Abstract
Endophytes represent a ubiquitous and magical world in plants. Almost all plant species studied by different researchers have been found to harbor one or more endophytes, which protect host plants from pathogen invasion and from adverse environmental conditions. They produce various metabolites that can directly inhibit the growth of pathogens and even promote the growth and development of the host plants. In this review, we focus on the biological control of plant diseases, aiming to elucidate the contribution and key roles of endophytes and their metabolites in this field with the latest research information. Metabolites synthesized by endophytes are part of plant disease management, and the application of endophyte metabolites to induce plant resistance is very promising. Furthermore, multi-omics should be more fully utilized in plant–microbe research, especially in mining novel bioactive metabolites. We believe that the utilization of endophytes and their metabolites for plant disease management is a meaningful and promising research direction that can lead to new breakthroughs in the development of more effective and ecosystem-friendly insecticides and fungicides in modern agriculture.
1. Introduction
Plant diseases, caused by agricultural pests and pathogens, commonly result in crop losses and are a significant threat to food security. Agrochemicals are efficient in plant disease management. However, the intensive application of chemical fertilizers and pesticides has negative effects on the ecosystem and human beings, causing environmental pollution, pathogen resistance, and ecological imbalance [1]. Biocontrols, unlike chemical methods, are environmentally friendly through the action of natural control agents, such as beneficial microorganisms and their products, metabolites [2]. Endophytes and their bioactive metabolites have received considerable attention due to their potential as biological control agents (BCAs) [3,4].
Endophytes, including endophytic fungi, endophytic bacteria, and endophytic actinomycetes, exist in various organs, tissues, and intercellular spaces of plants without causing immediate signs of diseases [5,6]. They have established a mutually beneficial relationship with host plants during long-term coevolution. Plants provide nutrients for endophytes, while endophytes contribute to maintaining the health of plants [7,8]. Compared with soil microorganisms, endophytes within plants may have more positive and direct impacts on plants because of their special ecological niches. The results from different research teams have provided good evidence for the effects of endophytes on plant growth promotion, stress mitigation, and disease resistance in host plants [9,10,11,12]. The mechanisms proposed for disease prevention include competition with pathogens for niche and nutrition, induction of plant resistance, secretion of bioactive metabolites, and promotion of plant growth, usually working in concert [13,14,15]. Endophytic microorganisms, such as Bacillus, Burkholderia, Enterobacter, Pseudomonas, Streptomyces, etc., are used as microbial formulations against various phytopathogens [16].
Endophytes can produce metabolites with a variety of biological activities, such as alkaloids, polypeptides, polyketides, terpenoids, etc., which are of great significance and value in different fields, especially in agriculture and the pharmaceutical industry [14,17,18]. These metabolites have attracted a lot of attention and increased interest, because they can serve as antibiotics, insecticidal agents, natural antioxidants, antitumor agents, antidiabetic products, and so on [19,20]. In plant health protection, the major function of these bioactive metabolites is to directly or indirectly help the host plants resist biotic and abiotic stresses. For example, some antimicrobial compounds produced by endophytes are well known for strongly inhibiting pathogens, hydrolases secreted by endophytic bacteria can decompose the cell wall of pathogens, and phytohormones released by endophytes play a vital role in plant development and stress response [21,22]. Certain microorganisms called plant growth-promoting microbes (PGPM) are well-known elicitors of prime systemic resistance in plants, and they perform this function through producing metabolites such as antimicrobials and volatile organic compounds [23]. It is worth noting that studies related to the application of metabolites from endophytes in eliciting plant resistance are still limited and need to be explored and enriched in future research.
Overall, a precise understanding of these “biocontrol agents” is important for helping plants to overcome different stress conditions. Here, we summarize several critical mechanisms employed by endophytes, particularly their metabolites, that enhance host plant disease resistance. Using endophytes and their metabolites as biological control agents can be one of the most effective ways to improve crop quality, increase yields, and achieve sustainable agriculture under the premise of minimizing the harm to the environment and humans.
2. The Concept and Types of Endophytes
The definition of an endophyte was originally proposed by De Bary (1866) as “any organism that grows within plant tissues”, which is distinguished from an epiphyte living on the surface of plants [24]. One hundred years later, Carroll (1986) proposed a new definition for endophytes as organisms that inhabit the aerial parts and living tissues of plants without causing visible infection or diseases, emphasizing the mutualistic relationship between endophytes and plants; pathogenic and mycorrhizal fungi were excluded [25]. Petrini (1991) further expanded Carroll’s definition as all organisms that colonize within plant tissues for some part of their lifecycle and do not cause symptomatic infections to the host plants, from which latent pathogens are also known as endophytes [26]. Furthermore, endophytes have been defined in several different ways [5,27]. What can be seen is that the concept of endophytes has always been controversial. The definition from Petrini is commonly used in most endophyte studies.
The major members of endophytic fungi include Ascomycota, Zygomycota, and Basidiomycota. In general, endophytic fungi have been recognized as two broad groups according to the life history traits and evolutionary relatedness, namely, (1) the clavicipitaceous endophytes colonizing within some grasses and (2) the non-clavicipitaceous endophytes from asymptomatic tissues of nonvascular plants, conifers, ferns, and angiosperms [28,29]. Endophytic fungi are well known to produce various bioactive compounds for anti-inflammatory, antioxidant, anti-fibrosis and antivirus drug development. Isopestacin, extracted from the fungal endophyte Pestalotiopsis microspore, has pronounced antioxidant properties [30]. Taxol (paclitaxel), an effective and important anticancer drug, is extracted from Taxomyces andreanae [31]. Another example is Podophyllotoxin, synthesized from Alternaria tenuissima, which exhibits excellent antitumor activity [32].
Endophytic bacteria belong to a diverse group of species, ranging from gram-positive to gram-negative bacteria, such as Bacillus, Agrobacterium, Brevibacterium, Pseudomonas, etc. [33]. The recent study conducted by Liu et al., revealed that a wide variety of endophytes inhabited wild rice, among which Proteobacteria, Bacteroidetes, and Firmicutes were dominant [34]. The diversity of endophytic bacteria in plants is affected by the host and environment related factors, e.g., plant growth stages, geographical location, and climatic conditions [35].
Endophytic actinomycetes are commonly isolated from a variety of plants, particularly from mangrove plants and medicinal plants in tropical rain forest [36]. Generally, the number and species of endophytic actinomycetes in plant roots are more than those in other parts of plants. Streptomyces and Micromonospora are the dominant genera and have been acknowledged as valuable resources for antibiotics as well as other bioactive metabolites, such as Munumbicin D, produced by Streptomyces NRRL 30562, and coronamycin, derived from Streptomyces sp. MSU-2110 [37]. Numerous species of endophytic microbes still need to be explored and identified.
3. Multifunctions of Endophytes and Their Metabolites in Plant Disease Management
Endophytes secrete various metabolites that directly or indirectly enhance the tolerance of the host to different stresses, thus making them beneficial to the plants, and they potentially serve as promising biological agents in controlling plant diseases. For example, Pyricularia oryzae Cav., a widely studied pathogenic fungus in rice blast can be efficiently controlled by the application of endophytic microbes [38]. The fungal endophytes of Populus alba enhanced the host’s tolerance to the pathogen Venturia tremulae, as described by Martínez-Arias et al. [39]. Romeralo et al., isolated several endophytes and proved their ability to protect Aleppo pine (Pinus halepensis) against Gremmeniella abietina [40,41]. The infection and colonization of European ash (Fraxinus excelsior) by the pathogen Hymenoscyphus fraxineus could be affected by endophytic microbes via toxin secretion and/or activation of the host defense response [42]. Researchers are increasingly interested in this field, and the biocontrol roles of endophytes and their metabolites against diseases have been discussed and reported in the literature from time to time. In this section, we summarize the key mechanisms of (1) competing with pathogens for niche and nutrition, (2) producing antimicrobial compounds, (3) secreting lytic enzymes, (4) inducing systemic resistance in host plants, and (5) producing plant hormones and plant growth-promoting regulators. There are still more problems to be solved. An overview of the main functions, future prospects, and challenges in using endophytes and their metabolites in plant disease management is shown in Figure 1.
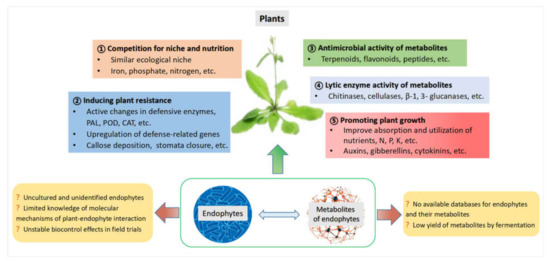
Figure 1.
