Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella
Abstract
1. Introduction
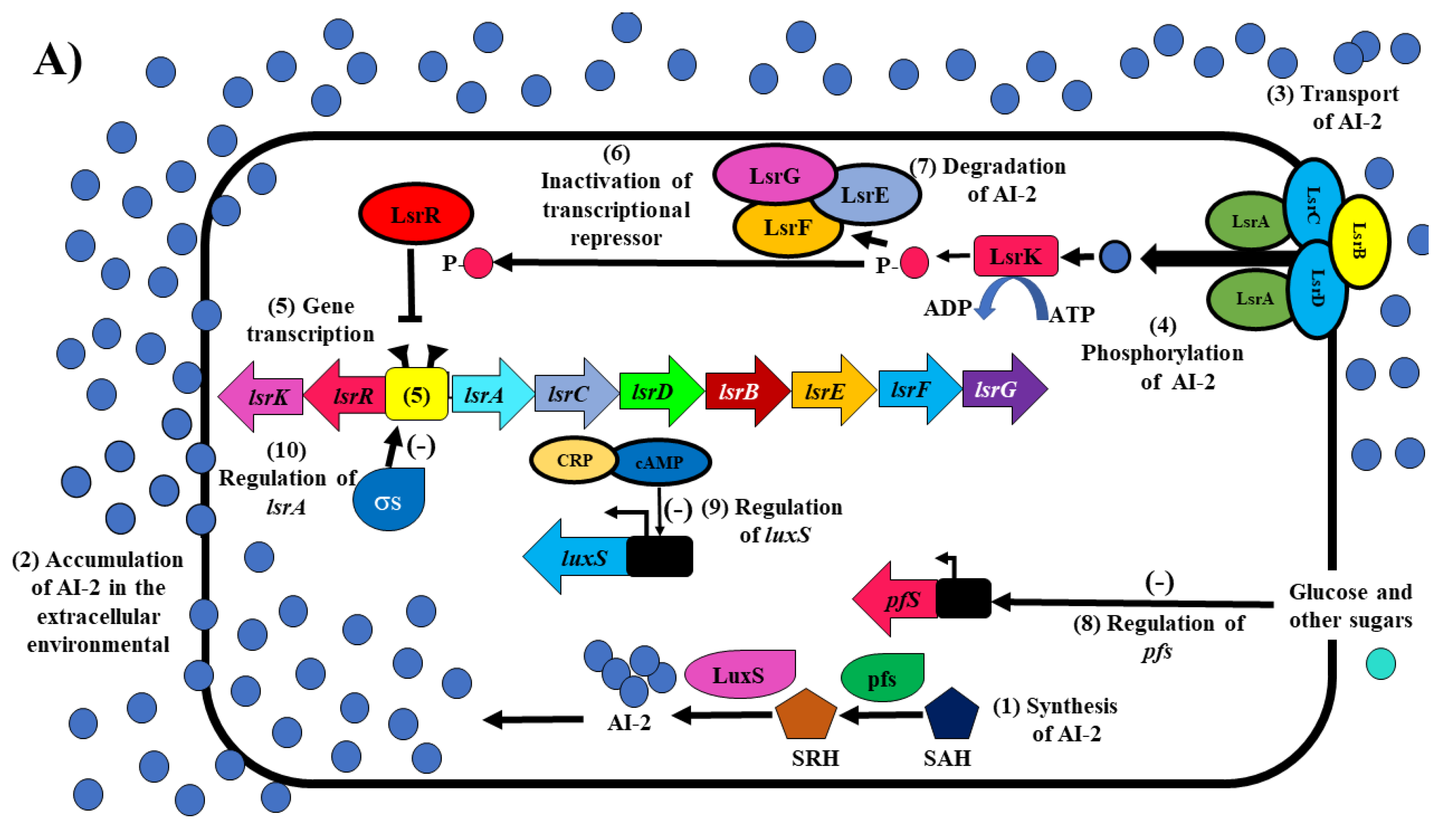
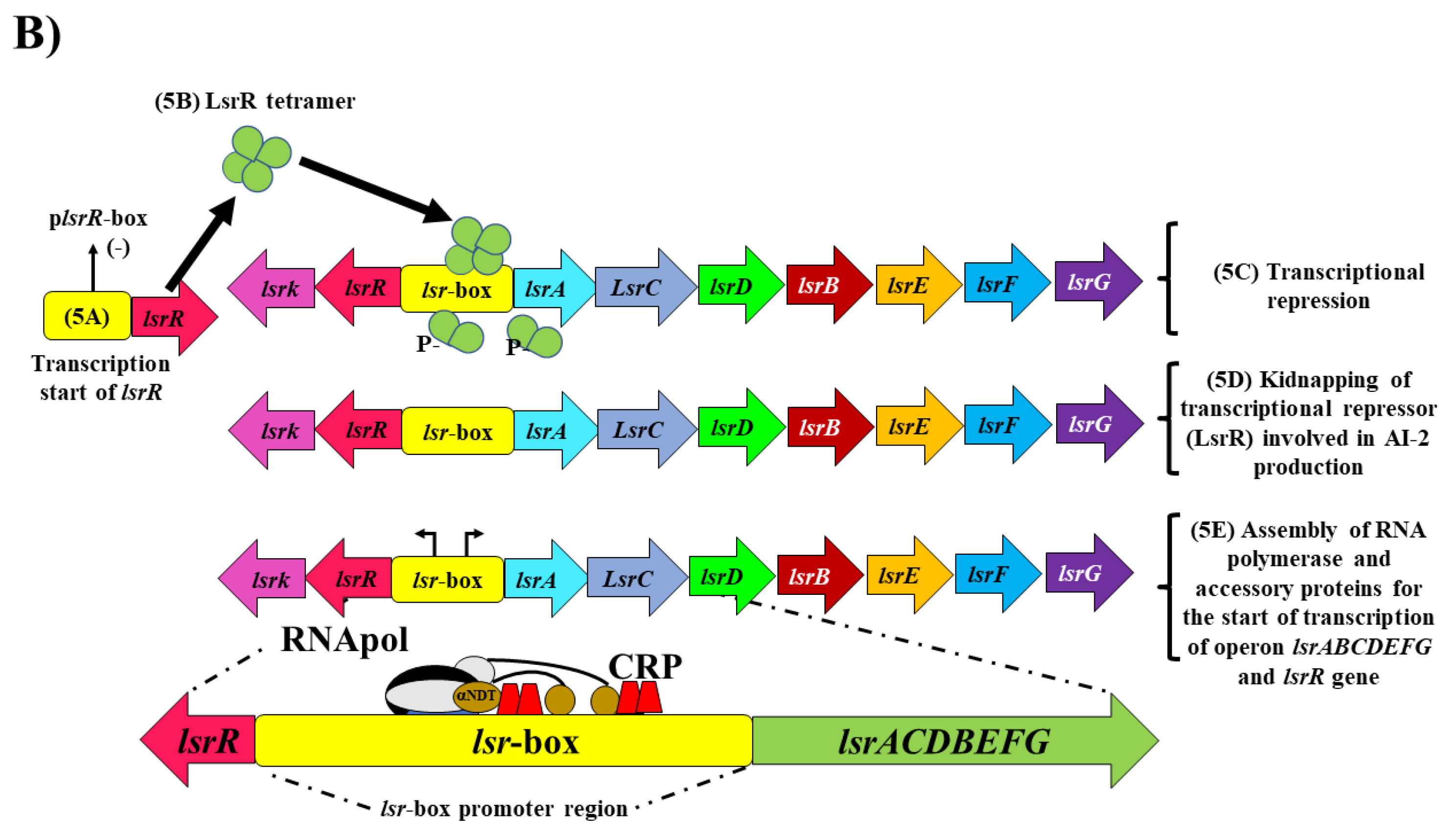
2. The QS System Based on Autoinducer-2 (AI-2) in E. coli and Salmonella spp.
3. Inhibition of Quorum Sensing
4. Types of Inhibitors of QS
4.1. Natural Inhibitors
4.2. Synthetic Inhibitors
5. Mechanisms of QS Inhibition
5.1. Inhibition of AI-2 Synthesis
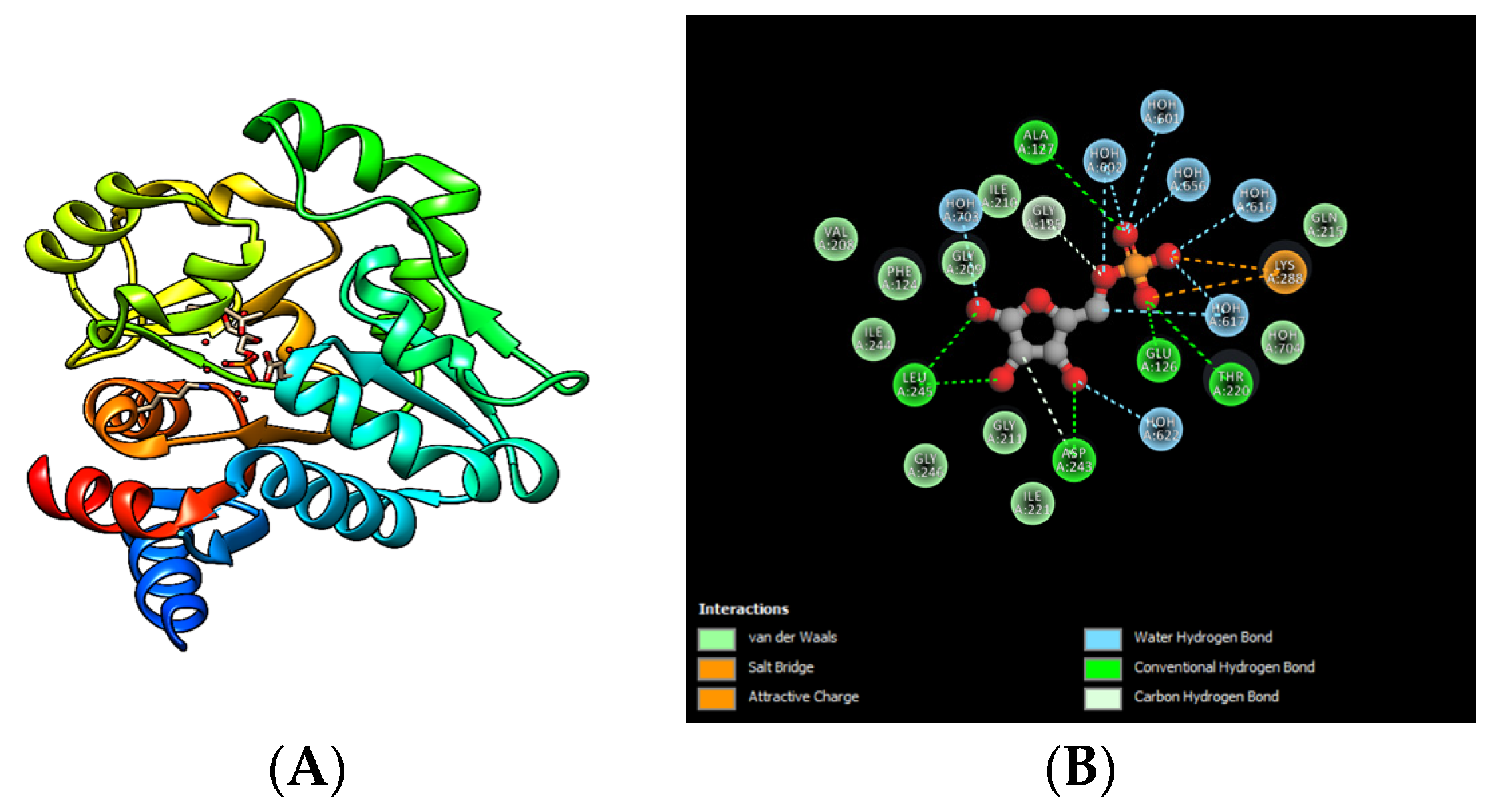
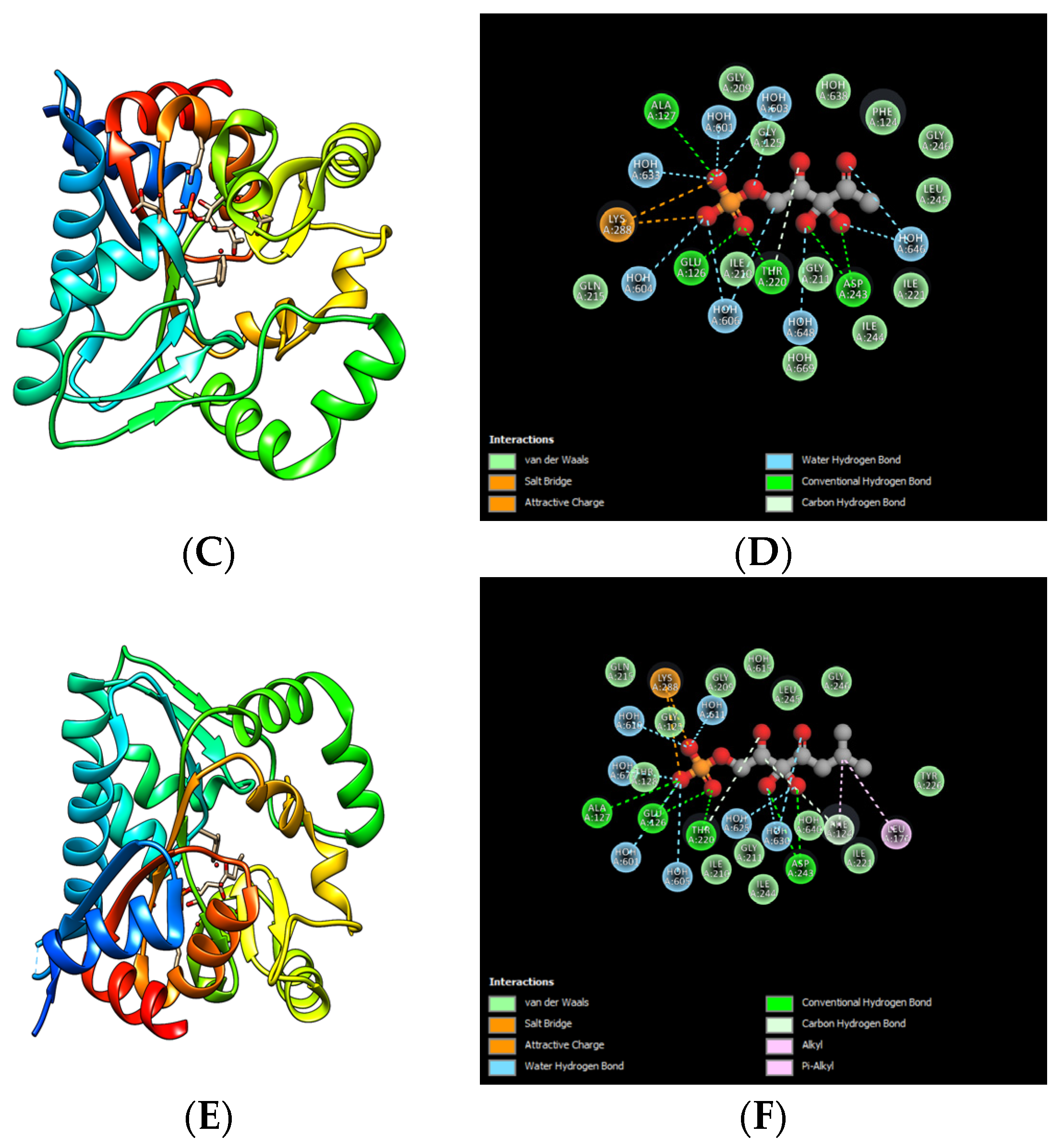
5.2. Inhibitors of the Incomplete QS System
6. Strategies Used for the Study of QSIs
7. Studies of QSI Uses
8. Resistance to QSIs
9. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Schuster, M.; Rumbaugh, K.P. Working together for the common good: Cell-cell communication in bacteria. J. Bacteriol. 2012, 194, 2131–2141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibbs, K.A.; Federle, M.J. A social medium: ASM’s 5th cell-cell communication in bacteria meeting in review. J. Bacteriol. 2015, 197, 2084–2091. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajput, A.; Kaur, K.; Kumar, M. SigMol: Repertoire of quorum sensing signaling molecules in prokaryotes. Nucleic Acids Res. 2016, 44, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Luna-Guevara, J.J.; Arenas-Hernandez, M.M.; Martínez de la Peña, C.; Silva, J.L.; Luna-Guevara, M.L. The role of pathogenic E. coli in fresh vegetables: Behavior, contamination factors, and preventive measures. Int. J. Microbiol. 2019, 2019, 2894328. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernandez, M.M.; Enciso-Martínez, Y.; Martínez-de la Peña, C.F.; del C Rocha-Gracia, R.; Lozano-Zaraín, P.; Navarro-Ocaña, P.; Martínez-Laguna, Y.; de la Rosa-López, R. Virulence and resistance determinants of uropathogenic Escherichia coli strains isolated from pregnant and non-pregnant women from two states in Mexico. Infect. Drug. Resist. 2020, 13, 295–310. [Google Scholar] [CrossRef]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial quorum sensing during infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Sheng, L.; Olsen, S.A.; Hu, J.; Yue, W.; Means, W.J.; Zhu, M.J. Inhibitory effects of grape seed extract on growth, quorum sensing, and virulence factors of CDC “top-six” non-O157 Shiga toxin producing E. coli. Int. J. Food Microbiol. 2016, 229, 24–32. [Google Scholar] [CrossRef]
- Zohar, B.A.; Kolodkin-Gal, I. Quorum sensing in Escherichia coli: Interkingdom, inter-and intraspecies dialogues, and a suicide-inducing peptide. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Springer: New Delhi, India, 2015; pp. 85–99. [Google Scholar]
- Witsø, I.L.; Rukke, H.V.; Benneche, T.; Scheie, A.A. Thiophenone attenuates enteropathogenic Escherichia coli O103:H2 virulence by interfering with AI-2 signaling. PLoS ONE 2016, 11, e0157334. [Google Scholar] [CrossRef]
