Imported Pet Reptiles and Their “Blind Passengers”—In-Depth Characterization of 80 Acinetobacter Species Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Isolates and DNA Extraction
2.3. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.4. Antimicrobial Susceptibility Testing
2.5. Whole-Genome Sequencing Analysis and Bacterial Species Confirmation
2.6. Antimicrobial Resistance Gene Screening
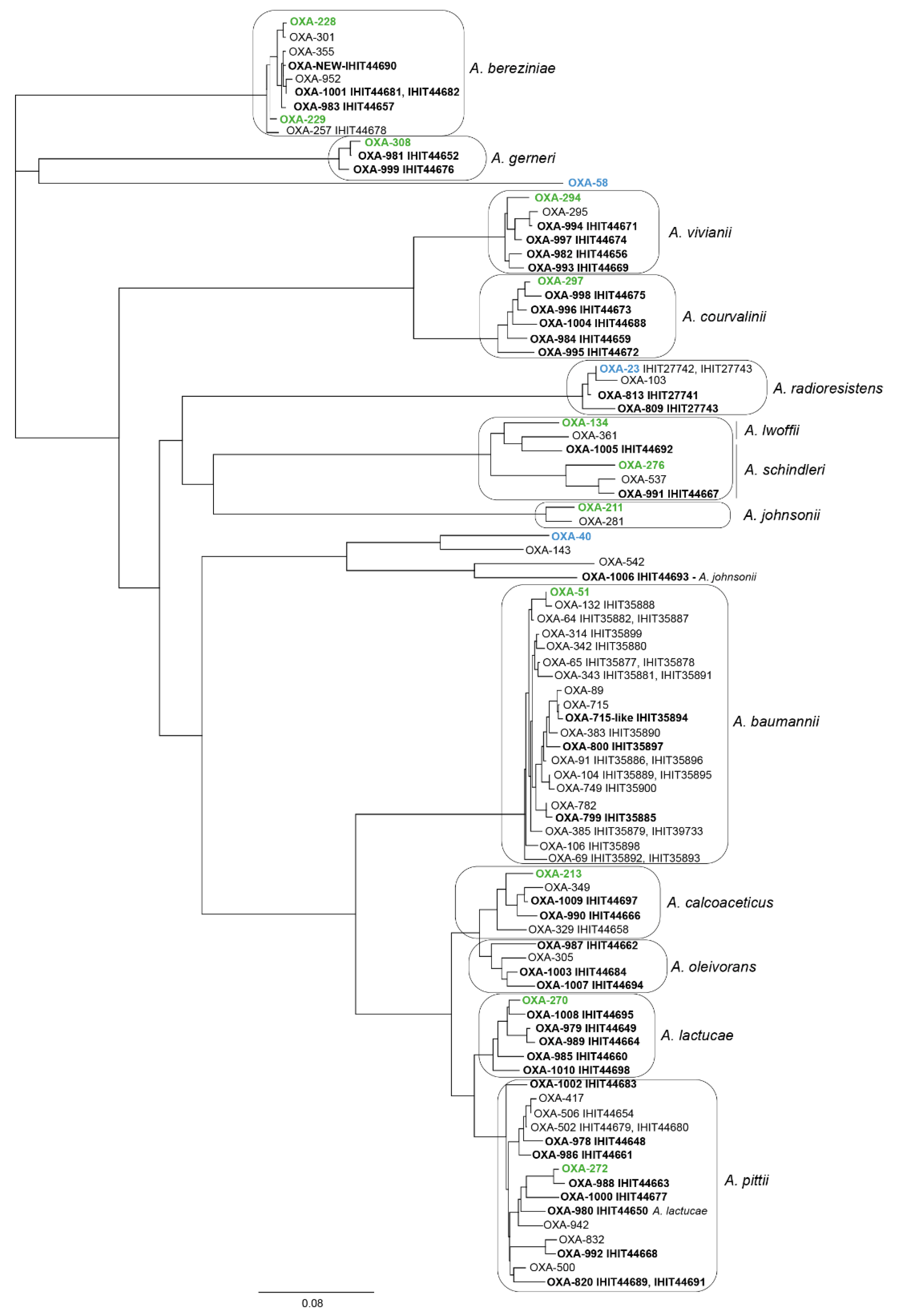
2.7. Detection of Oxacillinase Genes and Phylogenetic Analysis
2.8. Multilocus Sequence Typing of Acinetobacter Species and Assignment of A. baumannii Isolates to International Clones
3. Results
3.1. Sample Collection
3.2. Species Identification Based on MALDI-TOF MS Analysis and Whole-Genome Sequence Analysis
3.3. Phenotypic Antimicrobial Resistance
3.4. Antimicrobial Resistance Genes
3.5. Intrinsic Oxacillinase Genes and Novel OXA Protein Variants
3.6. MLST and International Clones
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Touchon, M.; Cury, J.; Yoon, E.J.; Krizova, L.; Cerqueira, G.C.; Murphy, C.; Feldgarden, M.; Wortman, J.; Clermont, D.; Lambert, T.; et al. The genomic diversification of the whole Acinetobacter genus: Origins, mechanisms, and consequences. Genome Biol. Evol. 2014, 6, 2866–2882. [Google Scholar] [CrossRef] [PubMed]
- Pulami, D.; Schauss, T.; Eisenberg, T.; Blom, J.; Schwengers, O.; Bender, J.K.; Wilharm, G.; Kämpfer, P.; Glaeser, S.P. Acinetobacter stercoris sp. nov. isolated from output source of a mesophilic German biogas plant with anaerobic operating conditions. Antonie Van Leeuwenhoek 2021, 114, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Pulami, D.; Schauss, T.; Eisenberg, T.; Wilharm, G.; Blom, J.; Goesmann, A.; Kampfer, P.; Glaeser, S.P. Acinetobacter baumannii in manure and anaerobic digestates of German biogas plants. FEMS Microbiol. Ecol. 2020, 96, fiaa176. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Fung, C.P.; Wang, F.D.; Chen, C.P.; Chen, T.L.; Cho, W.L. Outbreak of imipenem-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex harboring different carbapenemase gene-associated genetic structures in an intensive care unit. J. Microbiol. Immunol. Infect. 2012, 45, 43–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection—An emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Nemec, A.; Radolfova-Krizova, L.; Maixnerova, M.; Sedo, O. Acinetobacter colistiniresistens sp. nov. (formerly genomic species 13 sensu Bouvet and Jeanjean and genomic species 14 sensu Tjernberg and Ursing), isolated from human infections and characterized by intrinsic resistance to polymyxins. Int. J. Syst. Evol. Microbiol. 2017, 67, 2134–2141. [Google Scholar] [CrossRef]
- Carr, E.L.; Kämpfer, P.; Patel, B.K.C.; Gurtler, V.; Seviour, R.J. Seven novel species of Acinetobacter isolated from activated sludge. Int. J. Syst. Evol. Microbiol. 2003, 53, 953–963. [Google Scholar] [CrossRef]
- Anandham, R.; Weon, H.Y.; Kim, S.J.; Kim, Y.S.; Kim, B.Y.; Kwon, S.W. Acinetobacter brisouii sp. nov., isolated from a wetland in Korea. J. Microbiol. 2010, 48, 36–39. [Google Scholar] [CrossRef]