Multiple mechanisms employed by endophytes and their metabolites in plant disease management.
3.1. Competition with Pathogens for Niche and Nutrition
Colonization in plant tissues is one of the basic properties for endophytes [43]. Endophytes generally enter the host plant in the form of thalli or spores through epidermal penetration or stomata entry, which is similar to the way pathogenic bacteria infect plants. These beneficial “micro-guests” may preferentially occupy the invasion sites of pathogens in plants and utilize nutrients, thereby reducing pathogen invasion [28]. An early case in point is that a control system using the bacterial endophyte Bacillus subtilis (Ehrenberg) Cohn developed by Bacon et al. [44] showed great promise for reducing invasion and mycotoxin accumulation of Fusarium moniliforme. The reason was that these two microorganisms occupied a similar ecological niche in maize [44]. Simultaneously, endophytes compete with pathogenic microbes for nutrition, which tends to slow the growth of pathogens. The secretion of siderophores, peptides that have high affinity for iron, is good evidence for nutrient competition. This strategy was employed by some Pseudomonas species in biocontrol of carnation fusarium wilt caused by Fusarium oxysporum f. sp. dianthi (Fod) [45]. That is to say, these beneficial microbes of host plants are capable of competing for nutrients and resources when they inhabit the same ecological niche as pathogens. Similarly, the study carried out by Zeng et al. [46] confirmed that the strong antagonistic activity of the rice endophyte Streptomyces sporocinereus OsiSh-2 towards Magnaporthe oryzae was associated with the competition for iron. More examples of endophytes used in biological control of plant diseases are shown in Table 1.
Competition with pathogenic microorganisms for niche and nutrition, namely niche exclusion, is a promising mechanism for the use of endophytes in plant disease control [47]. Even so, limitations could be encountered, and it might be ineffective when disease is caused by a high presence of pathogens, which was further buttressed in the study of Lahlali [48]. Strategies to solve this problem include (i) prior and extensive inoculation of endophytes to host plants through various approaches, such as seed coating, soil drench, root dip, and foliar spray application and (ii) a combination of suitable endophytes or microbes instead of individual ones [49,50].

Table 1.
Endophytes and their metabolites used in biological control of plant diseases.
Table 1.
Endophytes and their metabolites used in biological control of plant diseases.
| Metabolites/Compounds | Endophytic Strain | Host Plant/Isolated From | Properties/Mechanisms | References |
|---|---|---|---|---|
| ND | Ten endophytes functionally annotated | Pine | Niche exclusion | [51] |
| ND | Bacillus cereus BCM2, B. cereus SZ5, B. altitudinis CCM7 etc. | Strawberry, persimmon, chili, tomato | Niche exclusion | [52] |
| ND | Pyrenochaeta cava, M. nivalis var. neglecta | Elm | Niche exclusion | [53] |
| ND | Burkholderia gladioli E39CS3 | Crocus sativus Linn. | Inducing plant resistance | [54] |
| ZhiNengCong, ZNC | Paecilomyces Variotii SJ1 | Tobacco | Inducing plant resistance | [55] |
| ND | Bacillus sp. 2P2 | Tomato | Inducing plant resistance | [56] |
| Antimicrobial compounds, cell wall degradation enzymes, etc. | Streptomyces albidoflavus OsiLf-2 | Rice | Inducing plant resistance; lytic enzyme activity; antimicrobial activity | [57] |
| Hydrolytic enzymes, protease, siderophore, IAA, etc. | Klebsiella pneumoniae HR1 | Vigna mungo L. | Inducing plant resistance; lytic enzyme activity; promoting plant growth | [58] |
| Antimicrobial compounds | Pseudomonas viridiflava | Canola | Antimicrobial activity; inducing plant resistance | [59] |
| Antifungal compounds | Pseudomonas aeruginosa H40, Stenotrophomonas maltophila H8, Bacillus subtilis H18 | P. sativum, B. oleracea, C. annuum | Antimicrobial activity; inducing plant resistance | [60] |
| Antimicrobial compounds | Penicillium, Colletotrichum, Diaporthe, Daldinia, Alternaria, Didymella | Zanthoxylum simulans Hance | Antimicrobial activity | [61] |
| Eugenol, myristaldehyde, lauric acid, caprylic acid | Neopestalotiopsis sp., Diaporthe sp. | Cinnamomum loureiroi | Antimicrobial activity | [62] |
| Ethyl acetate, chloroform, methanol | Proteus mirabilis, Bacillus | Moringa peregrina | Antimicrobial activity | [63] |
| Erythromycin, ketoconazole, fluconazole, chloramphenicol etc. | Streptomyces olivaceus BPSAC77, Streptomyces sp. BPSAC121 etc. | Rhynchotoechum ellipticum | Antimicrobial activity | [64] |
| Volatile substances | Pseudomonas putida BP25 | Black pepper | Antimicrobial activity | [65] |
| Antifungal compounds | Phomopis cassia | Cassia spectabilis | Antimicrobial activity | [66] |
| Lipases, proteases, amylases, cellulases, pectinases, xylanases | Pseudomonas, Micrococcus, Paenibacillus, Streptococcus, Curtobacterium, Chryseobacterium, Bacillus | Some poaceae plants | Lytic enzyme activity | [67] |
| Amylase, protease, cellulase, pectinase, lipase | Doritis pulcherrima, Dendrobiuma phyllum, Dendrobium anosmum, Ascocentrum curvifolium, Aerides falcata | Thai orchids | Lytic enzyme activity | [68] |
| Proteolytic enzymes, cellulase | Phoma putaminum, Penicillium, Myrmecridium schulzeri | Bauhinia forficata | Lytic enzyme activity | [69] |
| Chitinase | Streptomyces sp. P4 | Sweet pea | Lytic enzyme activity | [70] |
| IAA | Staphylococcus pasteuri MBL_B3; Kocuria sp. MBL_B19 etc. | Corchorus olitorius | Promoting plant growth | [71] |
| Siderophore, IAA | Ralstonia sp. | Poaceae | Promoting plant growth | [72] |
| Siderophore, IAA, gibberellic acid | Streptomyces spp. | Terfezia leonis Tul | Promoting plant growth | [73] |
| Gibberellins | Bacillus amyloliquefaciens RWL-1 | Rice seeds | Promoting plant growth | [74] |
| Indol acetic acid | B. subtilis NA-108 | Fragaria ananassa | Promoting plant growth | [75] |
ND: no data.
3.2. Induction of Plant Disease Resistance
Two critical patterns of plants in response to attacks of parasites or pathogens are induced systematic resistance (ISR), which is generally dependent on jasmonic acid (JA) and ethylene (ET) signaling, and systemic acquired resistance (SAR) that is commonly dependent on salicylic acid (SA) signaling. The JA/ET pathway mainly controls resistance to necrotrophic pathogens, while resistance to biotrophic pathogens is mediated by the SA pathway [76,77,78]. As described by Kloepper and Ryu, ISR mediated by some endophytes could be dependent on the SA pathway instead of the JA or ET pathways, and the signaling crosstalk between these pathways indicates that ISR is not fully separated from SAR [79]. Other plant hormones such as methyl jasmonate (MeJA) and brassinosteroids (BRs) are also involved in the plant defense system [76,80].
It has attracted a lot of interest and attention that endophytes control plant diseases through inducing plant resistance. Endophyte-inoculated plants usually have a stronger immunity to pathogens compared to uninfected plants. Utilization of endophytes in a certain part of the plant leads to a significant reduction in the disease index even though pathogens are infected in different parts of the host. The fungal endophytes Penicillium citrinum LWL4 and Aspergillus terreus LWL5 of the sunflower family (Helianthus annuus L.) visibly enhanced host resistance to stem rot caused by Sclerotium rolfsii through the SA and JA signaling networks [81]. The bacterial endophyte Azospirillum sp. B510, isolated from rice (Oryza sativa cv. Nipponbare), triggered host systemic resistance against rice blast disease and bacterial blight [82]. Similarly, the Bacillus strain YC7010T isolated from rice by Chung et al., was developed as a novel BCA against rice bacterial blight [83]. Representative examples of endophytic strains in eliciting plant resistance are shown in Table 1.
