveals CroRS as an Essential Regulator of Antimicrobial Tolerance. Msphere 2019, 4, e00228-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, A.; Prior, S.H.; Iyer, A.; Vincent, C.S.; Van Lysebetten, D.; Breukink, E.; Madder, A.; Taylor, E.J.; Singh, I. Defining the molecular structure of teixobactin analogues and understanding their role in antibacterial activities. Chem. Commun. 2017, 53, 2016–2019. [Google Scholar] [CrossRef]
- Parmar, A.; Iyer, A.; Lloyd, D.G.; Vincent, C.S.; Prior, S.H.; Madder, A.; Taylor, E.J.; Singh, I. Syntheses of potent teixobactin analogues against methicillin-resistant Staphylococcus aureus (MRSA) through the replacement of l-allo-enduracididine with its isosteres. Chem. Commun. 2017, 53, 7788–7791. [Google Scholar] [CrossRef] [Green Version]
- Ramchuran, E.J.; Somboro, A.M.; Abdel Monaim, S.A.; Amoako, D.G.; Parboosing, R.; Kumalo, H.M.; Agrawal, N.; Albericio, F.; de La Torre, B.G.; Bester, L.A. In Vitro Antibacterial Activity of Teixobactin Derivatives on Clinically Relevant Bacterial Isolates. Front. Microbiol. 2018, 9, 1535. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [Green Version]
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol. 2008, 9, R110. [Google Scholar] [CrossRef] [Green Version]
- Dale, J.L.; Beckman, K.B.; Willett, J.L.; Nilson, J.L.; Palani, N.P.; Baller, J.A.; Hauge, A.; Gohl, D.M.; Erickson, R.; Manias, D.A.; et al. Comprehensive Functional Analysis of the Enterococcus faecalis Core Genome Using an Ordered, Sequence-Defined Collection of Insertional Mutations in Strain OG1RF. Msystems 2018, 3, e00062-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoe, J.A.; Wysome, J.; West, A.P.; Heritage, J.; Wilcox, M.H. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J. Antimicrob. Chemother. 