- Kim, D.; Baik, K.S.; Kim, M.S.; Park, S.C.; Kim, S.S.; Rhee, M.S.; Kwak, Y.S.; Seong, C.N. Acinetobacter soli sp. nov., isolated from forest soil. J. Microbiol. 2008, 46, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Vaneechoutte, M.; Nemec, A.; Musilek, M.; van der Reijden, T.J.; van den Barselaar, M.; Tjernberg, I.; Calame, W.; Fani, R.; De Baere, T.; Dijkshoorn, L. Description of Acinetobacter venetianus ex Di Cello et al. 1997 sp. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Anand, S.; Jindal, S.; Rajagopal, R.; Lal, R. Acinetobacter indicus sp. nov., isolated from a hexachlorocyclohexane dump site. Int. J. Syst. Evol. Microbiol. 2012, 62, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Moreira, I.; Novo, A.; Hantsis-Zacharov, E.; Lopes, A.R.; Gomila, M.; Nunes, O.C.; Manaia, C.M.; Halpern, M. Acinetobacter rudis sp. nov., isolated from raw milk and raw wastewater. Int. J. Syst. Evol. Microbiol. 2011, 61, 2837–2843. [Google Scholar] [CrossRef]
- Radolfova-Krizova, L.; Maixnerova, M.; Nemec, A. Acinetobacter pragensis sp. nov., found in soil and water ecosystems. Int. J. Syst. Evol. Microbiol. 2016, 66, 3897–3903. [Google Scholar] [CrossRef]
- Ewers, C.; Klotz, P.; Leidner, U.; Stamm, I.; Prenger-Berninghoff, E.; Göttig, S.; Semmler, T.; Scheufen, S. OXA-23 and ISAba1-OXA-66 class D β-lactamases in Acinetobacter baumannii isolates from companion animals. Int. J. Antimicrob. Agents 2017, 49, 37–44. [Google Scholar] [CrossRef]
- Vale, A.P.; Leggett, B.; Smyth, D.; Leonard, F. Challenges in the veterinary microbiology diagnostic laboratory: A novel Acinetobacter species as presumptive cause for feline unilateral conjunctivitis. Access Microbiol. 2020, 2, acmi000118. [Google Scholar] [CrossRef]
- Gentilini, F.; Turba, M.E.; Pasquali, F.; Mion, D.; Romagnoli, N.; Zambon, E.; Terni, D.; Peirano, G.; Pitout, J.D.D.; Parisi, A.; et al. Hospitalized pets as a source of carbapenem-resistance. Front. Microbiol. 2018, 9, 2872. [Google Scholar] [CrossRef]
- Schwarz, S.; Mensing, N.; Hörmann, F.; Schneider, M.; Baumgärtner, W. Polyarthritis caused by Acinetobacter kookii in a Rothschild’s giraffe calf (Giraffa camelopardalis rothschildi). J. Comp. Pathol. 2020, 178, 56–60. [Google Scholar] [CrossRef]
- Pereira, D.G.; Batista, E.; de Cristo, T.G.; Santiani, F.; Sfaciotte, R.A.P.; Ferraz, S.M.; de Moraes, A.N.; Casagrande, R.A. Aspiration bronchopneumonia by Acinetobacter baumannii in a wildlife European hare (Lepus europaeus) in Brazil. J. Zoo Wildl. Med. 2020, 51, 253–256. [Google Scholar] [CrossRef]
- Wilharm, G.; Skiebe, E.; Higgins, P.G.; Poppel, M.T.; Blaschke, U.; Leser, S.; Heider, C.; Heindorf, M.; Brauner, P.; Jackel, U.; et al. Relatedness of wildlife and livestock avian isolates of the nosocomial pathogen Acinetobacter baumannii to lineages spread in hospitals worldwide. Environ. Microbiol. 2017, 19, 4349–4364. [Google Scholar] [CrossRef] [PubMed]