Endophytes play an important role in plant disease control, because they can trigger plant resistance through upregulation of defense-related genes (pathogenesis-related genes such as PR1, PR2, and PR3, phenylpropanoid pathway genes such as chalcone synthase CHS, and phenylalanine ammonia-lyase gene PAL involved in phytoalexin biosynthesis, etc.), modifications of plant cell walls (callose deposition, stomata closure, etc.), and enhanced levels of defense-related antioxidant enzymes [84,85,86]. High levels of polyphenol oxidase (PPO), peroxidase, and phenylalanine ammonia lyase (PAL) were observed in tomato plants treated with two endophytic strains that activated systemic resistance in the host plant against Fusarium wilt [87].
Moreover, as mentioned in some reports, metabolites of endophytes can not only inhibit pathogenic microorganisms but also induce the expression of plant defense-related genes and activate the host immune response [7,88]. ZhiNengCong (ZNC), extracted from endophytic fungi Paecilomyces Variotii SJ1, has been proven to be an ultrahigh activity immune inducer in tobacco in a recent study [55]. More metabolites from endophytes are yet to be exploited as elicitors, and these bioactive components could provide a promising alternative resource for plant disease management. Attention has already been given to the secondary metabolites of certain non-endophytic microbes, since they have been confirmed as elicitors of plant resistance. For example, Bacillus amyloliquefaciens SQR9 isolated from cucumber rhizosphere produced secondary metabolites, such as fengycin, surfactin, and 2,3-butanediol, and could elicit systemic resistance in Arabidopsis through different signaling pathways [89]. C15 surfactin A, the major secondary metabolite of Bacillus velezensis HN-2 isolated from soil, not only showed strong antibacterial activity against Xanthomonas oryzae pv. Oryzae (Xoo) but also effectively initiated rice resistance to pathogens [90]. Another interesting example is the production of glycoprotein GP-1 obtained from Streptomyces sp. ZX01 isolated from soil that triggered early plant immune responses in tobacco [91]. These findings are critical for the application of metabolites from endophytes in stimulating plant resistance.
3.3. Antimicrobial Properties of Metabolites from Endophytes
Endophytes are well known for their potential to produce a large number of secondary metabolites with antifungal and antibacterial properties that can directly suppress pathogens [92]. In 1993, Stierle et al., isolated an endophytic fungus from Pacific yew-Taxus brevifolia and found that it could produce the same substance as the host, thus inspiring researchers to find biologically active ingredients from plant endophytes [93]. The lipopeptide antibacterial components derived from the fermentation broth of the Chinese medicinal Ginkgo biloba endophytic strain Bacillus amyloliquefaciens CGMCC 5569 inhibited growth of Lasiodiplodia rubropurpurea and L. theobromae [94]. The results obtained by Mousa et al. [95] indicated that an endophytic fungus, WF4, isolated from the finger millet crop exhibited notable antagonist activity towards F. graminearum because of the production of four antifungal compounds. Endophytic actinomycetes are among the most extensively studied “producers” of antibacterial substances. Four major compounds with antibacterial activity against Staphylococcus aureus were obtained from the endophytic actinomycete strain LGMB491 (closely related to Aeromicrobium ponti) that was isolated from Vochysia divergens, a medicinal plant in Pantanal, Brazil [96]. Endophytic Bacillus and Streptomyces isolated from diverse environments are exploited as the most abundant antimicrobial compounds producers among Gram-positive bacteria [97]. Endophytic Bacillus has also been reported to produce surfactins, iturins, and fengycins [98]. The bioactive metabolites produced by endophytes are shown in Table 1.
Over the past decades, different bioactive metabolites such as terpenoids, flavonoids, peptides, and alkaloids have been exploited from endophytes for their properties to inhibit phytopathogens. Altersetin, a kind of alkaloid produced by endophyte Alternaria spp., displayed strong potential in inhibiting many pathogenic gram-positive bacteria [99]. Volatile substances (methanethiol, ketones, etc.) produced by Panax notoginseng-associated endophytic T. gamsii YIM PH30019 prevented the growth of pathogenic fungi [100]. The crosstalk between homologous gene clusters of endophytes and host plants could lead to production of novel metabolites; yet, the mechanisms involved still need to be identified [101]. Studies are still ongoing to explore endophytes and their metabolites for possible use in plant disease management.
3.4. Lytic Enzyme Activity of Metabolites from Endophytes
Endophytes are isolated from the seeds, roots, stems, leaves, or other tissues of the host plants. They produce various enzymes such as chitinases, cellulases, β-1, 3- glucanases, pectinases, glucanases, and proteases [102,103,104]. These enzymes can degrade the cell wall of pathogens or inhibit spore germination. This is an effective way to suppress phytopathogens and enable the host to obtain protection from biotic stress. In the study of Zhu and She, 45 endophytic bacteria were isolated from the Ammodendron bifolium plant, 40% of which showed significant activities for amylase and cellulose production, 13.3% and 53.3% of which exhibited protease and lipase activity, respectively [105]. An endophytic isolate of Actinoplanes missouriensis was reported to secret large amounts of chitinase, and inhibited the growth of the pathogen Plectosporium tabacinum by degrading the hyphae and causing plasmolysis and cell wall lysis [106]. Streptomyces produced several lytic enzymes that acted as antagonizing agents in M. perniciosa in cacao Witches’ broom disease [107]. More examples of endophytic microbes reported to have lytic enzyme production are presented in Table 1.
The chitinase genes of some biocontrol bacteria have been cloned and introduced into plants to improve host disease resistance [108]. Achari et al., reported that an eggplant (Solanum melongena L.) endophyte Bacillus cereus XB177R was able to produce endoglucanase and pectinase enzymes to facilitate colonization in host plants [109]. Even though many endophytes have been isolated and identified for increasing host resistance against phytopathogens because of their ability to produce metabolites with lytic enzyme activity, it remains unknown whether the lytic enzymes act as elicitors of plant systemic resistance in controlling pathogens. Mostly, these enzymes may present much stronger antagonistic activities when they are integrated with other mechanisms. Metabolites derived from endophytes are good alternative sources for numerous extracellular hydrolytic enzymes, and microbial production of enzymes is an exciting prospect for building sustainable agriculture systems [110].
3.5. Promotion of Plant Growth by Metabolites from Endophytes
Resistance of host plants to different stresses can be enhanced along with the promotion of plant growth and is recognized as one of the strategies employed by plants in response to pathogen attacks [111]. It is well known that endophytes and their metabolites act as promotors of plant growth. On one hand, endophytes substantially improve plant absorption and utilization of nutrients, such as nitrogen (N), phosphorus (P), and potassium (K). In particular, endophytic diazotrophic bacteria associated with gramineous crops convert atmospheric nitrogen into ammonia by nitrogen fixation; in this way, they stimulate host growth and disease resistance. Studies showed that corn seedlings inoculated with Paenibacillus polymyxa P2b-2R from lodgepole pine seedlings obtained 30% of foliar nitrogen from the atmosphere, and the seedling length increased by 52% [112]. On the other hand, endophytes have also been reported to accelerate plant growth by producing substances such as auxin, ethylene, gibberellin, and cytokinin. Endophytic bacteria of Staphylococcus, Azotobacter, and Azospirillum produce secondary metabolites, including a variety of plant hormones, which can regulate and promote the growth and development of host plants [113]. Shan et al. [114] isolated a total of 46 actinomycetes from tissue samples of 15 tea cultivars, the majority of which were able to produce IAA. More growth-promoting endophytes have been found to be common in different plants [115,116,117,118] (Table 1). Therefore, it is believed that the plant growth promotion initiated by endophytes can indirectly protect host plants against pathogens.
In general, growth regulators and phytohormones are commonly extracted from plants or chemically synthesized in the agriculture industry. Microbial fermentation has been considered a more convenient and powerful tool to increase productivity and minimize production costs of plant metabolites. Even through numerous reports have shown successes in the production of plant metabolites by endophytes in vitro, few products have been commercially produced on a mass scale. At the same time, we must address the issue of whether bioactive metabolites are produced by the endophytes or by host plants. We lack a complete understanding of plant–endophyte interaction mechanisms. When endophytes are cultured in vitro isolated from the host, the native plant–endophyte network is correspondingly disrupted. Elaborations of the relative roles of the “host plants” and the “micro-guests” in the production of certain metabolites need to be studied in greater depth.
