The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis
Abstract
:1. Introduction
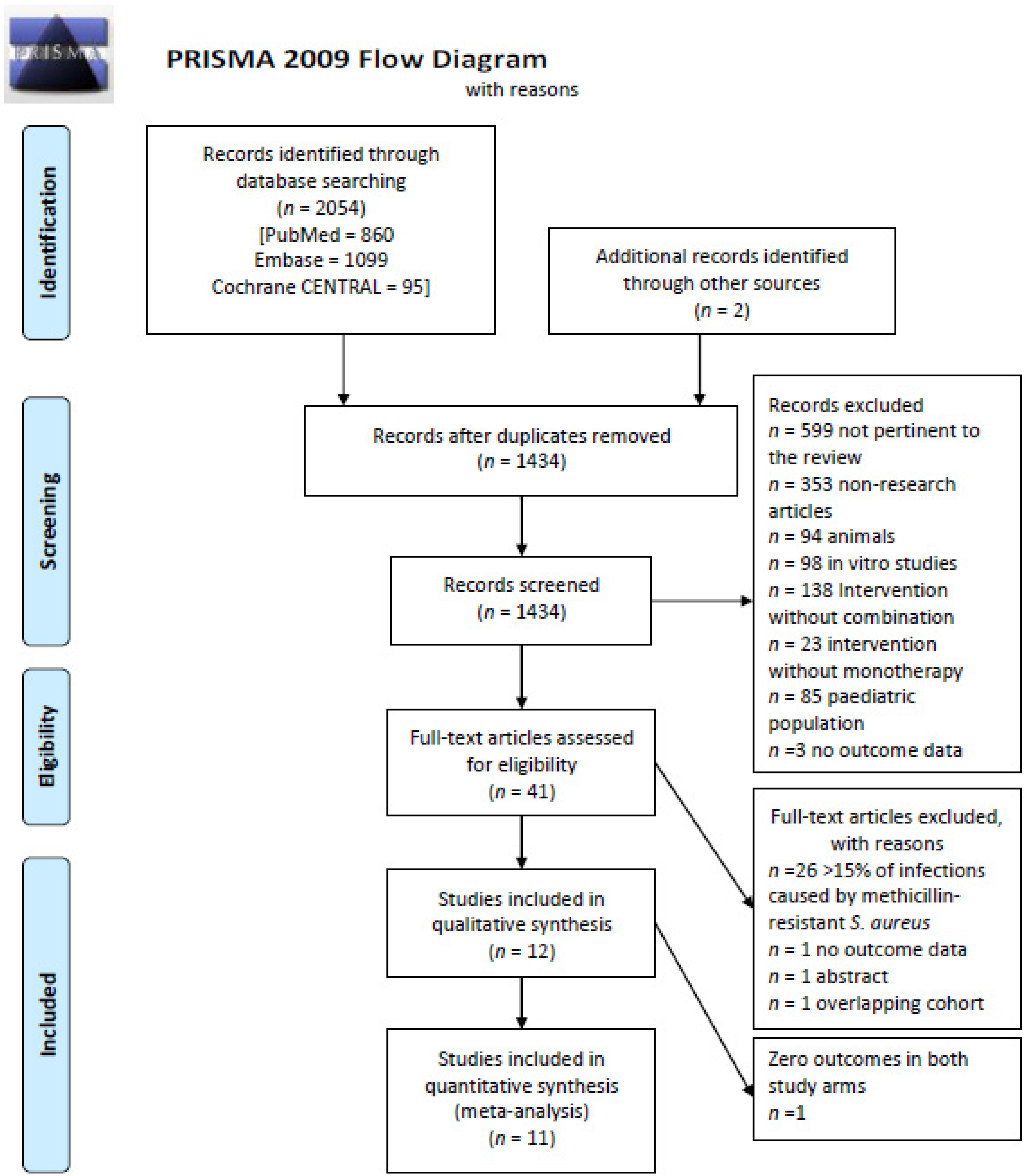
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Outcomes
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Groups
3.2. Start of Combination Therapy
3.3. Sources of Infection
3.4. Compliance with Good Clinical Practices
3.5. Risk of Bias
3.6. Effectiveness of Combination Therapy
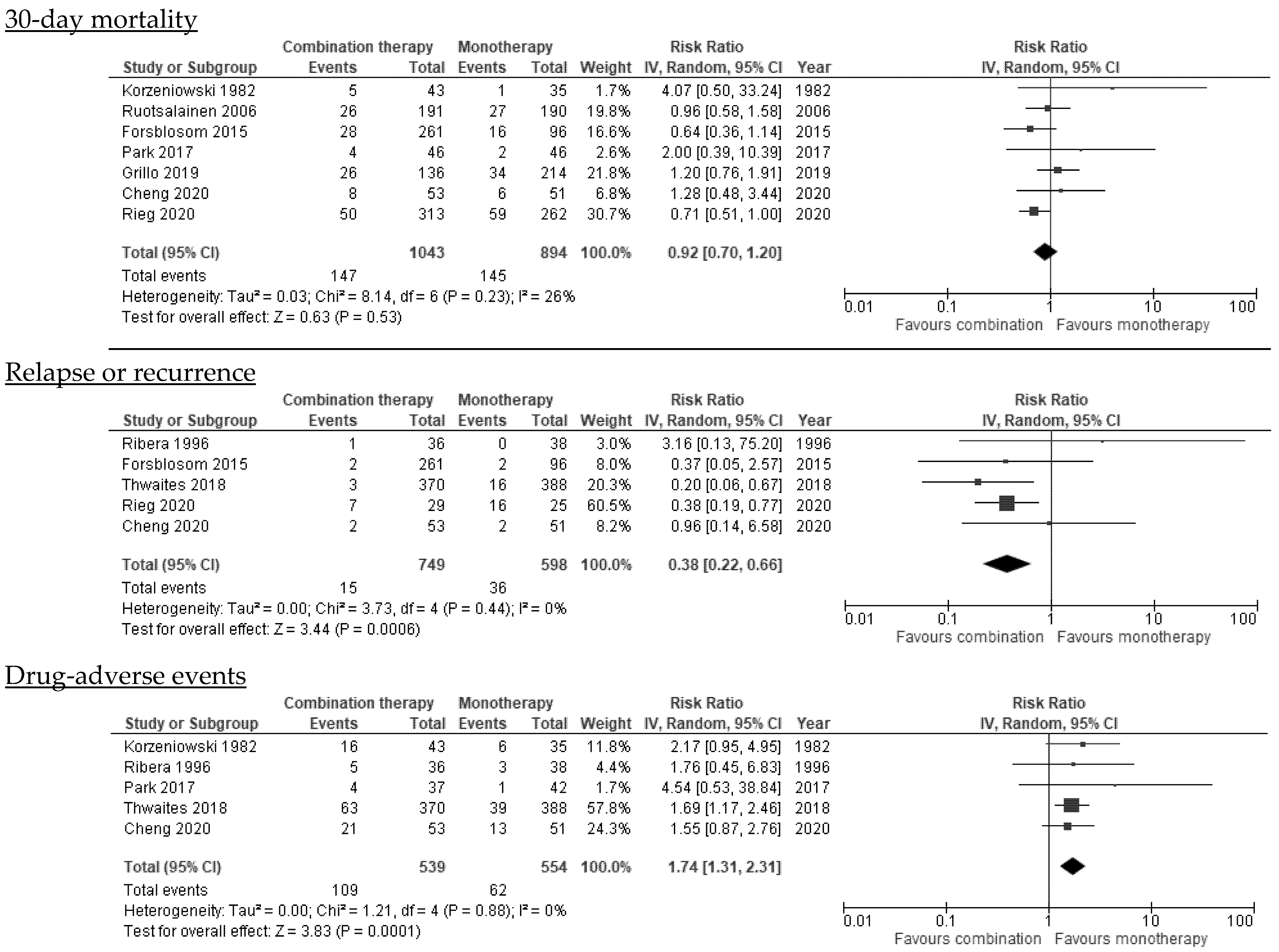
3.6.1. Mortality
3.6.2. Duration of Bacteremia
3.6.3. Persistent Bacteremia
3.6.4. Relapse or Recurrence
3.7. Drug-Related Adverse Events
3.8. Effectiveness per Type of Antibiotic in Combination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souli, M.; Ruffin, F.; Choi, S.-H.; Park, L.P.; Gao, S.; Lent, N.C.; Sharma-Kuinkel, B.K.; Thaden, J.T.; Maskarinec, S.A.; Wanda, L.; et al. Changing Characteristics of Staphylococcus aureus Bacteremia: Results from a 21-Year, Prospective, Longitudinal Study. Clin. Infect. Dis. 2019, 69, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Lyytikäinen, O.; Søgaard, M.; Kennedy, K.J.; Knudsen, J.D.; Ostergaard, C.; Galbraith, J.C.; Valiquette, L.; Jacobsson, G.; Collignon, P.; et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: A multinational population-based surveillance study. Clin. Microbiol. Infect. 2013, 19, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, R.; Croydon, E.A.P.; Rolinson, G.N. Flucloxacillin, a New Isoxazolyl Penicillin, Compared with Oxacillin Cloxacillin, and Dicloxacillin. Br. Med. J. 1970, 4, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Gudiol, F.; Aguado, J.M.; Almirante, B.; Bouza, E.; Cercenado, E.; Domínguez, M.Á.; Gasch, O.; Lora-Tamayo, J.; Miró, J.M.; Palomar, M.; et al. Executive summary of the diagnosis and treatment of bacteremia and endocarditis due to Staphylococcus aureus. A clinical guideline from the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2015, 33, 626–632. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, L.E.; Del Toro, M.D.; Gálvez-Acebal, J.; Bereciartua-Bastarrica, E.; Fariñas, M.C.; Sanz-Franco, M.; Natera, C.; Corzo, J.E.; Lomas, J.M.; Pasquau, J.; et al. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of staphylococcus aureus bacteremia. Clin. Infect. Dis. 2013, 57, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of mortality in staphylococcus aureus bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sande, M.A.; Courtney, K.B. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis-PubMed. J. Lab. Clin. Med. 1976, 88, 118–124. [Google Scholar]
- Licht, J.H. Penicillinase-Resistant Penicillin/Gentamicin Synergism: Effect in Patients With Staphylococcus aureus Bacteremia. Arch. Intern. Med. 1979, 139, 1094–1098. [Google Scholar] [CrossRef]