- Choi, J.; Shin, D.; Kim, M.; Park, J.; Lim, S.; Ryu, S. LsrR-mediated quorum sensing controls invasiveness of Salmonella typhimurium by regulating SPI-1 and flagella genes. PLoS ONE 2012, 7, e37059. [Google Scholar] [CrossRef]
- Abed, N.; Grépinet, O.; Canepa, S.; Hurtado-Escobar, G.A.; Guichard, N.; Wiedemann, A.; Velge, P.; Virlogeux-Payant, I. Direct regulation of the pefI-srgC operon encoding the Rck invasin by the quorum-sensing regulator SdiA in Salmonella Typhimurium. Mol. Microbiol. 2014, 94, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Habyarimana, F.; Sabag-Daigle, A.; Ahmer, B.M. The SdiA-regulated gene srgE encodes a type III secreted effector. J. Bacteriol. 2014, 96, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Miyamoto, C.M.; Wood, T.K.; Meighen, E.A.; Sorgeloos, P.; Verstraete, W.; Bossier, P. The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2 (5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein LuxR. Environ. Microbiol. 2007, 9, 2486–2495. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.; Park, H.Y.; Park, H.J.; Lee, J.H.; Kim, C.K.; Yoon, J. Furanone derivatives as quorum-sensing antagonists of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 80, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kalia, D. Synthetic Quorum sensing inhibitors: Signal analogues. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Springer: New Delhi, India, 2015; pp. 285–302. [Google Scholar]
- Proctor, C.R.; McCarron, P.A.; Ternan, N.G. Furanone quorum-sensing inhibitors with potential as novel therapeutics against Pseudomonas aeruginosa. Int. J. Med. Microbiol. 2020, 69, 195–206. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella Typhimurium. LWT. Food Sci. Technol. 2016, 66, 560–564. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of organic acids on biofilm formation and quorum signaling of pathogens from fresh fruits and vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Wang, R.; Vega, P.; Xu, Y.; Chen, C.Y.; Irudayaraj, J. Exploring the anti-quorum sensing activity of ad-limonene nanoemulsion for Escherichia coli O157: H7. J. Biomed. Mate. Res. 2018, 106, 1979–1986. [Google Scholar] [CrossRef]
- Rasko, D.A.; Moreira, C.G.; Reading, N.C.; Ritchie, J.M.; Waldor, M.K.; Williams, N.; Huntley, J.F. Targeting QseC signaling and virulence for antibiotic development. Science 2008, 321, 1078–1080. [Google Scholar] [CrossRef]
- Brackman, G.; Nelis, H.J.; Coenye, T. Inhibition of quorum sensing as a novel antimicrobial strategy. In Antimicrobial Drug Discovery: Emerging Strategies; CAB eBooks: Wallingford, UK, 2012; pp. 115–134. [Google Scholar] [CrossRef]
- Hirakawa, H.; Tomita, H. Interference of bacterial cell-to-cell communication: A new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front. Microbiol. 2013, 4, 114. [Google Scholar] [CrossRef]