4. Application of Endophytes and Their Metabolites as Novel BCAs in Agriculture
Endophytes that colonize within a plant without causing symptoms of diseases and also have the ability to produce abundant bioactive metabolites are potential biological control agents [119]. They are appropriate substitutes for agrochemicals to minimize the impact on the environment and to ensure a sustainable agricultural system. Many studies on the exploitation of endophytes for biological control of plant diseases have been carried out in recent years. In a previous study, using Epichloë typhina isolated from Timothy-grass reduced the disease incidence of host plants caused by Cladosporium phlei; this was the first record of fungal endophytes used for the biocontrol of foliar cereal diseases [120]. It is interesting to note that a certain endophyte has the potential to become a biological control agent (BCA), while the same species might also promote growth and improve stress tolerance of the host plants [119]. Other microorganisms such as Bacillus spp., Enterobacter spp., Pantoea spp., Pseudomonas spp., and Streptomyces spp. have recently been manipulated and applied for various biocontrol programs in agriculture [119,121,122]. There is still not yet a trend to exploit and commercialize endophytes and their metabolites as BACs in modern agriculture.
The application of plant endophytes in agriculture can be achieved by microbial pesticides, that is, mass propagation of living microbes and processing into preparations for use. These novel BCAs are derived from plants, act on plants, and do not contain the toxic ingredients of traditional pesticides; so, they are environmentally friendly [123]. Many factors in the field and a plant’s micro-ecological environment can affect the performance of endophytic microbe resistance to stress and diseases. Some endophytes have been found to be highly host-specific and can only adapt to the specific tissues of specific plants [124,125,126]. Moreover, the infection rate of endophytes to non-natural host plants is also a critical issue when applied to plant disease management. Geographical factors cause physiological changes in plants, resulting in significant changes in the colonization and diversity of endophytes within the same plant in different sampling sites [127]. Therefore, the ecological, pathological, and other factors (e.g., complex life cycles of pathogens, inter-species variation/diversity/interrelationship) must be considered in the practical application of endophytic pesticides [128,129,130].
Endophytes are a rich source of diverse and functional metabolites [131]. On the one hand, natural active substances such as plant hormones, antibiotics, and alkaloids can be used to resist diseases and promote growth of plants. After separation and extraction, these compounds can be directly applied to agricultural production. On the other hand, the secondary metabolites of plant endophytes can be used as lead compounds of green pesticides (biopesticides). A recent paper noted that 51% of bioactive substances isolated from endophyte fungi were previously undiscovered, while only 38% of those isolated from soil microorganisms were [132]. Accordingly, exhaustive exploitation for endophyte metabolites with biopesticide activity is a promising direction to develop new biopesticides.
Plant endophytes in different extreme environments such as cold, drought, and high-latitude often produce secondary metabolites with special physiological functions, which are of great value to agriculture [6,133]. Therefore, specific active substances can be obtained by screening endophytes from special ecological environments. Researchers have conducted significant work on the isolation, extraction, and identification of these bioresources. At present, reports of lead compounds structures are relatively limited, and it is believed that there will be breakthrough progress in the near future.
What concerns us most is the potential toxicity of these expected products to plants, animals, and humans. In fact, both plants and animals are eukaryotes. Endophytes belong to the core microbiome in plants, as they establish a symbiotic relationship with the host. Studies have shown that most antimicrobial agents produced by endophytes are toxic to phytopathogens, eco-friendly, and do not harm plants and the human [21,134]. Plants automatically act as selection systems for nontoxic biologically active substances. Overall, the use of plant endophytes and their metabolites as sources of novel BCAs in plant disease management has obvious advantages and great potential.
5. Multi-Omics Approaches for Mining Bioactive Metabolites from Endophytes
With the progress in high-throughput sequencing and systems biology, multi-omics, encompassing genomics, transcriptomics, proteomics, and metabolomics, are becoming more and more essential in plant–microbe interaction research [135,136,137]. Genomics deals with the entire genome sequences, while transcriptomics studies RNA and gene expression patterns [138,139]. Proteomics examines the structures, functions, and interactions of dynamic proteins, while metabolomics aims to understand the synthesis, decomposition, and transformation of metabolites in a particular organism [140,141,142].
Genome analysis is critically important for identification and characterization of the genes involved in the beneficial plant–endophyte interaction. It has revealed the genes responsible for their colonizing preferences within plants as well as the synthesis of various bioactive compounds, for example, genes for plant hormones and antibiotics production, nitrogen fixation, and nutrition acquisition (K, P, Fe, etc.) [143,144]. Genome analysis of Piriformospora indica revealed its potential as a bioactive agent for plant production [145]. Complete genome sequences of many endophytes have been summarized by Kaul et al. [146]. Multigenome analysis or genome comparison analysis has provided a new tool to unravel some of the mysteries, namely, closely related endophytic species perform different roles in host plants, and the different metabolite gene clusters illustrate the diversity of endophyte metabolites to a certain extent [147].
Genomics provides the genomic sequencing information, while transcriptomics links gene functions with specific conditions. The dynamics and regulation of actively transcribed genes can be achieved by transcriptomic analysis. For example, the sequencing of mRNA is a useful approach to understand the different responses of plants in the presence and absence of endophytes [148]. Proteomics is aimed at the analysis of entire proteins from a tissue or an organism under a specific condition [141]. Similarly, total protein content of endophyte-free and endophyte-inhabited plants can be extracted and assessed to investigate the specific proteins involved in the relationship between the two groups. As shown by Lery et al., 78 differentially expressed proteins related to the endophytic Gluconacetobacter-sugarcane interaction were identified by proteomic analysis based on mass spectrometry [149]. Yuan et al., employed transcriptomics and proteomics of the host Atractylodes lancea inoculated with and without endophyte Gilmaniella sp. AL12 to decode the effect of endophyte treatment [150].
Metabolomics, widely used in bioanalytical procedures, enables the identification and quantification of the metabolites in a sample. It can complement transcriptomic and proteomic data, thus facilitating a better understanding of host phenotypic features and unveiling the mechanisms of plant–microbe interactions [151]. Metabolomics are often classified into two groups: untargeted metabolomics, a comprehensive analysis of numerous known/unknown metabolites in the sample, and targeted metabolomics, the measurement of the specific metabolites in the sample [152]. Untargeted metabolomics can be performed to discover novel compounds, and targeted metabolomics is able to validate the results of untargeted metabolomics [153]. As described by Liu et al., the high-performance liquid chromatography-mass spectrometry (HPLC-MS) based analysis showed that 88 secondary metabolites including 70 novel natural products were produced by Pestalotiopsis fici isolated from healthy Camellia sinensis (Theaceae) [154]. Other analytical platforms for metabolomics such as liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) have been recently reviewed by Segers et al. [155].
Integrated analysis of transcriptomics and metabolomics is able to analyze both the genes and metabolites that are differentially expressed along a time curve in exploring the causal relationships between genes and metabolites. The key genes and metabolites and key signaling and metabolic pathways can be found through bioinformatic tools such as functional annotation and pathway enrichment, leading to an improved understanding of the physiological and molecular mechanisms [156,157]. In recent years, transcriptomics–metabolomics combined analysis has been widely used in studies of rice, potato, tobacco, and other plants [158,159,160,161,162]. Sade et al. [163] conducted a study on susceptible and resistant tomato plants to determining the differences in the regulated pathways and the levels of specialized metabolites between these two samples in response to tomato yellow leaf curl virus invasion using both comparative transcriptomics and metabolomics. In another example, transcriptomic and metabolomic analyses were performed by Yang et al., to detect the differentially expressed genes (DEGs) and metabolites of anthracnose-resistant and susceptible varieties of Camellia oleifera, suggesting the important role of flavonoid biosynthesis in the defense against anthracnose [164]. As mentioned in the report of Kaul et al. [146], differential expression analysis regarding endophyte-free and endophyte-inoculated plants can be helpful to fully understand the mechanisms of plant–endophyte interactions and endophyte-mediated disease resistance.
In terms of plant–endophyte research, omics technology and multi-omics joint analysis will definitely continue to play irreplaceable roles not only in the study of host growth and stress tolerance but also in discovery of bioactive metabolites (Figure 2). However, multi-omics data analysis still faces great challenges. How to better correlate the data of various omics and mine more useful information remains one of the main problems that needs to be continuously explored [165].
