Detection of Antibodies to Ehrlichia spp. in Dromedary Camels and Co-Grazing Sheep in Northern Kenya Using an Ehrlichia ruminantium Polyclonal Competitive ELISA
Abstract
:1. Introduction
2. Materials and Methods
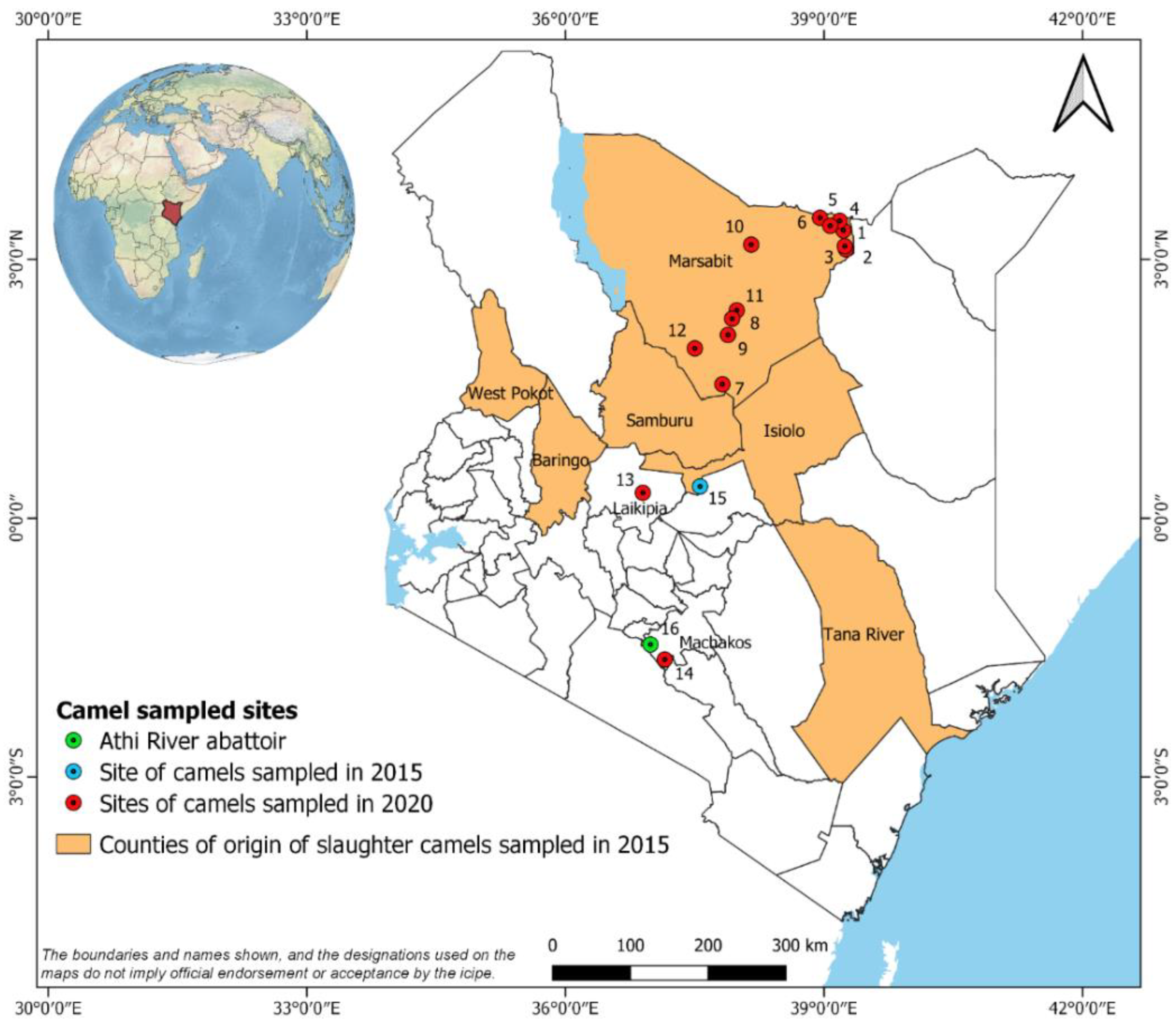
2.1. Sample Collection
2.2. Ethical Approval
2.3. E. ruminantium PC-ELISA