- Klotz, P.; Higgins, P.G.; Schaubmar, A.R.; Failing, K.; Leidner, U.; Seifert, H.; Scheufen, S.; Semmler, T.; Ewers, C. Seasonal occurrence and carbapenem susceptibility of bovine Acinetobacter baumannii in Germany. Front. Microbiol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Wareth, G.; Neubauer, H.; Sprague, L.D. Acinetobacter baumannii—A neglected pathogen in veterinary and environmental health in Germany. Vet. Res. Commun. 2019, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Al Bayssari, C.; Dabboussi, F.; Hamze, M.; Rolain, J.M. Emergence of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii in livestock animals in Lebanon. J. Antimicrob. Chemother. 2015, 70, 950–951. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Hamze, M.; Pailhories, H.; Eveillard, M.; Marsollier, L.; Joly-Guillou, M.L.; Dabboussi, F.; Kempf, M. Extrahuman epidemiology of Acinetobacter baumannii in Lebanon. Appl. Environ. Microbiol. 2015, 81, 2359–2367. [Google Scholar] [CrossRef]
- Linz, B.; Mukhtar, N.; Shabbir, M.Z.; Rivera, I.; Ivanov, Y.V.; Tahir, Z.; Yaqub, T.; Harvill, E.T. Virulent epidemic pneumonia in sheep caused by the human pathogen Acinetobacter baumannii. Front. Microbiol. 2018, 9, 2616. [Google Scholar] [CrossRef]
- Maboni, G.; Seguel, M.; Lorton, A.; Sanchez, S. Antimicrobial resistance patterns of Acinetobacter spp. of animal origin reveal high rate of multidrug resistance. Vet. Microbiol. 2020, 245, 108702. [Google Scholar] [CrossRef]
- Klotz, P.; Jacobmeyer, L.; Leidner, U.; Stamm, I.; Semmler, T.; Ewers, C. Acinetobacter pittii from companion animals co-harboring blaOXA-58, the tet(39) region, and other resistance genes on a single plasmid. Antimicrob. Agents Chemother. 2018, 62, e01993-17. [Google Scholar] [CrossRef]
- Nocera, F.P.; Addante, L.; Capozzi, L.; Bianco, A.; Fiorito, F.; De Martino, L.; Parisi, A. Detection of a novel clone of Acinetobacter baumannii isolated from a dog with otitis externa. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101471. [Google Scholar] [CrossRef]
- Santaniello, A.; Sansone, M.; Fioretti, A.; Menna, L.F. Systematic Review and Meta-Analysis of the Occurrence of ESKAPE Bacteria group in dogs, and the related zoonotic risk in animal-assisted therapy, and in animal-assisted activity in the health context. Int. J. Environ. Res. Public Health 2020, 17, 3278. [Google Scholar] [CrossRef]
- Klotz, P.; Gottig, S.; Leidner, U.; Semmler, T.; Scheufen, S.; Ewers, C. Carbapenem-resistance and pathogenicity of bovine Acinetobacter indicus-like isolates. PLoS ONE 2017, 12, e0171986. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, C.Y.; Wu, X.T.; Fang, L.X.; He, Q.; He, B.; Long, T.F.; Liao, X.P.; Chen, L.; Liu, Y.H.; et al. Spread of tet(X5) and tet(X6) genes in multidrug-resistant Acinetobacter baumannii strains of animal origin. Vet. Microbiol. 2021, 253, 108954. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Dawoud, T.M.; Mubarak, A.S.; Al-Sarar, D.; Alsubki, R.A.; Alhaji, J.H.; Hamada, M.; Abalkhail, A.; et al. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Feng, J.; Liu, Y.; Yang, B.; Li, R.; Bai, L.; He, T.; Wang, X.; Yang, Z. Characterization of three porcine Acinetobacter towneri strains co-harboring tet(X3) and blaOXA-58. Front. Cell. Infect. Microbiol. 2020, 10, 586507. [Google Scholar] [CrossRef] [PubMed]
- Candan, O.; Candan, E.D. Bacterial diversity of the green turtle (Chelonia mydas) nest environment. Sci. Total Environ. 2020, 720, 137717. [Google Scholar] [CrossRef]