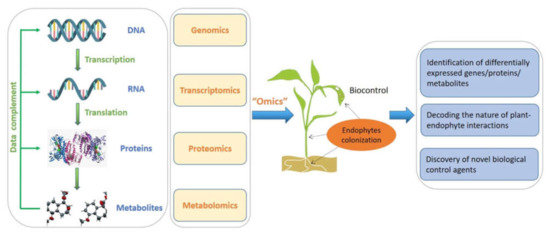
Figure 2.
Multi-omics approaches for the research of endophytes and their metabolites in plant disease biocontrol.
6. Conclusions and Future Prospects
In the past three decades, remarkable progress has been made in research on plant disease resistance mechanisms and plant–microbe interactions. Endophytes, colonizing plant tissues, are regarded as naturally occurring agents in plant disease suppression. Most of their success is attributed to the production of a vast array of metabolites. These metabolites have an abundance of biological activities, making them a promising resource collection, and they play an increasingly important role in different fields. Multi-omics joint analysis could achieve data complementation from genes to metabolites; thus, it has been used extensively as an approach to comprehensively discover physiological and molecular mechanisms in plant disease resistance. In this review, we reported the multifunctions of endophytes and their metabolites in the biocontrol of plants. We provided extensive evidence and recent examples and described the importance of these bioresources for future agricultural development. As we have outlined, one of the mechanisms of endophytes in plant protection is the elicitation of plant resistance. In addition, various metabolites from non-endophytic microorganisms have been identified as elicitors of plant resistance. However, the current research on endophyte metabolites in biological control is mainly focused on antibacterial, hydrolase activities, and growth-promoting value, and the reports on the induction of plant resistance are relatively limited. So we propose using multi-omics approaches to isolate and identify more metabolites from endophytes, especially as plant resistance inducers, for increasing plant fitness and crop yields.
Biological control agents are reliable and environmentally friendly in plant disease management and crucial for sustainable agriculture. Using endophytes and their metabolites for plant protection has many advantages over chemical pesticides and conventional bioformulations. The metabolites of plant endophytes contain a variety of bioactive ingredients that enhance the host defense against pathogens, rather than simple toxic properties. Therefore, application of one or several natural active substances as the lead compound is among the most promising approaches for green pesticide discovery in the future. However, in order to truly achieve their large-scale commercial production and application, we still have some challenges to overcome, such as the following:
- (i)
- Many endophytes are uncultured and unidentified.
- (ii)
- There are no available databases for endophytes and their metabolites.
- (iii)
- Knowledge of the molecular mechanisms of plant–endophyte interactions is limited.
- (iv)
- Biocontrol effects of endophytes are not definitely stable in field trials.
- (v)
- Yield of metabolites by fermentation is low.
It is necessary to elevate the exploitation of endophytes and their metabolites in the biological control of plant diseases to the multi-omics level as a promising research frontier.
Author Contributions
Writing—original draft preparation, Y.X.; writing—review and editing, J.L., C.C. and X.M.; visualization, Q.T., Y.H. and Z.W.; supervision, J.Y. and G.Z.; project administration, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 31971661), Natural Science Foundation of Hunan Province, China (Grant No. 2021JJ31145), and Hunan Province University Innovation Platform Open Fund Project (Grant No. 20K145).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hasan, S.; Anand, S. Biopotential of microbial antagonists against soilborne fungal plant pathogens. Int. J. Agric. Food Sci. Technol. 2013, 4, 37–39. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Dutta, D.; Puzari, K.C.; Gogoi, R.; Dutta, P. Endophytes: Exploitation as a tool in plant protection. Braz. Arch. Biol. Technol. 2014, 57, 621–629. [Google Scholar] [CrossRef]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs tree pathogens and pests: Can they be used as biological control agents to improve tree health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [Green Version]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Khan, Z.; Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil 2009, 322, 197–207. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.; Glick, B. Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J. Appl. Microbiol. 2012, 113, 1139–1144. [Google Scholar] [CrossRef]
- Ullah, A.; Nisar, M.; Ali, H.; Hazrat, A.; Hayat, K.; Keerio, A.A.; Ihsan, M.; Laiq, M.; Ullah, S.; Fahad, S.; et al. Drought tolerance improvement in plants: An endophytic bacterial approach. Appl. Microbiol. Biotechnol. 2019, 103, 7385–7397. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ning, Q.; Yang, Y.; Liu, Y.; Niu, S.; Hu, X.; Pan, H.; Bu, Z.; Chen, N.; Guo, J.; et al. Endophytic Streptomyces hygroscopicus OsiSh-2-Mediated Balancing between Growth and Disease Resistance in Host Rice. mBio 2021, 12, e01566-21. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, N.; Kloepper, J.W.; Quadt-Hallman, A.; Tuzun, S. Induction of Defense-Related Ultrastructural Modifications in Pea Root Tissues Inoculated with Endophytic Bacteria. Plant Physiol. 1996, 112, 919–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Plata, J.S.; Ormeño-Moncalvillo, S.; Gil, L.; Calcerrada, J.R.; Martín, J. Endophyte inoculation enhances Ulmus minor resistance to Dutch elm disease. Fungal Ecol. 2020, 50, 101024. [Google Scholar] [CrossRef]
- Jacob, J.; Krishnan, G.V.; Thankappan, D.; Amma, D.K.B.N.S. 4-Endophytic bacterial strains induced systemic resistance in agriculturally important crop plants. In Microbial Endophytes; Kumar, A., Radhakrishnan, E.K., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 75–105. [Google Scholar] [CrossRef]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Biotechnological potential of plant-associated endophytic fungi: Hope versus hype. Trends Biotechnol. 2014, 32, 297–303. [Google Scholar] [CrossRef]
- Joseph, B.; Priya, R.M. Bioactive Compounds from Endophytes and their Potential in Pharmaceutical Effect: A Review. Am. J. Biochem. Mol. Biol. 2011, 1, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, D.; Kharwar, R.; White, J.; Gond, S. Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2018, 103, 9–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bary, A.D. Morphologie und Physiologie der Pilze, Flechten und Myxomyceten; Wilhelm Engelmann: Leipzig, Germany, 1866. [Google Scholar] [CrossRef]
- Carroll, G.C. The biology of endophytism in plants with particular reference to woody perennials. In Microbiology of the Phyllosphere; Cambridge University Press: Cambridge, UK, 1986; pp. 205–222. [Google Scholar]
- Petrini, O. Fungal Endophytes of Tree Leaves. In Microbial Ecology of Leaves; Springer: New York, NY, USA, 1991; pp. 179–2197. [Google Scholar]
- Stone, J.K.; Bacon, C.W.; White, J. An overview of endophytic microbes: Endophytism defined. Microb. Endophytes 2000, 1, 29–33. [Google Scholar]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Strobel, G.; Ford, E.; Worapong, J.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Fung, P.C.; Chau, R.M.W. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 179–183. [Google Scholar] [CrossRef]
- Prakash, V.; Rana, S.; Sagar, A. Taxomyces andreanae: A source of anticancer drug. Int. J. Bot. Stud 2016, 1, 2455–2541X. [Google Scholar]
- Liang, Z.; Zhang, J.; Zhang, X.; Li, J.; Zhang, X.; Zhao, C. Endophytic Fungus from Sinopodophyllum emodi (Wall.) Ying that Produces Podophyllotoxin. J. Chromatogr. Sci. 2016, 54, 175–178. [Google Scholar]
- Sun, H.-q.; He, Y.; Xiao, Q.; Ye, R.; Tian, Y.J.A.J.o.M.R. Isolation, characterization, and antimicrobial activity of endophytic bacteria from Polygonum cuspidatum. Afr. J. Microbiol. Res. 2013, 7, 1496–1504. [Google Scholar]