- Wang, C.; Ye, C.; Liao, L.; Wang, Z.; Hu, Y.; Deng, C.; Liu, L. Adjuvant β-Lactam Therapy Combined with Vancomycin or Daptomycin for Methicillin-Resistant Staphylococcus aureus Bacteremia: A Systematic Review and Meta-analysis. Antimicrob. Agents Chemother. 2020, 64, 11. [Google Scholar] [CrossRef]
- Falagas, M.E.; Matthaiou, D.K.; Bliziotis, I.A. The role of aminoglycosides in combination with a β-lactam for the treatment of bacterial endocarditis: A meta-analysis of comparative trials. J. Antimicrob. Chemother. 2006, 57, 639–647. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, J.; Peng, L.; Gao, Y.; Zhang, G.; Luoi, Z. Adjunctive rifampin for the treatment of staphylococcus aureus bacteremia with deep infections: A meta-analysis. PLoS ONE 2020, 15, e0230383. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.; Wang, C.; Li, Z.; Li, X.; Pan, J.; Liu, L.; Wang, Z. The Effect of Combination Therapy on Mortality and Adverse Events in Patients with Staphylococcus aureus Bacteraemia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Infect. Dis. Ther. 2021, 10, 2643–2660. [Google Scholar] [CrossRef]
- Cosgrove, S.E.; Sakoulas, G.; Perencevich, E.N. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: A meta-analysis. Clin. Infect. Dis. 2003, 36, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, A.D.; Showler, A.; Burry, L.; Steinberg, M.; Ricciuto, D.R.; Fernandes, T.; Chiu, A.; Raybardhan, S.; Science, M.; Fernando, E.; et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in staphylococcus aureus bacteremia: Results from a large multicenter cohort study. Clin. Infect. Dis. 2015, 60, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne., J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrams, B.; Sklaver, A.; Hoffman, T.; Greenman, R. Single or combination therapy of staphylococcal endocarditis in intravenous drug abusers. Ann. Intern. Med. 1979, 90, 789–791. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Scarborough, M.; Szubert, A.; Nsutebu, E.; Tilley, R.; Greig, J.; Wyllie, S.A.; Wilson, P.; Auckland, C.; Cairns, J.; et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Ruotsalainen, E.; Järvinen, A.; Koivula, I.; Kauma, H.; Rintala, E.; Lumio, J.; Kotilainen, P.; Vaara, M.; Nikoskelainen, K.; Valtonen, V.; et al. Levofloxacin does not decrease mortality in Staphylococcus aureus bacteraemia when added to the standard treatment: A prospective and randomized clinical trial of 381 patients. J. Intern. Med. 2006, 259, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowski, O.; Sande, M.A. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts. A prospective study. Ann. Intern. Med. 1982, 97, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Ribera, E.; Gómez-Jimenez, J.; Cortes, E.; Del Valle, O.; Planes, A.; Teresa Gonzalez-Alujas, M.; Almirante, B.; Ocana, I.; Pahissa, A. Effectiveness of cloxacillin with and without gentamicin in short-term therapy for right-sided Staphylococcus aureus endocarditis: A randomized, controlled trial. Ann. Intern. Med. 1996, 125, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.P.; Lawandi, A.; Butler-Laporte, G.; De l’Étoile-Morel, S.; Paquette, K.; Lee, T.C. Adjunctive Daptomycin in the Treatment of Methicillin-susceptible Staphylococcus aureus Bacteremia: A Randomized, Controlled Trial. Clin. Infect. Dis. 2021, 72, e196–e203. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Ernst, A.; Peyerl-Hoffmann, G.; Joost, I.; Camp, J.; Hellmich, M.; V Kern, W.; J Kaascj, A.; Seifert, H. Combination therapy with rifampicin or fosfomycin in patients with Staphylococcus aureus bloodstream infection at high risk for complications or relapse: Results of a large prospective observational cohort. J. Antimicrob. Chemother. 2020, 75, 2282–2290. [Google Scholar] [CrossRef]
- Forsblom, E.; Ruotsalainen, E.; Järvinen, A. Improved outcome with early rifampicin combination treatment in methicillin-sensitive Staphylococcus aureus bacteraemia with a deep infection focus-A retrospective cohort study. PLoS ONE 2015, 10, e0122824. [Google Scholar] [CrossRef] [PubMed]
- Rajashekaraiah, K.R.; Rice, T.; Marsh, D. Clinical significance of tolerant strains of Staphylococcus aureus in patients with endocarditis. Ann. Intern. Med. 1980, 93, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Grillo, S.; Cuervo, G.; Carratalà, J.; Grau, I.; Pallarès, N.; Tebé, C.; Tió, L.G.; Murillo, O.; Ardanuy, C.; Domínguez, M.A.; et al. Impact of β-Lactam and Daptomycin Combination Therapy on Clinical Outcomes in Methicillin-susceptible Staphylococcus aureus Bacteremia: A Propensity Score-matched Analysis. Clin. Infect. Dis. 2019, 69, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Park, G.E.; Ko, J.H.; Cho, S.Y.; Ha, Y.E.; Lee, N.Y.; Kang, C.I.; Chung, D.R.; Song, J.H.; Peck, K.R. Empirical combination of a β-lactam to vancomycin may not improve outcomes of methicillin-susceptible Staphylococcus aureus bacteremia, compared to vancomycin monotherapy. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1091–1096. [Google Scholar] [CrossRef]
- Watanakunakorn, C.; Baird, I. Prognostic factors in Staphylococcus aureus endocarditis and results of therapy with a penicillin and gentamicin. Am. J. Med. Sci. 1976, 273, 133–139. [Google Scholar] [CrossRef]
- Lebeaux, D.; Fernández-Hidalgo, N.; Pilmis, B.; Tattevin, P.; Mainardi, J.L. Aminoglycosides for infective endocarditis: Time to say goodbye? Clin. Microbiol. Infect. 2020, 26, 723–728. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; Khoury, G.E.; Erba, P.A.; Lung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Matthews, P.C.; Berendt, A.R.; McNally, M.A.; Byren, I. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the infectious diseases Society of America. BMJ 2009, 338, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedel, D.J.; Weekes, E.; Forrest, G.N. Addition of rifampin to standard therapy for treatment of native valve infective endocarditis caused by Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2463–2467. [Google Scholar] [CrossRef] [Green Version]