- Soslau, G.; Russell, J.A.; Spotila, J.R.; Mathew, A.J.; Bagsiyao, P. Acinetobacter sp. HM746599 isolated from leatherback turtle blood. FEMS Microbiol. Lett. 2011, 322, 166–171. [Google Scholar] [CrossRef][Green Version]
- Morrison, B.J.; Rubin, J.E. Detection of multidrug-resistant Gram-negative bacteria from imported reptile and amphibian meats. J. Appl. Microbiol. 2020, 129, 1053–1061. [Google Scholar] [CrossRef]
- Brockmann, M.; Aupperle-Lellbach, H.; Muller, E.; Heusinger, A.; Pees, M.; Marschang, R.E. Aerobic bacteria from skin lesions in reptiles and their antimicrobial susceptibility. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2020, 48, 78–88. [Google Scholar] [CrossRef]
- Tang, P.K.; Divers, S.J.; Sanchez, S. Antimicrobial susceptibility patterns for aerobic bacteria isolated from reptilian samples submitted to a veterinary diagnostic laboratory: 129 cases (2005–2016). J. Am. Vet. Med. Assoc. 2020, 257, 305–312. [Google Scholar] [CrossRef]
- Eibach, D.; Nagel, M.; Lorenzen, S.; Hogan, B.; Belmar Campos, C.; Aepfelbacher, M.; Sarpong, N.; May, J. Extended-spectrum β-lactamase-producing Enterobacteriaceae among geckos (Hemidactylus brookii) in a Ghanaian hospital. Clin. Microbiol. Infect. 2019, 25, 1048–1050. [Google Scholar] [CrossRef]
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Ludwig, M.L.; Rothe, M.; Semmler, T.; Ewers, C. Imported reptiles as a risk factor for the global distribution of Escherichia coli harbouring the colistin resistance gene mcr-1. Int. J. Antimicrob. Agents 2017, 49, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Fraport. The 2020 Fiscal Year at a Glance. Fraport Annual Report 2020. Available online: https://www.annualreports.com/HostedData/AnnualReports/PDF/OTC_FPRUY_2020.pdf (accessed on 2 February 2022).
- CITES Glossary. Available online: https://cites.org/eng/resources/terms/glossary.php#r (accessed on 2 March 2022).
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Goker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Goker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucl. Acids Res. 2021, 50, D801–D807. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef]
- Lagesen, K.; Hallin, P.; Rodland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucl. Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinf. 2009, 10, 421. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Goker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinf. 2013, 14, 60. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Farris, J.S. Estimating phylogenetic trees from distance matrices. Am. Nat. 1972, 106, 951. [Google Scholar] [CrossRef]
- Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A phylogenetic tree viewer with extended phyloXML support for functional genomics data visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Rossello-Mora, R.; Oliver Glockner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- ResFinder 4.2, CGE (Center for Genomic Epidemiology) Server. Available online: https://cge.cbs.dtu.dk/services/ResFinder/ (accessed on 22 March 2022).