- García-Contreras, R.; Martínez-Vázquez, M.; Velázquez Guadarrama, N.; Villegas Pañeda, A.G.; Hashimoto, T.; Maeda, T.; Wood, T.K. Resistance to the quorum-quenching compounds brominated furanone C-30 and 5-fluorouracil in Pseudomonas aeruginosa clinical isolates. Pathog. Dis. 2013, 68, 8–11. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, X.L. An alternative strategy as quorum-sensing inhibitor: Pheromone-guided antimicrobial peptides. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Kalia, V., Ed.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Mellini, M.; Di Muzio, E.; D’Angelo, F.; Baldelli, V.; Ferrillo, S.; Visca, P.; Rampioni, G. In silico selection and experimental validation of FDA-approved drugs as anti-quorum sensing agents. Front. Microbiol. 2019, 10, 2355. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.D.; Van Gennip, M.; Jakobsen, T.H.; Alhede, M.; Hougen, H.P.; Høiby, N.; Givskov, M. Synergistic antibacterial efficacy of early combination treatment with tobramycin and quorum-sensing inhibitors against Pseudomonas aeruginosa in an intraperitoneal foreign-body infection mouse model. J. Antimicrob. Chemother. 2012, 67, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.; Kang, Y.C.; Lee, J.K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Hu, J.; Che, C.; Jiang, W.; Chen, Z.; Tu, J.; Han, X.; Qi, K. Avian pathogenic Escherichia coli through Pfs affects the tran-scription of membrane proteins to resist β-lactam antibiotics. Vet. Sci. 2020, 9, 98. [Google Scholar] [CrossRef]
- Senan, S.; Prajapati, J.B. Mechanism and manifestation of bacterial quorum sensing in food environment. Int. J. Food. Ferment. Technol. 2012, 2, 103–112. [Google Scholar]
- Wu, M.; Tao, Y.; Liu, X.; Zang, J. Structural basis for phosphorylated autoinducer-2 modulation of the oligomerization state of the global transcription regulator LsrR, Escherichia coli. J. Biol. Chem. 2013, 288, 15878–15887. [Google Scholar] [CrossRef]
- Marques, J.C.; Oh, I.K.; Ly, D.C.; Lamosa, P.; Ventura, M.R.; Miller, S.T.; Xavier, K.B. LsrF, a coenzyme A-dependent thiolase, catalyzes the terminal step in processing the quorum sensing signal autoinducer-2. Proc. Natl. Acad. Sci. USA 2014, 111, 14235–14240. [Google Scholar] [CrossRef]
- Pereira, C.S.; Santos, A.J.; Bejerano-Sagie, M.; Correia, P.B.; Marques, J.C.; Xavier, K.B. Phosphoenolpyruvate phosphotransferase system regulates detection and processing of the quorum sensing signal autoinducer-2. Mol. Microbiol. 2012, 84, 93–104. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, X.; Sun, B. An EAL domain protein and cyclic AMP contribute to the interaction between the two quorum sensing systems in Escherichia coli. Cell Res. 2008, 18, 937–948. [Google Scholar] [CrossRef]
- Torres-Escobar, A.; Juárez-Rodríguez, M.D.; Lamont, R.J.; Demuth, D.R. Transcriptional regulation of Aggregatibacter actinomycetemcomitans lsrACDBFG and lsrRK operons and their role in and formation. J. Bacteriol. 2013, 195, 56–65. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Xu, X.; Fu, Y.V. Microbe social skill: The cell-to-cell communication between microorganisms. Sci. Bull. 2017, 62, 516–524. [Google Scholar] [CrossRef]