2.4. Statistical Analysis
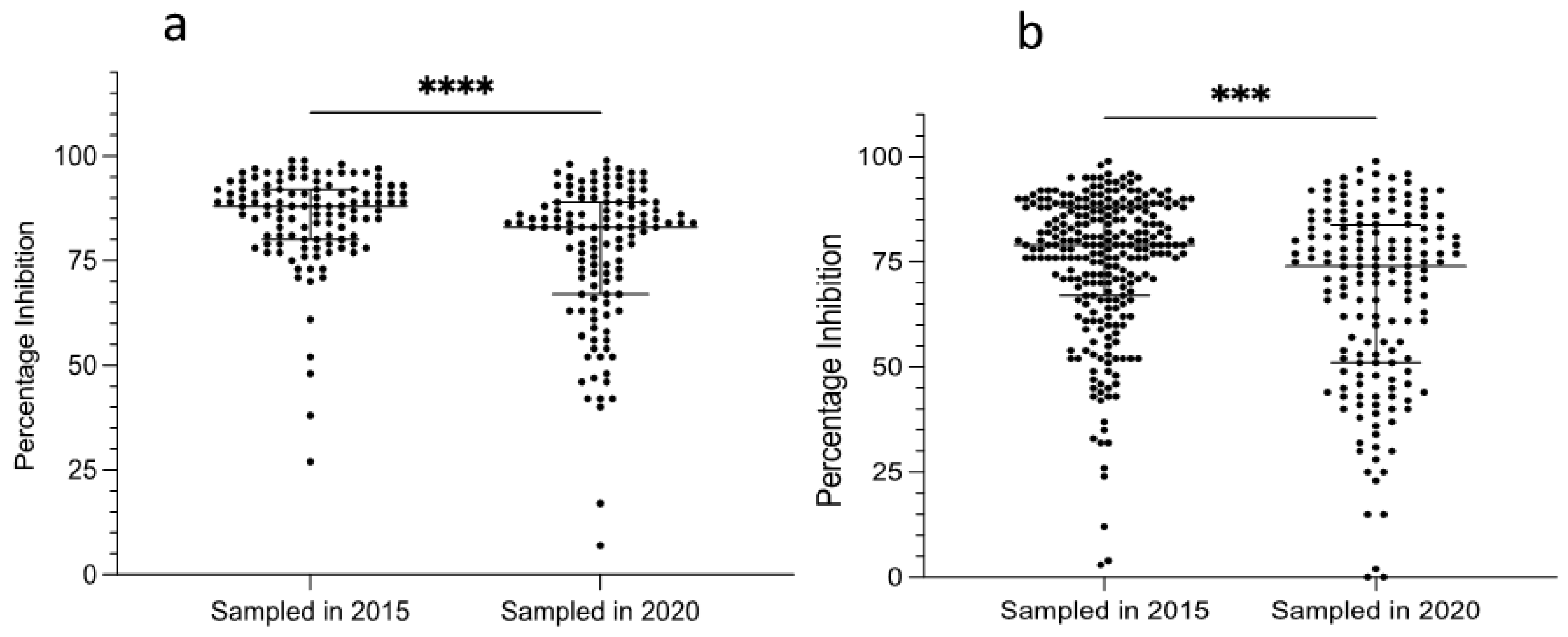
3. Results
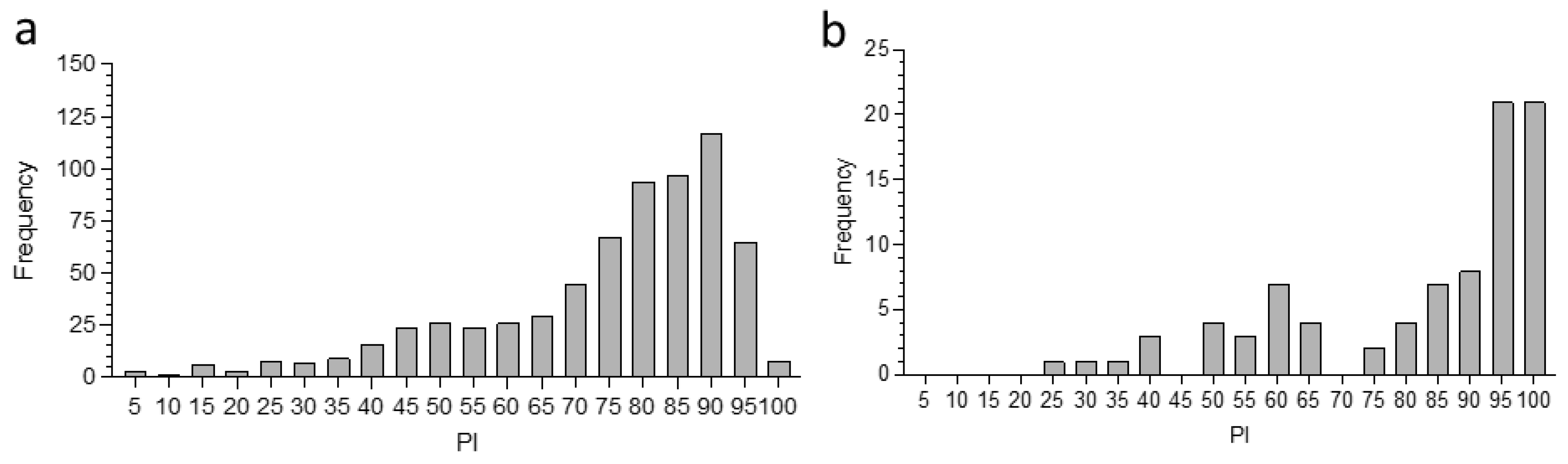
3.1. Application of the PC-ELISA for Detection of Exposure of Camels to Ehrlichia spp.
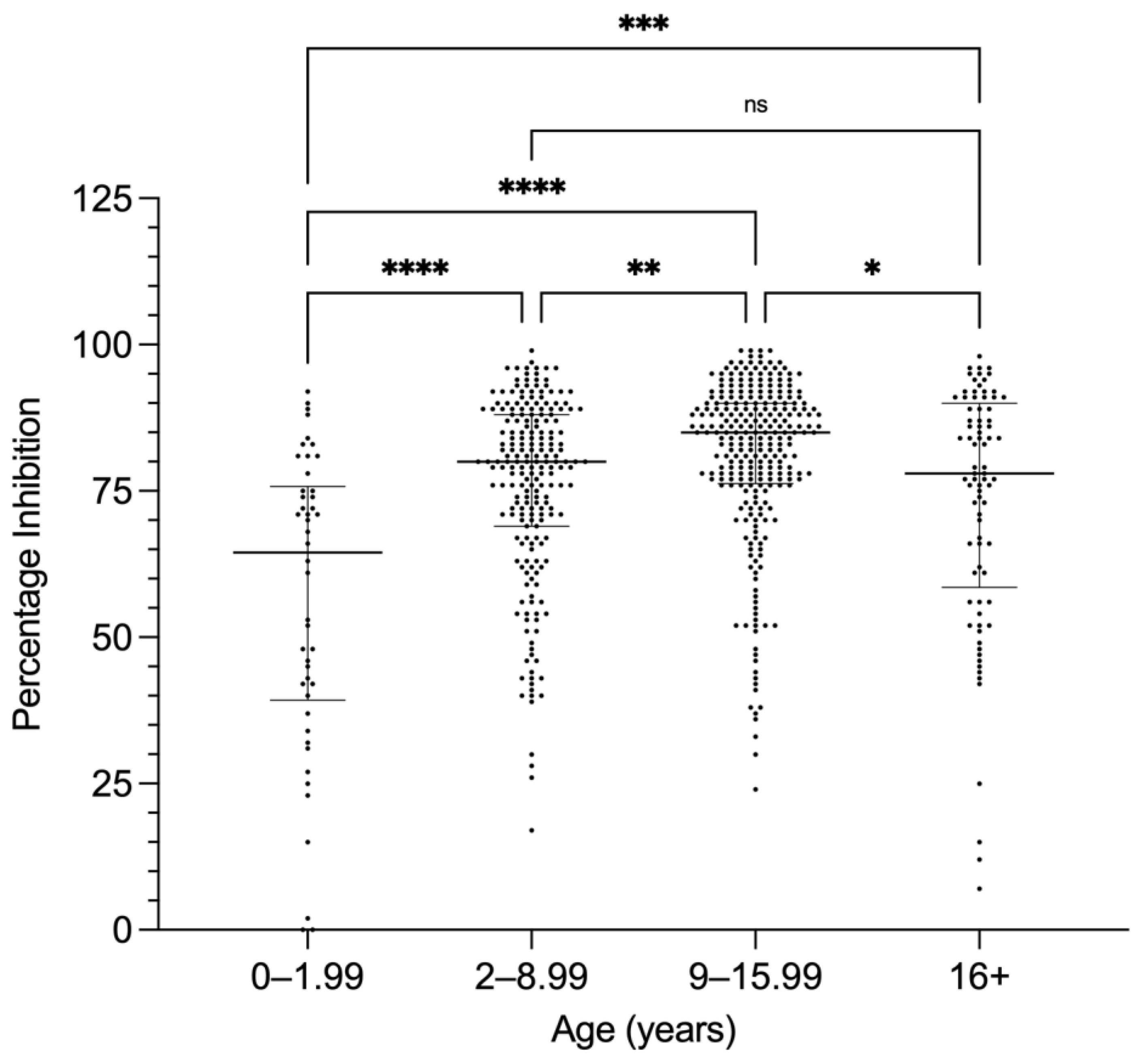
3.2. PC-ELISA Seropositivity in Camels Related to Age and Sex