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucl. Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef]
- Turton, J.F.; Gabriel, S.N.; Valderrey, C.; Kaufmann, M.E.; Pitt, T.L. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 2007, 13, 807–815. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucl. Acids Res. 2021, 49, D644–D650. [Google Scholar] [CrossRef]
- Zander, E.; Nemec, A.; Seifert, H.; Higgins, P.G. Association between beta-lactamase-encoding blaOXA-51 variants and DiversiLab rep-PCR-based typing of Acinetobacter baumannii isolates. J. Clin. Microbiol. 2012, 50, 1900–1904. [Google Scholar] [CrossRef][Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucl. Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Klotz, P.; Jacobmeyer, L.; Stamm, I.; Leidner, U.; Pfeifer, Y.; Semmler, T.; Prenger-Berninghoff, E.; Ewers, C. Carbapenem-resistant Acinetobacter baumannii ST294 harbouring the OXA-72 carbapenemase from a captive grey parrot. J. Antimicrob. Chemother. 2018, 73, 1098–1100. [Google Scholar] [CrossRef]
- Principe, L.; Piazza, A.; Giani, T.; Bracco, S.; Caltagirone, M.S.; Arena, F.; Nucleo, E.; Tammaro, F.; Rossolini, G.M.; Pagani, L.; et al. Epidemic diffusion of OXA-23-producing Acinetobacter baumannii isolates in Italy: Results of the first cross-sectional countrywide survey. J. Clin. Microbiol. 2014, 52, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Interplay between colistin resistance, virulence and fitness in Acinetobacter baumannii. Antibiotics 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Di Lallo, G.; D′Andrea, M.M.; Sennati, S.; Thaller, M.C.; Migliore, L.; Gentile, G. Evidence of another anthropic impact on Iguana delicatissima from the Lesser Antilles: The presence of antibiotic resistant enterobacteria. Antibiotics 2021, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Anonym. Annual Report 2019 of the Hessian State Laboratory (LHL). Available online: https://lhl.hessen.de/presse/jahresberichte-des-lhl-2016-2020 (accessed on 22 March 2022).
- Sahl, J.W.; Del Franco, M.; Pournaras, S.; Colman, R.E.; Karah, N.; Dijkshoorn, L.; Zarrilli, R. Phylogenetic and genomic diversity in isolates from the globally distributed Acinetobacter baumannii ST25 lineage. Sci. Rep. 2015, 5, 15188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Hagen, R.M.; Kreikemeyer, B.; Warnke, P.; Podbielski, A.; Frickmann, H.; Loderstadt, U. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates from Northern Africa and the Middle East. Antibiotics 2021, 10, 291. [Google Scholar] [CrossRef]
- Lupo, A.; Chatre, P.; Ponsin, C.; Saras, E.; Boulouis, H.J.; Keck, N.; Haenni, M.; Madec, J.Y. Clonal spread of Acinetobacter baumannii sequence type 25 carrying blaOXA-23 in companion animals in France. Antimicrob. Agents Chemother. 2017, 61, e01881-16. [Google Scholar] [CrossRef]
- Jacobmeyer, L.; Stamm, I.; Semmler, T.; Ewers, C. First report of NDM-1 in an Acinetobacter baumannii strain from a pet animal in Europe. J. Glob. Antimicrob. Resist. 2021, 26, 128–129. [Google Scholar] [CrossRef]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in captive reptiles and their environment-can we tame the dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef]
- Nieto-Claudin, A.; Deem, S.L.; Rodriguez, C.; Cano, S.; Moity, N.; Cabrera, F.; Esperon, F. Antimicrobial resistance in Galapagos tortoises as an indicator of the growing human footprint. Environ. Pollut. 2021, 284, 117453. [Google Scholar] [CrossRef]