- Ravichandiran, V.; Shanmugam, K.; Princy Solomon, A. Screening of SdiA inhibitors from Melia dubia seeds extracts towards the hold back of uropathogenic E. coli quorum sensing-regulated factors. Med. Chem. 2013, 9, 819–827. [Google Scholar] [CrossRef]
- Yao, Y.; Martinez-Yamout, M.A.; Dickerson, T.J.; Brogan, A.P.; Wright, P.E.; Dyson, H.J. Structure of the Escherichia coli quorum sensing protein SdiA: Activation of the folding switch by acyl homoserine lactones. J. Mol. Biol. 2006, 355, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Eo, Y.; Grishaev, A.; Guo, M.; Smith, J.A.; Sintim, H.O.; Ryu, K.S. Crystal structures of the LsrR proteins complexed with phospho-AI-2 and two signal-interrupting analogs reveal distinct mechanisms for ligand recognition. J. Am. Chem. Soc. 2014, 135, 15526–15539. [Google Scholar] [CrossRef]
- Vinothkannan, R.; Tamizh, M.M.; Raj, C.D.; Princy, S.A. Fructose furoic acid ester: An effective quorum sensing inhibitor against uropathogenic Escherichia coli. Bioor. Chem. 2018, 79, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef]
- Maurer, C.K.; Lu, C.; Empting, M.; Hartmann, R.W. Synthetic quorum sensing inhibitors (QSIs) blocking receptor signaling or signal molecule biosynthesis in Pseudomonas aeruginosa. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Springer: New Delhi, India, 2015; pp. 303–317. [Google Scholar] [CrossRef]
- Guo, M.; Gamby, S.; Zheng, Y.; Sintim, H.O. Small molecule inhibitors of AI-2 signaling in bacteria: State-of-the-art and future perspectives for anti-quorum sensing agents. Int. J. Mol. Sci. 2013, 14, 17694–17728. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, C.; Bi, C.; Chen, L.; Di Costanzo, L.; Zardecki, C. RCSB Protein Data Bank: Biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, 464–474. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 2006, 296, 149–161. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbio. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.A.K.; Arnason, J.T. Mini review of phytochemicals and plant taxa with activity as microbial biofilm and quorum sensing inhibitors. Molecules 2016, 21, 29. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; McLean, R.J. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Al-Hussaini, R.; Mahasneh, A.M. Microbial growth and quorum sensing antagonist activities of herbal plant extracts. Molecules 2009, 14, 3425–3435. [Google Scholar] [CrossRef]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.T.; Lou, Z.; Zhang, J.; Yu, F.; Timilsena, Y.P.; Zhang, C.; Zhang, Y.; Bakry, A.M. Star Anise (Illicium verum Hook. F.) As quorum sensing and formation inhibitor n foodborne bacteria: Study in Milk. J. Food Prot. 2017, 80, 645–653. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, D.S.; Patil, B.S. Grapefruit juice and its furocoumarins inhibits autoinducer signaling and formation in bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotech. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Lian, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on antibacterial and quorum-sensing inhibition activities of Cinnamomum camphora leaf essential oil. Molecules 2019, 24, 3792. [Google Scholar] [CrossRef]