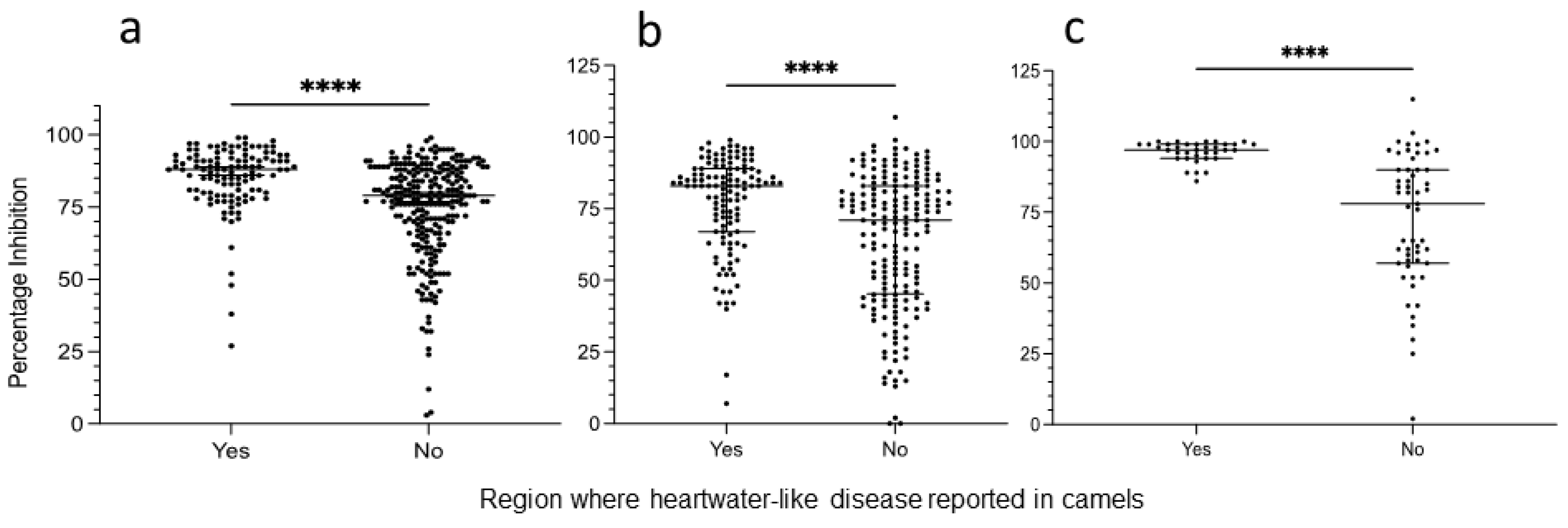
3.3. Comparison between Animals Originating from Areas with and without Reported Heartwater-like Disease in Camels
3.4. Relationship between PC-ELISA Seropositivity and Presence of Ehrlichia spp. DNA in Camel Blood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faye, B. How many large camelids in the world? A synthetic analysis of the world camel demographic changes. Pastoralism Res. Policy Pract. 2020, 10, 25. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 13 February 2022).
- FAOSTAT. Available online: http://faostat3.fao.org (accessed on 16 April 2015).