- McConeghy, K.W.; Bleasdale, S.C.; Rodvold, K.A. The empirical combination of vancomycin and a β-lactam for staphylococcal bacteremia. Clin. Infect. Dis. 2013, 57, 1760–1765. [Google Scholar] [CrossRef] [Green Version]
- Hornak, J.P.; Anjum, S.; Reynoso, D. Adjunctive ceftaroline in combination with daptomycin or vancomycin for complicated methicillin-resistant Staphylococcus aureus bacteremia after monotherapy failure. Ther. Adv. Infect. Dis. 2019, 6, 2049936119886504. [Google Scholar] [CrossRef] [Green Version]
- McCreary, E.K.; Kullar, R.; Geriak, M.; Zasowski, E.J.; Rizvi, K.; Schulz, L.T.; Ouellette, K.; Vasina, L.; Haddad, F.; Rybak, M.J.; et al. Multicenter cohort of patients with methicillin-resistant Staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum Infect. Dis. 2020, 7, ofz538. [Google Scholar] [CrossRef] [Green Version]
- Sakoulas, G.; Moise, P.A.; Casapao, A.M.; Nonejuie, P.; Olson, J.; Okumura, C.Y.M.; Rybak, M.J.; Kullar, R.; Dhand, A.; Rose, W.E.; et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin. Ther. 2014, 36, 1317–1333. [Google Scholar] [CrossRef]
- García-De-La-Mària, C.; Gasch, O.; Castañeda, X.; García-González, J.; Soy, D.; Cañas, M.A.; Ambrosioni, J.; Almela, M.; Pericàs, J.M.; Téllez, A.; et al. Cloxacillin or fosfomycin plus daptomycin combinations are more active than cloxacillin monotherapy or combined with gentamicin against MSSA in a rabbit model of experimental endocarditis. J. Antimicrob. Chemother. 2020, 75, 3586–3592. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, E.R.; Singh, K.V.; Geriak, M.; Haddad, F.; Murray, B.E.; Nizet, V.; Sakoulas, G. Cefazolin and ertapenem salvage therapy rapidly clears persistent methicillin-susceptible staphylococcus aureus bacteremia. Clin. Infect. Dis. 2020, 71, 1413–1418. [Google Scholar] [CrossRef]
- Sakoulas, G.; Olson, J.; Yim, J.; Singh, N.B.; Kumaraswamy, M.; Quach, D.T.; Rybak, M.J.; Pogliano, J.; Nizet, V. Cefazolin and ertapenem, a synergistic combination used to clear persistent Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2016, 60, 6609–6618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, T.L.; Chambers, H.F.; Boucher, H.W.; Corey, G.R.; Coleman, R.; Castaneda-Ruiz, B.; Fowler, V.G. Considerations for clinical trials of staphylococcus aureus bloodstream infection in adults. Clin. Infect. Dis. 2019, 68, 865–872. [Google Scholar] [CrossRef]