- Pan, J.; Xie, X.; Tian, W.; Bahar, A.A.; Lin, N.; Song, F.; Jing, A.; Ren, D. (Z)-4-Bromo-5-(bromomethylene)-3-methylfuran-2 (5H)-one sensitizes Escherichia coli persister cells to antibiotics. Appl. Microbiol. Biotechnol. 2013, 97, 9145–9154. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Krishnan, T.; Chan, K.G.; Lim, S.H.E. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Subramani, P.; Hari, B.N.V.; Gowrishankar, S.; Pandian, S.K.; Wilson, A.; Nithyanand, P.; Rubini, D.; Banu, S.F.; et al. Extracted chitosan disrupts quorum sensing mediated virulence factors in urinary tract infection causing pathogens. Pathog. Dis. 2019, 77, ftz009. [Google Scholar] [CrossRef]
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef]
- Defoirdt, T.; Boon, N.; Bossier, P. Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathog. 2010, 6, e1000989. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, X.H. Quorum quenching agents: Resources for antivirulence therapy. Mar. Drugs. 2014, 12, 3245–3282. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and of Gram-negative bacteria. Front. Microbiol. 2015, 6, 420. [Google Scholar] [CrossRef]
- Miller, L.C.; O’Loughlin, C.T.; Zhang, Z.; Siryaporn, A.; Silpe, J.E.; Bassler, B.L.; Semmelhack, M.F. Development of potent inhibitors of pyocyanin production in Pseudomonas aeruginosa. Med. Chem. 2015, 58, 1298–1306. [Google Scholar] [CrossRef]
- D’Angelo, F.; Baldelli, V.; Halliday, N.; Pantalone, P.; Polticelli, F.; Fiscarelli, E.; Williams, P.; Visca, P.; Leoni, L.; Rampioni, G. Identification of FDA-approved drugs as antivirulence agents. Antimicrob. Agents Chemother. 2018, 62, e01296-18. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Seleem, N.M.; Abd El Latif, H.K.; Shaldam, M.A.; El-Ganiny, A. Drugs with new lease of life as quorum sensing inhibitors: For combating MDR Acinetobacter baumannii infections. Eur. J. Clin. Microbiol. 2020, 39, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- Hentzer, M.; Givskov, M. Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1300–1307. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Bjarnsholt, T.; Skindersoe, M.E.; Hentzer, M.; Kristoffersen, P.; Kote, M.; Nielsen, J.; Ebert, L.; Givskov, M. Screening for Quorum-Sensing Inhibitors (QSI) by use of a novel genetic system, the QSI selector. J. Bacteriol. 2005, 187, 1799–1814. [Google Scholar] [CrossRef]
- Ren, D.; Sims, J.J.; Wood, T.K. Inhibition of and formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ. Microbiol. 2001, 3, 731–736. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P.; et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Rui, F.; Marques, J.C.; Miller, S.T.; Maycock, C.D.; Xavier, K.B.; Ventura, M.R. Stereochemical diversity of AI-2 analogs modulates quorum sensing in Vibrio harveyi and Escherichia coli. Bioorganic. Med. Chem. 2012, 20, 249–256. [Google Scholar] [CrossRef]