- Younan, M.; Ouso, D.O.; Bodha, B.; Keitany, E.K.; Wesonga, H.O.; Sitawa, R.; Kimutai, J.; Kuria, W.; Sake, W.S.; Svitek, N.; et al. Ehrlichia spp. close to Ehrlichia ruminantium, Ehrlichia canis, and “Candidatus Ehrlichia regneryi” linked to heartwater-like disease in Kenyan camels (Camelus dromedarius). Trop. Anim. Health Prod. 2021, 53, 147. [Google Scholar] [CrossRef] [PubMed]
- Gitonga, P.N. Acute Camel Death Syndrome: Outbreak Investigation Report for Wajir County; University of Nairobi: Nairobi, Kenya, 2016. [Google Scholar] [CrossRef]
- Camus, E.; Barre, N.; Martinez, D.; Uilenberg, G. Heartwater (Cowdriosis) A Review, 2nd ed.; O.I.E.: Paris, France, 1996. [Google Scholar]
- Van de Pypekamp, H.E.; Prozesky, L. Heartwater. An overview of the clinical signs, susceptibility and differential diagnoses of the disease in domestic animals. Onderstepoort J. Vet. Res. 1987, 54, 263–266. [Google Scholar] [PubMed]
- Daubney, R. Natural transmission of heart-water of sheep by Amblyomma variegatum (Fabricius 1794). Parasitology 1930, 22, 260–267. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.J.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Moshkovski, S.D. Comments by Readers. Science 1947, 106, 62. [Google Scholar]
- Cowdry, E.V. Studies on the etiology of heartwater. I. Observation of a rickettsia, Rickettsia ruminantium (N. Sp.) in the tissues of infected animals. J. Exp. Med. 1925, 42, 231–252. [Google Scholar]
- Ngumi, P.N.; Rumberia, R.M.; Williamson, S.M.; Sumption, K.J.; Lesan, A.C.; Kariuki, D.P. Isolation of the causative agent of heartwater (Cowdria ruminantium) from three Amblyomma species in eight districts of Kenya. Vet. Rec. 1997, 140, 13–16. [Google Scholar] [CrossRef]
- Bastos, A.D.S.; Mohammed, O.B.; Bennet, N.C.; Petevinos, C.; Alagaili, A.N. Molecular detection of novel Anaplasmataceae closely related to Anaplasma platys and Ehrlichia canis in the dromedary camel (Camelus dromedarius). Vet. Microbiol. 2015, 179, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Karrar, G. Rickettsial infection (heartwater) in sheep and goats in the Sudan. Brit. Vet. J. 1960, 116, 105–114. [Google Scholar] [CrossRef]
- Bechir, A. OIE Immediate Notification Report (31/12/2013). Available online: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=14588 (accessed on 14 July 2020).
- Getange, D.; Bargul, J.; Kanduma, E.; Collins, M.; Bodha, B.; Denge, D.; Chiuya, T.; Githaka, N.; Younan, M.; Fèvre, E.; et al. Ticks and tick-borne pathogens associated with one-humped camels (Camelus dromedarius) in northern Kenya. Microorganisms 2021, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Sumption, K.J.; Paxton, E.A.; Bell-Sakyi, L. Development of a polyclonal competitive enzyme-linked immunosorbent assay for detection of antibodies to Ehrlichia ruminantium. Clin. Diagn. Lab. Immunol. 2003, 10, 910–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell-Sakyi, L.; Koney, E.B.M.; Dogbey, O.; Walker, A.R. Ehrlichia ruminantium seroprevalence in domestic ruminants in Ghana. I. Longitudinal survey in the Greater Accra Region. Vet. Microbiol. 2004, 100, 175–188. [Google Scholar] [CrossRef]
- van Vliet, A.H.M.; Zeijst, B.A.M.; Camus, E.; Mahan, S.M.; Martinez, D.; Jongejan, F. Use of a specific immunogenic region on the Cowdria ruminantium MAP1 protein in a serological assay. J. Clin. Microbiol. 1995, 33, 2405–2410. [Google Scholar] [CrossRef] [Green Version]
- Bell-Sakyi, L.; Koney, E.B.M.; Dogbey, O.; Sumption, K.J.; Walker, A.R.; Bath, A.; Jongejan, F. Detection by two enzyme-linked immunosorbent assays of antibodies to Ehrlichia ruminantium in field sera collected from sheep and cattle in Ghana. Clin. Diagn. Lab. Immunol. 2003, 10, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Azwai, S.M.; Carter, S.D.; Woldehiwet, Z. The isolation and characterization of camel (Camelus dromedarius) immunoglobulin classes and subclasses. J. Comp. Pathol. 1993, 109, 187–195. [Google Scholar] [CrossRef]