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Price, J. Defining persistent Staphylococcus aureus bacteraemia: Secondary analysis of a prospective cohort study. Lancet Infect. Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author, Year Country a | Study Design; n° of Hospitals | Study Population | Monotherapy Group | Antibiotic/s Added in the Combination Group b | Reason for Starting the Combination Therapy | Duration of the Combination Therapy c | Sample Size (Combination/Monotherapy Groups) | Outcomes Assessed | Adjusted for the Following Confounders: | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Duration of Bacteremia | Persistent Bacteremia | Relapse | Adverse Events | |||||||||
| Watanakunakorn, 1977, US | Cohort study; 1 | SAB and endocarditis (possibly MSSA) | OXA/PEN/CFZ | GEN 3-5 mg/kg/day | Patients who had been treated recently | 2–3 weeks | 40 (15 vs. 25) | Yes, death during the 6 weeks of treatment | No | No | No | Nephrotoxicity | None |
| Abrams, 1979, US | RCT; 1 | Intravenous drug users with MSSA bacteremia and endocarditis | OXA or CFZ | GEN 80 mg/8 h | After randomization | 2 weeks PP | 25 (12 vs. 13) | Yes, all-cause mortality (end-point of assessment not reported) | No | No | No | Nephrotoxicity, drug-induced hepatitis, leukopenia, drug fever and rash. | None, study groups well-balanced |
| Rajashekaraiah, 1980, US | Cohort study; 1 | MSSA bacteremia and endocarditis | OXA/PEN/CFZ 4–6 weeks | GEN 4.5 mg/kg/day | GEN added because of lack of prompt clinical response Combination started ≤ 48 h after starting treatment | At least 7 days | 33 (21 vs. 12) | Yes, all-cause mortality (end-point of assessment not reported) | No | No | No | No | None |
| Korzeniowski, 1982, US | RCT; 1 | SAB and native valve endocarditis (possibly MSSA) | PEN 6 weeks | GEN 3 mg/kg/8 h | After randomization | 2 weeks PP | 78 (35 vs. 43) | Yes, 30-day | Yes | No | No | Nephrotoxicity | None |
| Ribera, 1996, Spain | RCT; 1 | Intravenous drug users with right-sided MSSA endocarditis | OXA 14 days | GEN 1 mg/kg/8 h | After randomization | 7 days PP | 74 (36 vs. 38) | Yes, death during treatment | No | Yes | Yes | Nephrotoxicity | None, study groups well-balanced |
| Ruotsalainen, 2006, Finland | RCT; 13 | MSSA bacteremia | BL+/− AMG (If endocarditis)+/−RIF | LVX 500 mg once or b.i.d according to weight. | After randomization | 42 days (28–58) | 381 (191 vs. 190) | Yes, 30 and 90-days | No | No | No | Yes, liver enzyme elevations, Clostridoides difficile, and allergic reactions evaluated. | None, study groups well-balanced |
| Ruotsalainen, 2006, Finland | Post-hoc analysis of a prospective cohort; 13 | MSSA bacteremia with deep seated infection | BL+/− AMG (If endocarditis) +/−LVX | LVX+ RIF 450 or 600 mg/day | NR | Unknown | 331 (265 vs. 66) | Yes, 90-day | No | No | No | No | NR |
| Forsblom, 2015, Finland | Retrospective cohort; 13 | MSSA bacteremia and deep infection focus | OXA (58%), CXM, CRO, VAN or CLI d +/−FLQ/AMG | RIF 450 or 600 mg/day | NR | Short (1–13 days) Long (≥14 days) | 357 (261 vs. 96) | Yes, 30 and 90-days | No | No | Yes | No | Age, severity of illness, ID consultation, endocarditis, ultimately-rapidly fatal disease |