- Hume, E.B.H.; Baveja, J.; Muir, B.; Schubert, T.L.; Kumar, N.; Kjelleberg, S.; Willcox, M.D.P. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials 2004, 25, 5023–5030. [Google Scholar] [CrossRef]
- Henly, E.L.; Norris, K.; Rawson, K.; Zoulias, N.; Jaques, L.; Chirila, P.G.; Forbes, S. Impact of long-term quorum sensing inhibition on uropathogenic Escherichia coli. J. Antimicrob. Chemother. 2021, 76, 909–919. [Google Scholar] [CrossRef]
- de Almeida, F.A.; Vargas, E.L.G.; Carneiro, D.G.; Pinto, U.M.; Vanetti, M.C.D. Virtual screening of plant compounds and nonsteroidal anti-inflammatory drugs for inhibition of quorum sensing and biofilm formation in Salmonella. Microb. Pathog. 2018, 121, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Lönn-Stensrud, J.; Møretrø, T.; Langsrud, S.; Aamdal-Scheie, A.; Benneche, T.; Nesse, L.L. A synthetic furanone potentiates the effect of disinfectants on Salmonella in biofilm. J. Appl. Microbiol. 2010, 108, 771–778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skandamis, P.N.; Nychas, G.J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef] [PubMed]
- Bové, M.; Bao, X.; Sass, A.; Crabbé, A.; Coenye, T. The quorum-sensing inhibitor furanone C-30 rapidly loses its tobramycin-potentiating activity against Pseudomonas aeruginosa biofilms during experimental evolution. Antimicrob. Agents Chemother. 2021, 65, e01296-18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Wang, Y.; Lin, Z.; Wang, D.; Sun, H. Resistance risk induced by quorum sensing inhibitors and their combined use with antibiotics: Mechanism and its relationship with toxicity. Chemosphere 2021, 265, 129153. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef]
- Koul, S.; Prakash, J.; Mishra, A.; Kalia, V.C. Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J. Microbiol. 2016, 56, 1–18. [Google Scholar] [CrossRef]
- Vasudevan, S.; Swamy, S.S.; Kaur, G.; Princy, S.; Balamurugan, P. Synergism between quorum sensing inhibitors and antibiotics: Combating the antibiotic resistance crisis. In Biotechnological Applications of Quorum Sensing Inhibitors; Editorial Springer: Singapore, 2018; pp. 209–225. [Google Scholar] [CrossRef]
- García-Contreras, R.; Wood, T.K.; Tomás, M. Quorum network (sensing/quenching) of multidrug-resistant pathogens. Front. Cell. Infect. Microb. 2019, 9, 80. [Google Scholar] [CrossRef]