- Perveen, N.; Muzaffar, S.B.; Al-Deeb, M.A. Ticks and tick-borne diseases of livestock in the Middle East and North Africa. Insects 2021, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Bell-Sakyi, L.; Paxton, E.; Wright, P.; Sumption, K. Immunogenicity of Ehrlichia ruminantium grown in tick cell lines. Exp. Appl. Acarol. 2002, 28, 177–185. [Google Scholar] [CrossRef]
- Koney, E.B.M.; Dogbey, O.; Walker, A.R.; Bell-Sakyi, L. Ehrlichia ruminantium seroprevalence in domestic ruminants in Ghana. II. Point prevalence survey. Vet. Microbiol. 2004, 103, 183–193. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Zweygarth, E.; Vancová, M.; Broniszewska, M.; Grubhoffer, L.; Passos, L.M.F.; Ribeiro, M.F.B.; Alberdi, P.; de la Fuente, J. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016, 66, 1426–1430. [Google Scholar] [CrossRef] [Green Version]
- Alanazi, A.D.; Nguyen, V.L.; Alyousif, M.S.; Manoj, R.R.S.; Alouffi, A.S.; Donato, R.; Sazmand, A.; Mendoza-Roldan, J.A.; Dantas-Torres, F.; Otranto, D. Ticks and associated pathogens in camels (Camelus dromedarius) from Riyadh Province, Saudi Arabia. Parasites Vectors 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mboloi, M.M.; Bekker, C.P.J.; Kriutwagen, C.; Greiner, M.; Jongejan, F. Validation of the indirect MAP1-B enzyme-linked immunosorbent assay for the detection of antibodies to Cowdria ruminantium infection. Clin. Diagn. Lab. Immunol. 1999, 6, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondry, R.; Martinez, D.; Camus, E.; Liebisch, A.; Katz, J.B.; Dewald, R.; Van Vliet, A.H.M.; Jongejan, F. Validation and comparison of three enzyme-linked immunosorbent assays for the detection of antibodies to Cowdria ruminantium infection. Ann. N. Y. Acad. Sci. 1998, 849, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.C.; Bah, M.; Reibel, R.; Jongejan, F. Cowdria ruminantium antibodies in acaricide-treated and untreated cattle exposed to Amblyomma variegatum ticks in The Gambia. Exp. Appl. Acarol. 2000, 24, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Semu, S.M.; Peter, T.F.; Mukwedeya, D.; Barbet, A.F.; Jongejan, F.; Mahan, S.M. Antibody responses to MAP 1B and other Cowdria ruminantium antigens are down regulated in cattle challenged with tick-transmitted heartwater. Clin. Diagn. Lab. Immunol. 2001, 8, 388–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, T.F.; O’Callaghan, C.J.; Medley, G.F.; Perry, B.D.; Semu, S.M.; Mahan, S.M. Population-based evaluation of the Ehrlichia ruminantium MAP 1B indirect ELISA. Exp. Appl. Acarol. 2002, 25, 881–897. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; Geysen, D.; Munstermann, S.; Bell-Sakyi, L.; Jongejan, F. Longitudinal monitoring of Ehrlichia ruminantium infection in lambs and kids by pCS20 PCR and MAP1-B ELISA in The Gambia. BMC Infect. Dis. 2007, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Swai, E.S.; Mtui, P.F.; Chang’a, A.K.; Machange, G.E. The prevalence of serum antibodies to Ehrlichia ruminantium infection in ranch cattle in Tanzania: A cross-sectional study. J. S. Afr. Vet. Ass. 2008, 79, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Boucher, F.; Moutroifi, Y.; Peba, B.; Ali, M.; Moindjie, Y.; Ruget, A.-S.; Abdouroihamane, S.; Kassim, A.M.; Soule, M.; Charafouddine, O.; et al. Tick-borne diseases in the Union of the Comoros are a hindrance to livestock development: Circulation and associated risk factors. Ticks Tick Borne Dis. 2020, 11, 101283. [Google Scholar] [CrossRef]