- Pace, A.; Dipineto, L.; Fioretti, A.; Hochscheid, S. Loggerhead sea turtles as sentinels in the western Mediterranean: Antibiotic resistance and environment-related modifications of Gram-negative bacteria. Mar. Pollut Bull. 2019, 149, 110575. [Google Scholar] [CrossRef]
- De Carvalho, M.P.N.; Moura, Q.; Fernandes, M.R.; Sellera, F.P.; Pagotto, A.H.; Stuginski, D.R.; Castro, R.A.; Sant’Anna, S.S.; Grego, K.F.; Lincopan, N. Genomic features of a multidrug-resistant Enterobacter cloacae ST279 producing CTX-M-15 and AAC(6′)-Ib-cr isolated from fatal infectious stomatitis in a crossed pit viper (Bothrops alternatus). J. Glob. Antimicrob. Resist. 2018, 15, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Cirilli, M.; Marinaro, M.; Bosak, S.; Diakoudi, G.; Ciccarelli, S.; Paci, S.; Buonavoglia, D.; Corrente, M. Detection of multi-drug resistance and AmpC β-lactamase/extended-spectrum β-lactamase genes in bacterial isolates of loggerhead sea turtles (Caretta caretta) from the Mediterranean Sea. Mar. Pollut Bull. 2021, 164, 112015. [Google Scholar] [CrossRef] [PubMed]
- Deems, A.; Du Prey, M.; Dowd, S.E.; McLaughlin, R.W. Characterization of the biodiesel degrading Acinetobacter oleivorans strain PT8 isolated from the fecal material of a painted turtle (Chrysemys picta). Curr. Microbiol. 2021, 78, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Barksdale, S.M.; Hrifko, E.J.; van Hoek, M.L. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev. Comp. Immunol. 2017, 70, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hitt, S.J.; Bishop, B.M.; van Hoek, M.L. Komodo-dragon cathelicidin-inspired peptides are antibacterial against carbapenem-resistant Klebsiella pneumoniae. J. Med. Microbiol. 2020, 69, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Nunes, E.; Frihling, B.; Barros, E.; de Oliveira, C.; Verbisck, N.; Flores, T.; de Freitas Júnior, A.; Franco, O.; de Macedo, M.; Migliolo, L.; et al. Antibiofilm activity of acidic phospholipase isoform isolated from Bothrops erythromelas snake venom. Toxins 2020, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Santana, F.L.; Arenas, I.; Haney, E.F.; Estrada, K.; Hancock, R.E.W.; Corzo, G. Identification of a crocodylian β-defensin variant from Alligator mississippiensis with antimicrobial and antibiofilm activity. Peptides 2021, 141, 170549. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Akhavan, M.M.; Fallah, F.; Karimi, A. A recombinant snake cathelicidin derivative peptide: Antibiofilm properties and expression in Escherichia coli. Biomolecules 2018, 8, 118. [Google Scholar] [CrossRef]
- Zhao, F.; Lan, X.Q.; Du, Y.; Chen, P.Y.; Zhao, J.; Lee, W.H.; Zhang, Y. King cobra peptide OH-CATH30 as a potential candidate drug through clinic drug-resistant isolates. Zool. Res. 2018, 39, 87–96. [Google Scholar] [CrossRef]
- Winnie, F.Y.M.; Siddiqui, R.; Sagathevan, K.; Khan, N.A. Identification of antibacterial molecule(s) from animals living in polluted environments. Curr. Pharm. Biotechnol. 2020, 21, 425–437. [Google Scholar] [CrossRef]

| Strain ID | Species * | STPast ** | OXA Type ** | Sample | Animal **** | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shipment | Batch | Type *** | Species | Country | Origin | ||||
| IHIT27741 | Ar | 2046 | 813 | 17 | 21 | S | Jackson’s chameleon | USA | CB |
| IHIT27742 | Ar | 2053 | 23 | 46 | 69 | F | Horsfield’s tortoise | UZB | FB |
| IHIT27743 | Ar | 2073 | 809 | 73 | 125 | F | Fat-tail gecko | USA | CB |
| IHIT27744 | Ar | 2085 | 23 | 85 | 151 | F | Horsfield’s tortoise | UKR | FB |
| IHIT33475 | Aind | 2061 | neg. | 60 | 100 | F | Leopard tortoise | TZA | FB |
| IHIT35877 | Ab | 727 | 65 | 2 | 5 | S | Sand monitor | USA | CB |