| Park, 2017, Korea | Retrospective matched cohort; 1 | MSSA bacteremia | BL | VAN e | NR, but combination was an empirical treatment | 2.8 days (2.1–3.8) | 92 (46 vs. 46) | Yes, 30-day | Yes | No | No | Nephrotoxicity | Charlson score, Pitt score, white blood cell count |
| Thwaites, 2018, UK | RCT; 29 | Patients with SAB who have received ≤ 96 h of active antibiotic treatment (94% MSSA; 6% MRSA bacteremia) | OXA (83%), VAN or TEC | RIF 600 or 900 mg/day | After randomization | 12.6 days (6.0–13.2) | 708 (370 vs. 388) | Yes, 90-day | Yes | No | Yes, recurrence | Yes, all type. Serious adverse events, drug-modifying adverse events, drug interactions | None, study groups well-balanced |
| Grillo, 2019, Spain | Post-hoc analysis of a prospective cohort; 1 | Patients with MSSA bacteremia who survived > 48h | BL | DAP 10 mg/kg/day | NR; but combination was administered for ≥72 h and started within the first 4 days of treatment | 9 days (4–15) | 350 (136 vs. 214) | Yes, 7, 30 and 90-days | No | Yes | No | No | Age, Pitt score, source of infection |
| Rieg, 2020, Germany | Post-hoc analysis of a prospective cohort; 2 | SAB with deep-seated infection (89% MSSA; 11% MRSA bacteremia) | OXA, DAP, VAN, LIN | RIF (450 mg/12 h) [77.3%] or FOS (5 g/8 h iv) | NR; combination started within 14 days after SAB onset | 23 days (13–33) | 578 (313 vs. 265) | Yes, 30 and 90-days | No | No | Yes | No | Age, Charlson, severe sepsis |
| Cheng, 2020, Canada | Double-blind RCT; 2 | Patients with MSSA bacteremia with ≤72 h from first positive blood culture | BL | DAP 6 mg/kg/day | After randomization | 5 days PP | 104 (53 vs. 51) | Yes, 30 and 90-days | Yes | No | Yes | Nephrotoxicity, hepatotoxicity, rhabdomlyolisis within 5 days of enrollment | None, study groups well-balanced |
| Subgroup Analyses | N°. of Studies | References | N°. of Patients (Combination/Monotherapy) | Pooled Risk Ratio (95% CI) | I2 Test | |
|---|---|---|---|---|---|---|
| 30-day mortality | All studies | 7 | [19,20,22,23,24,26,27] | 1043/894 | 0.92 (0.70–1.20) | 26% |
| RCTs | 3 | [19,20,22] | 287/276 | 1.08 (0.70–1.67) | 0% | |
| Observational studies | 4 | [23,24,26,27] | 756/618 | 0.85 (0.60–1.22) | 42% | |
| Adjusted observational studies + well-balanced RCTs | 3 | [19,22,27] | 290/287 | 1.04 (0.67–1.60) | 0% | |
| Early administration of combination therapy | 4 | [20,22,26,27] | 278/346 | 1.31 (0.88–1.95) | 0% | |
| After excluding studies published before 2000 | 6 | [19,22,23,24,26,27] | 1000/859 | 0.88 (0.69–1.13) | 18% | |
| 90-day mortality | All studies | 6 | [18,19,22,23,24,26] | 1336/1179 | 0.89 (0.74–1.06) | 27% |
| RCTs | 3 | [18,19,22] | 632/611 | 0.93 (0.72–1.20) | 0% | |
| Observational studies | 3 | [23,24,26] | 704/568 | 0.86 (0.60–1.22) | 68% | |