- Gajdács, M.; Spengler, G. The role of drug repurposing in the development of novel antimicrobial drugs: Non-antibiotic pharmacological agents as quorum sensing-inhibitors. Antibiotics 2019, 8, 270. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- McInnis, C.E.; Blackwell, H.E. Thiolactone modulators of quorum sensing revealed through library design and screening. Bioorg. Med. Chem. 2011, 19, 4820–4828. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yeo, S.; Ji, Y.; Lee, J.; Yang, J.; Park, S.; Shin, H.; Holzapfel, W. Autoinducer-2 associated inhibition by Lactobacillus sakei NR28 reduces virulence of enterohaemorrhagic Escherichia coli O157: H7. Food Control. 2014, 45, 62–69. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Borza, T.; Rathgeber, B.; Stratton, G.S.; Thomas, N.A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Red seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down regulate virulence factors of Salmonella enteritidis and induce immune responses in Caenorhabditis elegans. Front. Microbiol. 2016, 7, 421. [Google Scholar] [CrossRef]
- Hodgkinson, J.T.; Galloway, W.R.; Wright, M.; Mati, I.K.; Nicholson, R.L.; Welch, M.; Spring, D.R. Design, synthesis and biological evaluation of non-natural modulators of quorum sensing in Pseudomonas aeruginosa. Org. Biomol. Chem. 2012, 10, 6032–6044. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.M.; Sperandio, V. What a dinner party! mechanisms and functions of interkingdom signaling in host-pathogen associations. MBio 2016, 7, e01748-15. [Google Scholar] [CrossRef]
- Kim, J.; Park, W. Indole: A signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J. Microbiol. 2015, 53, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Kumar, P. The battle: Quorum-sensing inhibitors versus evolution of bacterial resistance. In Quorum Sensing vs. Quorum Quenching: A Battle with No End in Sight; Springer: New Delhi, India, 2015; pp. 385–391. [Google Scholar]
- Hudaiberdiev, S.; Choudhary, K.S.; Vera Alvarez, R.; Gelencsér, Z.; Ligeti, B.; Lamba, D.; Pongor, S. Census of solo LuxR genes in prokaryotic genomes. Front. Cell. Infect. Microbiol. 2015, 5, 20. [Google Scholar] [CrossRef]
- Subramoni, S.; Florez-Salcedo, D.V.; Suarez-Moreno, Z.R. A bioinformatic survey of distribution, conservation, and probable functions of LuxR solo regulators in bacteria. Front. Cell. Infect. Microbiol. 2015, 5, 16. [Google Scholar] [CrossRef]
- Ng, W.L.; Perez, L.; Cong, J.; Semmelhack, M.F.; Bassler, B.L. Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in vibrios. PLoS Pathog. 2012, 8, e1002767. [Google Scholar] [CrossRef]
- Wei, G.; Lo, C.; Walsh, C.; Hiller, N.L.; Marculescu, R. In Silico evaluation of the impacts of Quorum Sensing Inhibition (QSI) on strain competition and development of QSI resistance. Sci. Rep. 2016, 6, 35136. [Google Scholar] [CrossRef]


| Natural QSI | Microorganism | Effect on QS-Regulated Process | In Vitro/In Vivo Experiments | Reference |
|---|---|---|---|---|
| Grape seed extract Reduction in AI activity and synthesis | E. coli (STEC), E. coli (VTEC), and E. coli (EAEC) | Reduces the production of the flagellum and inhibits the production of the Shiga toxin | In vitro | [8,41,48] |
| Extracts of Melia dubia bark | E. coli (EHEC) | Hemolysin suppression, effect on mobility-type swarming, and prevents the formation of biofilm | In vitro | [37] |