- Liu, L.-H.; Yuan, T.; Zhang, J.-Y.; Tang, G.-X.; Lü, H.; Zhao, H.-M.; Li, H.; Li, Y.-W.; Mo, C.-H.; Tan, Z.-Y.; et al. Diversity of endophytic bacteria in wild rice (Oryza meridionalis) and potential for promoting plant growth and degrading phthalates. Sci. Total Environ. 2021, 806, 150310. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xing, K.; Jiang, J.-H.; Xu, L.-H.; Li, W.-J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2010, 89, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.M.; Remali, J.; Nasrom, M.N.; Ishak, S.A.; Baba, M.S.; Jalil, J. Bioactive compounds fractionated from endophyte Streptomyces SUK 08 with promising ex-vivo antimalarial activity. Asian Pac. J. Trop. Biomed. 2017, 7, 1062–1066. [Google Scholar] [CrossRef]
- Widiantini, F.; Herdiansyah, A.; Yulia, E. Biocontrol Potential of Endophytic Bacteria Isolated from Healthy Rice Plant against Rice Blast Disease (Pyricularia oryzae Cav.). KnE Life Sci. 2017, 2, 287–295. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Macaya-Sanz, D.; Witzell, J.; Martín, J.A. Enhancement of Populus alba tolerance to Venturia tremulae upon inoculation with endophytes showing in vitro biocontrol potential. Eur. J. Plant Pathol. 2018, 153, 1031–1042. [Google Scholar] [CrossRef]
- Romeralo, C.; Witzell, J.; Romeralo-Tapia, R.; Botella, L.; Diez, J.J. Antagonistic activity of fungal endophyte filtrates against Gremmeniella abietina infections on Aleppo pine seedlings. Eur. J. Plant Pathol. 2015, 143, 691–704. [Google Scholar] [CrossRef]
- Romeralo, C.; Santamaría, O.; Pando, V.; Diez, J. Fungal endophytes reduce necrosis length produced by Gremmeniella abietina in Pinus halepensis seedlings. Biol. Control 2015, 80, 30–39. [Google Scholar] [CrossRef]
- Schlegel, M.; Dubach, V.; Von Buol, L.; Sieber, T.N. Effects of endophytic fungi on the ash dieback pathogen. FEMS Microbiol. Ecol. 2016, 92, fiw142. [Google Scholar] [CrossRef]
- Latz, M.; Jensen, B.; Collinge, D.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Bacon, C.W.; Yates, I.E.; Hinton, D.M.; Meredith, F. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001, 109, 325–332. [Google Scholar] [CrossRef]
- Duijff, B.J.; Meijer, J.W.; Bakker, P.A.H.M.; Schippers, B. Siderophore-mediated competition for iron and induced resistance in the suppression of fusarium wilt of carnation by fluorescent Pseudomonas spp. Eur. J. Plant Pathol. 1993, 99, 277–289. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, T.; Cao, L.; Tong, C.; Zhang, X.; Luo, D.; Han, S.; Pang, P.; Fu, W.; Yan, J.; et al. The Role of Iron Competition in the Antagonistic Action of the Rice Endophyte Streptomyces sporocinereus OsiSh-2 Against the Pathogen Magnaporthe oryzae. Microb. Ecol. 2018, 76, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Liarzi, O.; Ezra, D. Endophyte-Mediated Biocontrol of Herbaceous and Non-herbaceous Plants. In Advances in Endophytic Research; Springer: Berlin/Heidelberg, Germany, 2013; pp. 335–369. [Google Scholar] [CrossRef]
- Lahlali, R.; McGregor, L.; Song, T.; Gossen, B.D.; Narisawa, K.; Peng, G. Heteroconium chaetospira Induces Resistance to Clubroot via Upregulation of Host Genes Involved in Jasmonic Acid, Ethylene, and Auxin Biosynthesis. PLoS ONE 2014, 9, e94144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.R. Biocontrol and Bioremediation: Two Areas of Endophytic Research Which Hold Great Promise. In Advances in Endophytic Research; Springer: Berlin/Heidelberg, Germany, 2014; pp. 257–282. [Google Scholar] [CrossRef]
- Oliva, J.; Ridley, M.; Redondo, M.A.; Caballol, M. Competitive exclusion amongst endophytes determines shoot blight severity on pine. Funct. Ecol. 2020, 35, 239–254. [Google Scholar] [CrossRef]
- Hu, H.-J.; Chen, Y.-L.; Wang, Y.-F.; Tang, Y.-Y.; Chen, S.-L.; Yan, S.-Z. Endophytic Bacillus cereus Effectively Controls Meloidogyne incognita on Tomato Plants Through Rapid Rhizosphere Occupation and Repellent Action. Plant Dis. 2017, 101, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Blumenstein, K.; Albrectsen, B.R.; Martín, J.A.; Hultberg, M.; Sieber, T.N.; Helander, M.; Witzell, J. Nutritional niche overlap potentiates the use of endophytes in biocontrol of a tree disease. BioControl 2015, 60, 655–667. [Google Scholar] [CrossRef]
- Ahmad, T.; Bashir, A.; Farooq, S.; Riyaz-Ul-Hassan, S. Burkholderia gladioli E39CS3, an endophyte of Crocus sativus Linn., induces host resistance against corm-rot caused by Fusarium oxysporum. J. Appl. Microbiol. 2021, 132, 495–508. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, A.; Wang, Q.; Song, Y.; Zhang, M.; Ding, X.; Li, Y.; Geng, Q.; Zhu, C. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020, 20, 169. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.K.; Singh, S.; Gupta, A.; Singh, U.B.; Brahmaprakash, G.; Saxena, A.K. Antagonistic potential of bacterial endophytes and induction of systemic resistance against collar rot pathogen Sclerotium rolfsii in tomato. Biol. Control 2019, 137, 104014. [Google Scholar] [CrossRef]
- Gao, Y.; Zeng, X.D.; Ren, B.; Zeng, J.R.; Xu, T.; Yang, Y.Z.; Hu, X.C.; Zhu, Z.Y.; Shi, L.M.; Zhou, G.Y.; et al. Antagonistic activity against rice blast disease and elicitation of host-defence response capability of an endophytic Streptomyces albidoflavus OsiLf-2. Plant Pathol. 2019, 69, 259–271. [Google Scholar] [CrossRef]
- Dey, S.; Dutta, P.; Majumdar, S. Biological Control of Macrophomina phaseolina in Vigna mungo L. by Endophytic Klebsiella pneumoniae HR1. Jordan J. Biol. Sci. 2019, 12, 219–227. [Google Scholar]
- Romero, F.M.; Rossi, F.R.; Gárriz, A.; Carrasco, P.; Ruíz, O.A. A Bacterial Endophyte from Apoplast Fluids Protects Canola Plants from Different Phytopathogens via Antibiosis and Induction of Host Resistance. Phytopathology 2019, 109, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, H.M.M.; Gomaa, N.M.; Essa, A.M.M. Application of endophytic bacteria for the biocontrol of Rhizoctonia solani (Cantharellales: Ceratobasidiaceae) damping-off disease in cotton seedlings. Biocontrol Sci. Technol. 2017, 27, 81–95. [Google Scholar] [CrossRef]
- Kuo, J.; Chang, C.-F.; Chi, W.-C. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Zanthoxylum simulans Hance. Folia Microbiol. 2021, 66, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Pripdeevech, P. Production of eugenol from fungal endophytes Neopestalotiopsis sp. and Diaporthe sp. isolated from Cinnamomum loureiroi leaves. PeerJ 2019, 7, e6427. [Google Scholar] [CrossRef] [Green Version]
- Aljuraifani, A.; Aldosary, S.; Ababutain, I. In Vitro Antimicrobial Activity of Endophytes, Isolated from Moringa peregrina Growing in Eastern Region of Saudi Arabia. Natl. Acad. Sci. Lett. 2018, 42, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Passari, A.; Mishra, V.K.; Singh, G.; Singh, P.; Kumar, B.; Gupta, V.K.; Sarma, R.K.; Saikia, R.; Donovan, A.O.; Singh, B.P. Insights into the functionality of endophytic actinobacteria with a focus on their biosynthetic potential and secondary metabolites production. Sci. Rep. 2017, 7, 11809. [Google Scholar] [CrossRef]
- Sheoran, N.; Nadakkakath, A.V.; Munjal, V.; Kundu, A.; Subaharan, K.; Venugopal, V.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol. Res. 2015, 173, 66–78. [Google Scholar] [CrossRef]
- Silva, G.H.; Teles, H.L.; Zanardi, L.M.; Young, M.C.M.; Eberlin, M.N.; Hadad, R.; Pfenning, L.H.; Costa-Neto, C.; Castro-Gamboa, I.; Bolzani, V.; et al. Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae). Phytochemistry 2006, 67, 1964–1969. [Google Scholar] [CrossRef]