| Adjusted observational studies + well-balanced RCTs | 5 | [18,19,22,23,26] | 1075/1083 | 0.96 (0.78–1.19) | 13% | |
| After excluding studies with late start of combination therapy | 3 | [18,22,26] | 577/635 | 1.07 (0.84–1.37) | 0% | |
| All participants with deep-seated infections (rifampicin) a | 3 | [19,23,24] | 833/420 | 0.62 (0.42–0.92) | 73% | |
| Any-time mortality b | All studies | 11 | [18,19,20,21,22,23,24,25,26,27,28] | 1503/1339 | 0.91 (0.76–1.08) | 0% |
| RCTs | 6 | [18,19,20,21,22,25] | 732/696 | 1.01 (0.78–1.31) | 0% | |
| Observational studies | 5 | [23,24,26,27,28] | 771/643 | 0.86 (0.64–1.15) | 25% | |
| After excluding studies with late start of combination therapy | 7 | [18,20,21,22,25,26,27] | 723/766 | 1.09 (0.85–1.41) | 0% | |
| After excluding studies published before 2000 | 7 | [18,19,22,23,24,26,27] | 1388/1229 | 0.89 (0.74–1.08) | 5% | |
| All participants with endocarditis (aminoglycosides) c | 4 | [20,21,25,28] | 115/110 | 1.17 (0.64–2.16) | 0% | |
| ≥30% left-sided endocarditis (aminoglycosides) | 3 | [20,25,28] | 79/72 | 1.12 (0.60–2.11) | 0% | |
| Relapse d | All studies | 5 | [18,21,22,23,24] | 749/598 | 0.38 (0.22–0.66) | 0% |
| Excluding Thwaites study that used a different definition (recurrence) | 4 | [21,22,23,24] | 379/210 | 0.45 (0.24–0.83) | 0% | |
| RCTs | 3 | [18,21,22] | 459/477 | 0.54 (0.12–2.51) | 46% | |
| Observational studies | 2 | [23,24] | 290/121 | NA | ||
| Drug adverse-events d | Any type of antibiotic adverse-event | 5 | [18,20,21,22,27] | 539/554 | 1.74 (1.31–2.31) | 0% |
| After excluding studies published before 2000 | 3 | [18,22,27] | 460/482 | 1.69 (1.24–2.30) | 0% | |
| Nephrotoxicity or AKI | 4 | [20,21,22,27] | 169/166 | 1.81 (1.17–2.79) | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillo, S.; Puig-Asensio, M.; Schweizer, M.L.; Cuervo, G.; Oriol, I.; Pujol, M.; Carratalà, J. The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis. Microorganisms 2022, 10, 848. https://doi.org/10.3390/microorganisms10050848
Grillo S, Puig-Asensio M, Schweizer ML, Cuervo G, Oriol I, Pujol M, Carratalà J. The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis. Microorganisms. 2022; 10(5):848. https://doi.org/10.3390/microorganisms10050848
Chicago/Turabian StyleGrillo, Sara, Mireia Puig-Asensio, Marin L. Schweizer, Guillermo Cuervo, Isabel Oriol, Miquel Pujol, and Jordi Carratalà. 2022. "The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis" Microorganisms 10, no. 5: 848. https://doi.org/10.3390/microorganisms10050848
APA StyleGrillo, S., Puig-Asensio, M., Schweizer, M. L., Cuervo, G., Oriol, I., Pujol, M., & Carratalà, J. (2022). The Effectiveness of Combination Therapy for Treating Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Systematic Literature Review and a Meta-Analysis. Microorganisms, 10(5), 848. https://doi.org/10.3390/microorganisms10050848