| Thymol-carvacrol-chemotype (I and II) oils from Lippia origanoides and Thymus vulgaris oil | E. coli | Prevents the formation of biofilm | In vivo: VERO cell line | [49] |
| Broccoli extracts, basil, oregano, thyme, rosemary, ginger, and turmeric | E. coli (EHEC) | Reduces AI-2 synthesis, with effects on mobility-type swarming and virulence | In vitro | [47,50,51] |
| Punicalagin from a component of pomegranate rind | S. enteritidis | Effect on mobility-type swimming and swarming | In vivo: human colonic HT-29 cell line | [52] |
| Star anise | S. typhimurium | Prevents mobility and biofilm formation | In vitro | [53] |
| Organic acids: acetic acid, citric acid, and lactic acid | S. typhimurium and E. coli | Decreases the production of AI-2 and biofilm formation | In vitro | [18,19] |
| Grapefruit juice/furocoumarin | S. typhimurium | Inhibition of AI-2 activity | In vitro | [54] |
| Synthetic QSI | Microorganism | Effect on QS Regulated Process | In Vitro/In Vivo Experiments | Reference |
|---|---|---|---|---|
| Thiophene inhibitor (TF101) | E. coli | Reduces virulence and prevents the formation of biofilm, cytotoxicity, and the expression of fimH and lsrB | In vitro and in vivo: Caco-2 cell line | [10] |
| Furanone | E. coli | Prevents AI-2 synthesis | In vivo: mice tissues of lung, liver, spleen, and kidney C57BL/6 cell line | [57] |
| Cinnamomum verum bark essential oil or combination with piperacillin | E. coli (multidrug-resistant) | Prevents the formation of biofilm | In vitro | [56,58] |
| Chitosan | E. coli (UPEC) | Reduces virulence, prevents the formation of biofilm, and reduces mobility | In vitro | [59] |
| Fructose-furoic acid ester | E. coli (UPEC) | Decreases toxicity and biofilm production | In vivo: kidney carcinoma A498 cell line | [40] |
| Limonene nanoemulsion | E. coli (EHEC) | Reduces AI-2 synthesis, effect on mobility-type swimming and swarming, and suppression of curli and the extracellular polymeric substance (EPS) | In vivo | [56] |
| N-phenyl-4-phenylamino-thioxomenthyl amino-benzenesulfonamide | E. coli (EHEC) and S. typhimurium | Inhibition of QseC-mediated activation of virulence gene expression | In vivo: mice strain 129 × 1/SvJ | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. https://doi.org/10.3390/microorganisms10050884
Escobar-Muciño E, Arenas-Hernández MMP, Luna-Guevara ML. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms. 2022; 10(5):884. https://doi.org/10.3390/microorganisms10050884
Chicago/Turabian StyleEscobar-Muciño, Esmeralda, Margarita M. P. Arenas-Hernández, and M. Lorena Luna-Guevara. 2022. "Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella" Microorganisms 10, no. 5: 884. https://doi.org/10.3390/microorganisms10050884
APA StyleEscobar-Muciño, E., Arenas-Hernández, M. M. P., & Luna-Guevara, M. L. (2022). Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms, 10(5), 884. https://doi.org/10.3390/microorganisms10050884