- Dogan, G.; Taskin, B. Hydrolytic Enzymes Producing Bacterial Endophytes of Some Poaceae Plants. Pol. J. Microbiol. 2021, 70, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sopalun, K.; Iamtham, S. Isolation and screening of extracellular enzymatic activity of endophytic fungi isolated from Thai orchids. S. Afr. J. Bot. 2020, 134, 273–279. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Nascimento, C.C.; Barbosa, R.; Da Silva, D.C.; Svedese, V.M.; Silva-Nogueira, E.B.; Gomes, B.S.; Paiva, L.M.; Souza-Motta, C.M. Endophytic fungi from medicinal plant Bauhinia forficata: Diversity and biotechnological potential. Braz. J. Microbiol. 2015, 46, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Tang-Um, J. Chitinase production and antifungal potential of endophytic Streptomyces strain P4. Maejo Int. J. Sci. Technol. 2012, 6, 95–104. [Google Scholar] [CrossRef]
- Haidar, B.; Ferdous, M.; Fatema, B.; Ferdous, A.S.; Islam, M.R.; Khan, H. Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiol. Res. 2018, 208, 43–53. [Google Scholar] [CrossRef]
- Patel, J.K.; Archana, G. Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 2017, 417, 99–116. [Google Scholar] [CrossRef]
- Goudjal, Y.; Zamoum, M.; Meklat, A.; Sabaou, N.; Mathieu, F.; Zitouni, A. Plant-growth-promoting potential of endosymbiotic actinobacteria isolated from sand truffles (Terfezia leonis Tul.) of the Algerian Sahara. Ann. Microbiol. 2015, 66, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Pereira, G.V.D.M.; Magalhães, K.T.; Lorenzetii, E.R.; Souza, T.P.; Schwan, R.F. A Multiphasic Approach for the Identification of Endophytic Bacterial in Strawberry Fruit and their Potential for Plant Growth Promotion. Microb. Ecol. 2011, 63, 405–417. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone Crosstalk in Plant Disease and Defense: More Than Just JASMONATE-SALICYLATE Antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, S.; Van Wees, S.; Pieterse, C.M. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloepper, J.W.; Ryu, C.-M. Bacterial Endophytes as Elicitors of Induced Systemic Resistance. In Microbial Root Endophytes; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–52. [Google Scholar]
- Soler, A.; Marie-Alphonsine, P.-A.; Corbion, C.; Quénéhervé, P. Differential response of two pineapple cultivars (Ananas comosus (L.) Merr.) to SAR and ISR inducers against the nematode Rotylenchulus reniformis. Crop Prot. 2013, 54, 48–54. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.-M.; Kim, J.-G.; Lee, I.-J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Kusajima, M.; Shima, S.; Fujita, M.; Minamisawa, K.; Che, F.-S.; Yamakawa, H.; Nakashita, H. Involvement of ethylene signaling in Azospirillum sp. B510-induced disease resistance in rice. Biosci. Biotechnol. Biochem. 2018, 82, 1522–1526. [Google Scholar] [CrossRef]
- Chung, E.J.; Hossain, M.T.; Khan, A.; Kim, K.H.; Jeon, C.O.; Chung, Y.R. Bacillus oryzicola sp. nov., an Endophytic Bacterium Isolated from the Roots of Rice with Antimicrobial, Plant Growth Promoting, and Systemic Resistance Inducing Activities in Rice. Plant Pathol. J. 2015, 31, 152–164. [Google Scholar] [CrossRef]
- Howlader, P.; Bose, S.K.; Jia, X.; Zhang, C.; Wang, W.; Yin, H. Oligogalacturonides induce resistance in Arabidopsis thaliana by triggering salicylic acid and jasmonic acid pathways against Pst DC3000. Int. J. Biol. Macromol. 2020, 164, 4054–4064. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S.; Zervakis, G.; Ehaliotis, C.; Haralampidis, K.; Papadopoulou, K.K. Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J. Exp. Bot. 2007, 58, 3853–3864. [Google Scholar] [CrossRef] [Green Version]
- Boava, L.P.; Cristofani-Yaly, M.; Stuart, R.M.; Machado, M.A. Expression of defense-related genes in response to mechanical wounding and Phytophthora parasitica infection in Poncirus trifoliata and Citrus sunki. Physiol. Mol. Plant Pathol. 2011, 76, 119–125. [Google Scholar] [CrossRef]
- Akram, W.; Anjum, T. Quantitative changes in defense system of tomato induced by two strains of Bacillus against Fusarium wilt. Indian J. Fundam. Appl. Life Sci. 2011, 1, 14–18. [Google Scholar]
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballare, C.L.; Gundel, P.E. Epichloë Fungal Endophytes and Plant Defenses: Not Just Alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions with Plant Signaling Pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, P.; Wang, Y.; Tan, Z.; Liu, W.; Miao, W. Antibacterial activity and rice-induced resistance, mediated by C15surfactin A, in controlling rice disease caused by Xanthomonas oryzae pv. oryzae. Pestic. Biochem. Physiol. 2020, 169, 104669. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sun, Y.; Zhou, X.; Hao, X.; Wu, M.; Zhang, X.; Feng, J. A novel glycoprotein from Streptomyces sp. triggers early responses of plant defense. Pestic. Biochem. Physiol. 2020, 171, 104719. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural Products from Plant-Associated Microorganisms: Distribution, Structural Diversity, Bioactivity, and Implications of Their Occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, Z.; Qin, S.; Zhao, G.-H.; Feng, Y.-J.; Wei, L.-H.; Jiang, J.-H. Study of the anti-sapstain fungus activity of Bacillus amyloliquefaciens CGMCC 5569 associated with Ginkgo biloba and identification of its active components. Bioresour. Technol. 2012, 114, 536–541. [Google Scholar] [CrossRef]
- Mousa, W.K.; Schwan, A.; Davidson, J.; Strange, P.; Liu, H.; Zhou, T.; Auzanneau, F.-I.; Raizada, M.N. An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front. Microbiol. 2015, 6, 1157. [Google Scholar] [CrossRef] [Green Version]
- Gos, F.M.W.R.; Savi, D.C.; Shaaban, K.A.; Thorson, J.; Aluizio, R.; Possiede, Y.M.; Rohr, J.; Glienke, C. Antibacterial Activity of Endophytic Actinomycetes Isolated from the Medicinal Plant Vochysia divergens (Pantanal, Brazil). Front. Microbiol. 2017, 8, 1642. [Google Scholar] [CrossRef]
- Ek-Ramos, M.J.; Gomez-Flores, R.; Orozco-Flores, A.A.; Rodríguez-Padilla, C.; González-Ochoa, G.; Tamez-Guerra, P. Bioactive Products from Plant-Endophytic Gram-Positive Bacteria. Front. Microbiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Hardoim, P.R. Biologically Active Compounds from Bacterial Endophytes. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–31. [Google Scholar]
- Hellwig, V.; Grothe, T.; Mayer-Bartschmid, A.; Endermann, R.; Geschke, F.-U.; Henkel, T.; Stadler, M. Altersetin, a New Antibiotic from Cultures of Endophytic Alternaria spp. Taxonomy, Fermentation, Isolation, Structure Elucidation and Biological Activities. ChemInform 2003, 34, 881–892. [Google Scholar] [CrossRef]
- Chen, J.-L.; Sun, S.-Z.; Miao, C.-P.; Wu, K.; Chen, Y.-W.; Xu, L.-H.; Guan, H.-L.; Zhao, L.-X. Endophytic Trichoderma gamsii YIM PH30019: A promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng. J. Ginseng Res. 2015, 40, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the Chemical Cues of Other Species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Dai, C.; Liu, X. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Ben Abdallah, R.A.; Jabnoun-Khiareddine, H.; Daami-Remadi, M. Exploring the Beneficial Endophytic Microorganisms for Plant Growth Promotion and Crop Protection: Elucidation of Some Bioactive Secondary Metabolites Involved in Both Effects. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 319–352. [Google Scholar] [CrossRef]
- Rajulu, M.B.G.; Thirunavukkarasu, N.; Suryanarayanan, T.S.; Ravishankar, J.P.; El Gueddari, N.E.; Moerschbacher, B.M. Chitinolytic enzymes from endophytic fungi. Fungal Divers. 2010, 47, 43–53. [Google Scholar] [CrossRef]
- Zhu, Y.; She, X. Evaluation of the plant-growth-promoting abilities of endophytic bacteria from the psammophyte Ammodendron bifolium. Can. J. Microbiol. 2018, 64, 253–264. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A. An endophytic chitinase-producing isolate of Actinoplanes missouriensis, with potential for biological control of root rot of lupin caused by Plectosporium tabacinum. Aust. J. Bot. 2003, 51, 257–266. [Google Scholar] [CrossRef]