| IHIT35878 | Ab | 1211 | 65 | 18 | 23 | S | Green basilisk | USA | FB |
| IHIT35879 | Ab | 866 | 385 | 21 | 27 | F | Common leopard gecko | CAN | CB |
| IHIT35880 | Ab | 1290 | 342 | 23 | 30 | F | Eastern collared lizard | USA | WC |
| IHIT35881 | Ab | 294 | 343 | 27 | 40 | F | Central bearded dragon | USA | CB |
| IHIT35882 | Ab | 25 | 64 | 33 | 50 | F | Ball python | CAN | CB |
| IHIT35884 | Aseif | 1291 | neg. | 37 | 54 | S | Rough green snake | USA | WC |
| IHIT35885 | Ab | 311 | 799 | 38 | 55 | F | Armored pricklenape | VNM | WC |
| IHIT35886 | Ab | 1111 | 91 | 39 | 62 | F | Chinese water dragon | VNM | WC |
| IHIT35887 | Ab | 25 | 64 | 42 | 64 | F | Rainbow boa | USA | CB |
| IHIT35888 | Ab | 1292 | 132 | 44 | 67 | F | Madagascar day gecko | UKR | CB |
| IHIT35889 | Ab | 1293 | 104 | 50 | 76 | F | Indigo snake | USA | pr. CB |
| IHIT35890 | Ab | 1294 | 383 | 50 | 77 | F | Fat-tail gecko | USA | CB |
| IHIT35891 | Ab | 294 | 343 | 51 | 80 | S | Rough green snake | USA | WC |
| IHIT35892 | Ab | 1295 | 69 | 52 | 81 | F | Common green iguana | SLV | FB |
| IHIT35893 | Ab | 1212 | 69 | 58 | 95 | F | Saw-scaled curly-tail | USA | WC/FB |
| IHIT35894 | Ab | 1296 | # | 70 | 118 | F | Boa constrictor | USA | CB |
| IHIT35895 | Ab | 46 | 104 | 72 | 122 | F | Common green iguana | SLV | FB |
| IHIT35896 | Ab | 1297 | 91 | 75 | 131 | F | Green spiny lizard | USA | pr. CB |
| IHIT35897 | Ab | 1298 | 800 | 75 | 135 | S | Savannah monitor | USA | CB |
| IHIT35898 | Ab | 1299 | 106 | 82 | 145 | F | Sand monitor | CAN | CB |
| IHIT35899 | Ab | 1300 | 314 | 83 | 146 | F | Common leopard gecko | CAN | CB |
| IHIT35900 | Ab | 1301 | 749 | 84 | 148 | F | Common leopard gecko | USA | CB |
| IHIT39733 | Ab | 866 | 385 | 21 | 28 | F | Crested gecko | CAN | CB |
| IHIT44648 | Ap | 2038 | 978 | 1 | 1 | F | Green pricklenape | VNM | WC |
| IHIT44649 | Alac-like | 2047 | 979 | 18 | 25 | F | Green spiny lizard | USA | pr. CB |
| IHIT44650 | Alac | 2048 | 980 | 27 | 40 | F | Central bearded dragon | USA | CB |
| IHIT44651 | Atown | 2049 | neg. | 35 | 52 | F | Red-footed tortoise | COL | WC/FB |
| IHIT44652 | Ager | 2050 | 981 | 39 | 60 | F | Eastern garden lizard | VNM | WC |
| IHIT44653 | Anoso | 1269 | neg. | 39 | 60 | F | Eastern garden lizard | VNM | WC |
| IHIT44654 | Ap | 2039 | 506 | 44 | 66 | F | Central bearded dragon | UKR | CB |
| IHIT44655 | Aber | 2051 | 301 | 44 | 66 | F | Central bearded dragon | UKR | CB |
| IHIT44656 | Aviv | 2052 | 982 | 44 | 66 | F | Central bearded dragon | UKR | CB |
| IHIT44657 | Aber | 2054 | 983 | 48 | 72 | F | Yellow mud turtle | USA | FB |
| IHIT44658 | Acalc | 2055 | 329 | 50 | 77 | F | Fat-tail gecko | USA | CB |
| IHIT44659 | Acour | 2056 | 984 | 51 | 80 | S | Rough green snake | USA | WC |
| IHIT44660 | Ap-like | 2057 | 985 | 51 | 80 | S | Rough green snake | USA | WC |
| IHIT44661 | Ap-like | 2040 | 986 | 52 | 81 | F | Common green iguana | SLV | FB |
| IHIT44662 | Aolei | 2058 | 987 | 53 | 82 | F | Red-footed tortoise | BRA | WC/FB |
| IHIT44663 | Ap | 2041 | 988 | 58 | 93 | F | Hispaniolan masked curly-tail | USA | WC/FB |
| IHIT44664 | Alac-like | 2059 | 989 | 58 | 95 | F | Saw-scaled curly-tail | USA | WC/FB |
| IHIT44665 | Atan | 2060 | neg. | 58 | 97 | F | Striped mud turtle | USA | FB |
| IHIT44666 | Acalc | 2062 | 990 | 64 | 110 | F | East African black mud turtle | MOZ | WC |
| IHIT44667 | Aschin | 2063 | 991 | 65 | 111 | F | Leopard tortoise | ECU | FB |
| IHIT44668 | Agem | 2064 | 992 | 66 | 113 | F | Yellow-headed gecko | NIC | WC |
| IHIT44669 | Aviv | 2065 | 993 | 66 | 113 | F | Yellow-headed gecko | NIC | WC |