2006, 57, 767–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussein, M.; Karas, J.A.; Schneider-Futschik, E.K.; Chen, F.; Swarbrick, J.; Paulin, O.K.A.; Hoyer, D.; Baker, M.; Zhu, Y.; Li, J.; et al. The Killing Mechanism of Teixobactin against Methicillin-Resistant Staphylococcus aureus: An Untargeted Metabolomics Study. Msystems 2020, 5, e00077-20. [Google Scholar] [CrossRef] [PubMed]
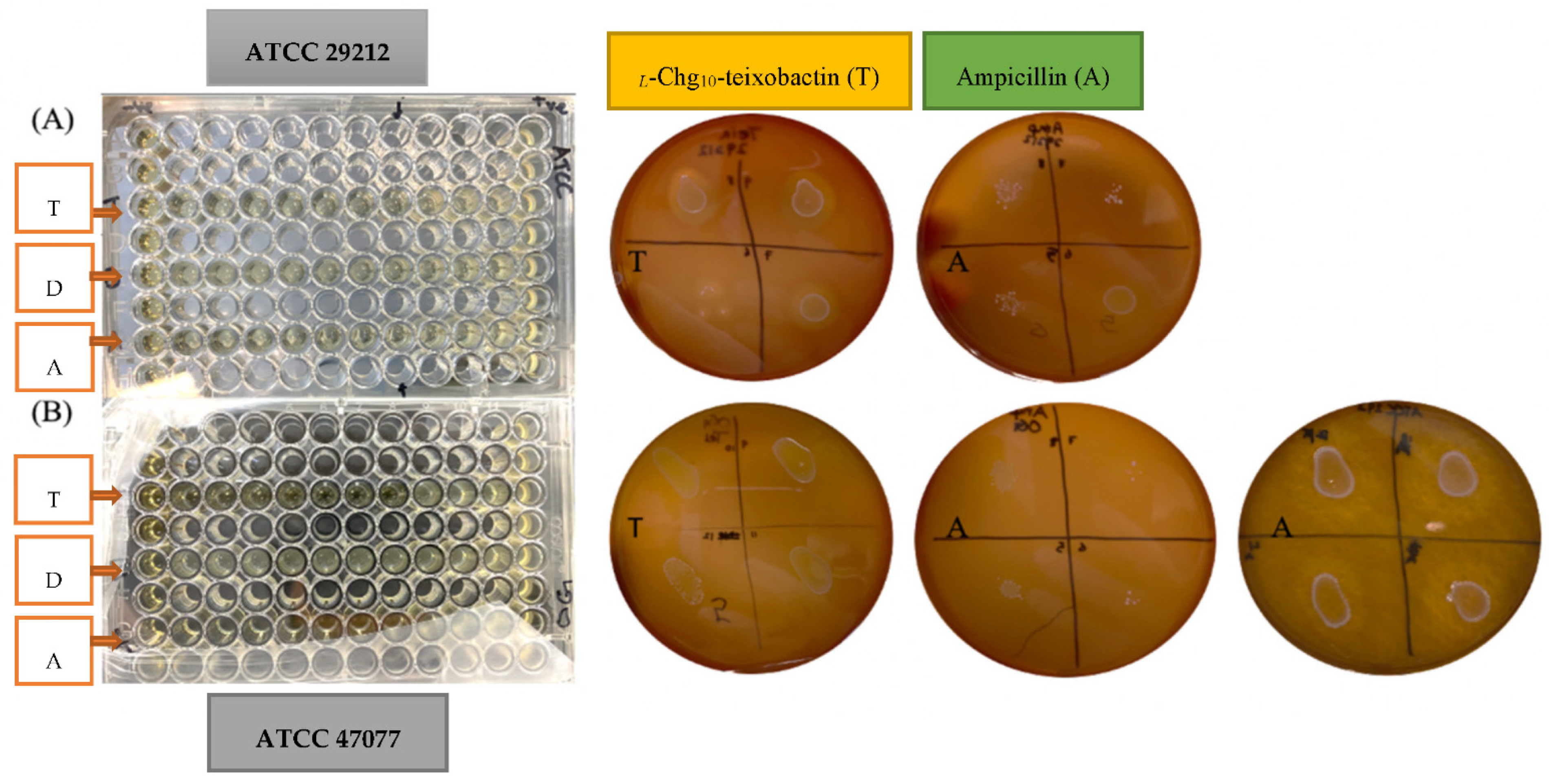
| Teixobactin Analogue | Ampicillin | DMSO | ||||
|---|---|---|---|---|---|---|
| Treatment Group | MIC | MBC | MIC | MBC | MIC | MBC |
| ATCC 29212 | 0.8 | 0.8 | 1.25 | 10 | N/A | N/A |
| ATCC 47077 | 0.8 | 0.8 | 1.25-5 | 20 | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarkhi, A.; Lee, A.H.C.; Sun, Z.; Hu, M.; Neelakantan, P.; Li, X.; Zhang, C. Antimicrobial Effects of L-Chg10-Teixobactin against Enterococcus faecalis In Vitro. Microorganisms 2022, 10, 1099. https://doi.org/10.3390/microorganisms10061099
Jarkhi A, Lee AHC, Sun Z, Hu M, Neelakantan P, Li X, Zhang C. Antimicrobial Effects of L-Chg10-Teixobactin against Enterococcus faecalis In Vitro. Microorganisms. 2022; 10(6):1099. https://doi.org/10.3390/microorganisms10061099
Chicago/Turabian StyleJarkhi, Alaa, Angeline Hui Cheng Lee, Zhenquan Sun, Mingxin Hu, Prasanna Neelakantan, Xuechen Li, and Chengfei Zhang. 2022. "Antimicrobial Effects of L-Chg10-Teixobactin against Enterococcus faecalis In Vitro" Microorganisms 10, no. 6: 1099. https://doi.org/10.3390/microorganisms10061099
APA StyleJarkhi, A., Lee, A. H. C., Sun, Z., Hu, M., Neelakantan, P., Li, X., & Zhang, C. (2022). Antimicrobial Effects of L-Chg10-Teixobactin against Enterococcus faecalis In Vitro. Microorganisms, 10(6), 1099. https://doi.org/10.3390/microorganisms10061099