- Awa, D.N. Serological survey of heartwater relative to the distribution of the vector Amblyomma variegatum and other tick species in north Cameroon. Vet. Parasitol. 1997, 68, 165–173. [Google Scholar] [CrossRef]
- Walker, J.B.; Olwage, A. The tick vectors of Cowdria ruminantium (Ixodoidea, Ixodidae, genus Amblyomma) and their distribution. Onderstepoort J. Vet. Res. 1987, 54, 353–379. [Google Scholar] [PubMed]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; University of Edinburgh: Edinburgh, UK, 2004; 221p. [Google Scholar]
- Bellabidi, M.; Benaissa, M.H.; Bissati-Bouafia, S.; Harrat, Z.; Brahmi, K.; Kernif, T. Coxiella burnetii in camels (Camelus dromedarius) from Algeria: Seroprevalence, molecular characterization, and ticks (Acari: Ixodidae) vectors. Acta Trop. 2020, 206, 105443. [Google Scholar] [CrossRef] [PubMed]
- Alsubki, R.A.; Albohairy, F.M.; Attia, K.A.; Kimiko, I.; Selim, A.; Sayed-Ahmed, M.Z. Assessment of seroprevalence and associated risk factors for anaplasmosis in Camelus dromedarius. Vet. Sci. 2022, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Mamlouk, A.; Ben Yahia, H.; Abdelaali, H.; Ben Said, M.; Sellami, K.; Daaloul-Jedidi, M.; Jemli, M.H.; Messadi, L. Coxiella burnetii in Tunisian dromedary camels (Camelus dromedarius): Seroprevalence, associated risk factors and seasonal dynamics. Acta Trop. 2018, 188, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, K.R.; Davis, J.; Alam, U.; Korman, A.; Rutherford, J.S.; Rosenberg, R.; Azad, A.F. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am. J. Trop. Med. Hyg. 2003, 68, 551–553. [Google Scholar] [CrossRef]
- Chiuya, T.; Masiga, D.K.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef]
- Lutomiah, J.; Musila, L.; Makio, A.; Ochieng, C.; Koka, H.; Chepkorir, E.; Mutisya, J.; Mulwa, F.; Khamadi, S.; Miller, B.R.; et al. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the eastern and northeastern parts of Kenya. J. Med. Entomol. 2014, 51, 269–277. [Google Scholar] [CrossRef]
- Younan, M. 2016 Investigation of Acute Camel Death Syndrome (ACDS) in Kenya Interim Report; FAO Technical Report; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, M.; Ngetich, C.; Owido, M.; Getange, D.; Harris, R.; Bargul, J.L.; Bodha, B.; Njoroge, D.; Muloi, D.; Martins, D.J.; et al. Detection of Antibodies to Ehrlichia spp. in Dromedary Camels and Co-Grazing Sheep in Northern Kenya Using an Ehrlichia ruminantium Polyclonal Competitive ELISA. Microorganisms 2022, 10, 916. https://doi.org/10.3390/microorganisms10050916
Collins M, Ngetich C, Owido M, Getange D, Harris R, Bargul JL, Bodha B, Njoroge D, Muloi D, Martins DJ, et al. Detection of Antibodies to Ehrlichia spp. in Dromedary Camels and Co-Grazing Sheep in Northern Kenya Using an Ehrlichia ruminantium Polyclonal Competitive ELISA. Microorganisms. 2022; 10(5):916. https://doi.org/10.3390/microorganisms10050916
Chicago/Turabian StyleCollins, Marisol, Collins Ngetich, Milton Owido, Dennis Getange, Robert Harris, Joel L. Bargul, Boku Bodha, Daniel Njoroge, Dishon Muloi, Dino J. Martins, and et al. 2022. "Detection of Antibodies to Ehrlichia spp. in Dromedary Camels and Co-Grazing Sheep in Northern Kenya Using an Ehrlichia ruminantium Polyclonal Competitive ELISA" Microorganisms 10, no. 5: 916. https://doi.org/10.3390/microorganisms10050916
APA StyleCollins, M., Ngetich, C., Owido, M., Getange, D., Harris, R., Bargul, J. L., Bodha, B., Njoroge, D., Muloi, D., Martins, D. J., Villinger, J., Githaka, N., Baylis, M., Fèvre, E. M., Kanduma, E., Younan, M., & Bell-Sakyi, L. (2022). Detection of Antibodies to Ehrlichia spp. in Dromedary Camels and Co-Grazing Sheep in Northern Kenya Using an Ehrlichia ruminantium Polyclonal Competitive ELISA. Microorganisms, 10(5), 916. https://doi.org/10.3390/microorganisms10050916