| IHIT44670 | Avar | 2066 | neg. | 67 | 114 | F | Horsfield’s tortoise | UZB | FB |
| IHIT44671 | Aviv | 2067 | 994 | 68 | 115 | F | Jackson’s chameleon | UGA | CB |
| IHIT44672 | Acour | 2068 | 995 | 69 | 116 | F | Cuvier’s Madagascar swift | MDG | WC |
| IHIT44673 | Acour | 2069 | 996 | 69 | 116 | F | Cuvier’s Madagascar swift | MDG | WC |
| IHIT44674 | Aviv | 2070 | 997 | 69 | 117 | F | Southeastern girdled lizard | MDG | WC |
| IHIT44675 | Acour | 2071 | 998 | 71 | 119 | F | Rough green snake | USA | WC |
| IHIT44676 | Ager-like | 2072 | 999 | 73 | 124 | F | Red corn snake | USA | CB |
| IHIT44677 | Ap | 2042 | 1000 | 73 | 125 | F | Fat-tail gecko | USA | CB |
| IHIT44678 | Aber | 2074 | 257 | 73 | 126 | F | New Caledonia giant gecko | USA | CB |
| IHIT44679 | Ap | 2043 | 502 | 73 | 126 | F | New Caledonia giant gecko | USA | CB |
| IHIT44680 | Ap | 2044 | 502 | 74 | 128 | F | Leopard tortoise | ZAF | FB |
| IHIT44681 | Aber | 2075 | 1001 | 74 | 128 | F | Leopard tortoise | ZAF | FB |
| IHIT44682 | Aber | 2075 | 1001 | 74 | 128 | F | Leopard tortoise | ZAF | FB |
| IHIT44683 | Ap | 2045 | 1002 | 74 | 128 | F | Leopard tortoise | ZAF | FB |
| IHIT44684 | Aolei | 2076 | 1003 | 75 | 129 | F | Cuban giant anole | USA | WC/FB |
| IHIT44685 | Aseif | 2077 | neg. | 75 | 130 | F | Argentine black and white tegu | USA | CB |
| IHIT44686 | Atan-like | n.d. | neg | 75 | 130 | F | Argentine black and white tegu | USA | CB |
| IHIT44687 | Anoso | 2078 | neg | 75 | 130 | F | Argentine black and white tegu | USA | CB |
| IHIT44688 | Acour | 2079 | 1004 | 75 | 131 | F | Green spiny lizard | USA | pr. CB |
| IHIT44689 | Ap | 220 | 820 | 76 | 136 | F | Shingleback lizard | JPN | CB |
| IHIT44690 | Aber | 2080 | 1052 | 78 | 138 | F | Chinese water dragon | VNM | WC |
| IHIT44691 | Ap | 220 | 820 | 79 | 142 | F | Painted wood turtle | NIC | WC |
| IHIT44692 | Aschin | 2081 | 1005 | 80 | 143 | F | Painted wood turtle | USA | pr. CB |
| IHIT44693 | Aj-like | 2082 | 1006 | 82 | 145 | F | Sand monitor | CAN | CB |
| IHIT44694 | Aolei | 2083 | 1007 | 82 | 145 | F | Sand monitor | CAN | CB |
| IHIT44695 | Alac-like | 2084 | 1008 | 84 | 148 | F | Common leopard gecko | USA | CB |
| IHIT44696 | Asol | 2086 | neg | 87 | 154 | F | Horsfield’s tortoise | UKR | FB |
| IHIT44697 | Acalc | 2087 | 1009 | 87 | 154 | F | Horsfield’s tortoise | UKR | FB |
| IHIT44698 | Alac | 2088 | 1010 | 91 | 159 | F | Central bearded dragon | UKR | CB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Semmler, T.; Ewers, C. Imported Pet Reptiles and Their “Blind Passengers”—In-Depth Characterization of 80 Acinetobacter Species Isolates. Microorganisms 2022, 10, 893. https://doi.org/10.3390/microorganisms10050893
Unger F, Eisenberg T, Prenger-Berninghoff E, Leidner U, Semmler T, Ewers C. Imported Pet Reptiles and Their “Blind Passengers”—In-Depth Characterization of 80 Acinetobacter Species Isolates. Microorganisms. 2022; 10(5):893. https://doi.org/10.3390/microorganisms10050893
Chicago/Turabian StyleUnger, Franziska, Tobias Eisenberg, Ellen Prenger-Berninghoff, Ursula Leidner, Torsten Semmler, and Christa Ewers. 2022. "Imported Pet Reptiles and Their “Blind Passengers”—In-Depth Characterization of 80 Acinetobacter Species Isolates" Microorganisms 10, no. 5: 893. https://doi.org/10.3390/microorganisms10050893
APA StyleUnger, F., Eisenberg, T., Prenger-Berninghoff, E., Leidner, U., Semmler, T., & Ewers, C. (2022). Imported Pet Reptiles and Their “Blind Passengers”—In-Depth Characterization of 80 Acinetobacter Species Isolates. Microorganisms, 10(5), 893. https://doi.org/10.3390/microorganisms10050893