- Macagnan, D.; Romeiro, R.D.S.; Pomella, A.W.; Desouza, J.T. Production of lytic enzymes and siderophores, and inhibition of germination of basidiospores of Moniliophthora (ex Crinipellis) perniciosa by phylloplane actinomycetes. Biol. Control 2008, 47, 309–314. [Google Scholar] [CrossRef]
- Cook, R.J. Making greater use of introduced microorganisms for biological control of plant pathogens. Annu. Rev. Phytopathol. 1993, 31, 53–80. [Google Scholar] [CrossRef]
- Achari, G.A.; Ramesh, R. Colonization of Eggplant by Endophytic Bacteria Antagonistic to Ralstonia solanacearum, the Bacterial Wilt Pathogen. Proc. Natl. Acad. Sci. USA India Sect. B Boil. Sci. 2018, 89, 585–593. [Google Scholar] [CrossRef]
- Khan, A.L.; Shahzad, R.; Al-Harrasi, A.; Lee, I.-J. Endophytic Microbes: A Resource for Producing Extracellular Enzymes. In Endophytes: Crop Productivity and Protection; Springer: Cham, Switzerland, 2017; Volume 16, pp. 95–110. [Google Scholar] [CrossRef]
- Kuldau, G.; Bacon, C. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Seedling growth promotion and nitrogen fixation by a bacterial endophyte Paenibacillus polymyxa P2b-2R and its GFP derivative in corn in a long-term trial. Symbiosis 2016, 69, 123–129. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Shan, W.; Zhou, Y.; Liu, H.; Yu, X. Endophytic Actinomycetes from Tea Plants (Camellia sinensis): Isolation, Abundance, Antimicrobial, and Plant-Growth-Promoting Activities. BioMed Res. Int. 2018, 2018, 1470305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing Bacterial Endophytes for Promotion of Plant Growth and Biotechnological Applications: An Overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Ramakumar, A.; Bartels, D.; Battistoni, F.; Bekel, T.; Boch, J.; Böhm, M.; Friedrich, F.; Hurek, T.; Krause, L.; et al. Complete genome of the mutualistic, N2-fixing grass endophyte Azoarcus sp. strain BH72. Nat. Biotechnol. 2006, 24, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.; Cai, S.; Cai, W.; Cheng, Z.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Borah, A.; Thakur, D. Phylogenetic and Functional Characterization of Culturable Endophytic Actinobacteria Associated with Camellia spp. for Growth Promotion in Commercial Tea Cultivars. Front. Microbiol. 2020, 11, 318. [Google Scholar] [CrossRef] [Green Version]
- De Silva, N.I.; Brooks, S.; Lumyong, S.; Hyde, K.D. Use of endophytes as biocontrol agents. Fungal Biol. Rev. 2019, 33, 133–148. [Google Scholar] [CrossRef]
- O’Hanlon, K.A.; Knorr, K.; Jørgensen, L.N.; Nicolaisen, M.; Boelt, B. Exploring the potential of symbiotic fungal endophytes in cereal disease suppression. Biol. Control 2012, 63, 69–78. [Google Scholar] [CrossRef]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Larran, S.; Simon, M.R.; Moreno, M.V.; Siurana, M.S.; Perelló, A. Endophytes from wheat as biocontrol agents against tan spot disease. Biol. Control 2016, 92, 17–23. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest. Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidke, S.A.; Kl, R.K.; Ramakrishna, D.; Kiran, S.; Kosturkova, G.; Gokare, R.A. Current Understanding of Endophytes: Their Relevance, Importance, and Industrial Potentials. IOSR J. Biotechnol. Biochem. 2017, 3, 43–59. [Google Scholar] [CrossRef]
- Li, J.-L.; Sun, X.; Chen, L.; Guo, L.-D. Community structure of endophytic fungi of four mangrove species in Southern China. Mycology 2016, 7, 180–190. [Google Scholar] [CrossRef]
- Collado, J.; Platas, G.; González, I.; Peláez, F. Geographical and seasonal influences on the distribution of fungal endophytes in Quercus ilex. New Phytol. 1999, 144, 525–532. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Zhang, L.; He, S.Y. Plant-Microbe Interactions Facing Environmental Challenge. Cell Host Microbe 2019, 26, 183–192. [Google Scholar] [CrossRef]
- Prospero, S.; Botella, L.; Santini, A.; Robin, C. Biological control of emerging forest diseases: How can we move from dreams to reality? For. Ecol. Manag. 2021, 496, 119377. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A.; Blumenstein, K. Ecological aspects of endophyte-based biocontrol of forest diseases. In Advances in Endophytic Research; Verma, V.C., Gange, A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 321–333. [Google Scholar]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, T.S.; Wittlinger, S.K.; Faeth, S.H. Endophytic fungi associated with cacti in Arizona1 1Dedicated to John Webster on the occasion of his 80th birthday. Mycol. Res. 2005, 109, 635–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musetti, R.; Polizzotto, R.; Vecchione, A.; Borselli, S.; Zulini, L.; D’Ambrosio, M.; di Toppi, L.S.; Pertot, I. Antifungal activity of diketopiperazines extracted from Alternaria alternata against Plasmopara viticola: An ultrastructural study. Micron 2007, 38, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Woo, H.R.; Nam, H.G. Toward Systems Understanding of Leaf Senescence: An Integrated Multi-Omics Perspective on Leaf Senescence Research. Mol. Plant 2016, 9, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Sudheer, S.; Usmani, Z.; Rani, R.; Gupta, P. Deciphering the Omics of Plant-Microbe Interaction: Perspectives and New Insights. Curr. Genom. 2020, 21, 343–362. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- de Vries, S.; Stukenbrock, E.H.; Rose, L.E. Rapid evolution in plant–microbe interactions—an evolutionary genomics perspective. New Phytol. 2020, 226, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Hall, R.D. Plant Metabolomics in a Nutshell: Potential and Future Challenges. Annu. Plant Rev. 2011, 43, 1–24. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Sanchez, J.-C.; Gooley, A.A.; Appel, R.D.; Humphery-Smith, I.; Hochstrasser, D.F.; Williams, K.L. Progress with Proteome Projects: Why all Proteins Expressed by a Genome Should be Identified and How to Do It. Biotechnol. Genet. Eng. Rev. 1996, 13, 19–50. [Google Scholar] [CrossRef] [Green Version]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Tyler, H.L.; DeBoy, R.T.; Daugherty, S.; Ren, Q.; Badger, J.H.; Durkin, A.S.; Huot, H.; Shrivastava, S.; Kothari, S.; et al. Complete Genome Sequence of the N2-Fixing Broad Host Range Endophyte Klebsiella pneumoniae 342 and Virulence Predictions Verified in Mice. PLoS Genet. 2008, 4, e1000141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firrincieli, A.; Eotillar, R.; Esalamov, A.; Eschmutz, J.; Ekhan, Z.; Redman, R.S.; Fleck, N.D.; Elindquist, E.; Grigoriev, I.V.; Doty, S.L. Genome sequence of the plant growth promoting endophytic yeast Rhodotorula graminis WP1. Front. Microbiol. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, X.; Weiss, M.; KOGEL, K.H.; Schäfer, P. Piriformospora indica—a mutualistic basidiomycete with an exceptionally large plant host range. Mol. Plant Pathol. 2011, 13, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M.K. “Omics” Tools for Better Understanding the Plant–Endophyte Interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef] [Green Version]
- Dinsdale, E.A.; Edwards, R.A.; Hall, D.; Angly, F.; Breitbart, M.; Brulc, J.M.; Furlan, M.; Desnues, C.; Haynes, M.; Li, L.; et al. Functional metagenomic profiling of nine biomes. Nature 2008, 452, 629–632. [Google Scholar] [CrossRef]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome Profiling of the Endophyte Burkholderia phytofirmans PsJN Indicates Sensing of the Plant Environment and Drought Stress. mBio 2015, 6, e00621-15. [Google Scholar] [CrossRef] [Green Version]
- Lery, L.M.S.; Hemerly, A.S.; Nogueira, E.M.; von Krüger, W.M.A.; Bisch, P.M. Quantitative Proteomic Analysis of the Interaction Between the Endophytic Plant-Growth-Promoting Bacterium Gluconacetobacter diazotrophicus and Sugarcane. Mol. Plant-Microbe Interactions 2011, 24, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, W.; Sun, K.; Tang, M.-J.; Chen, P.-X.; Li, X.; Dai, C.-C. Comparative Transcriptomics and Proteomics of Atractylodes lancea in Response to Endophytic Fungus Gilmaniella sp. AL12 Reveals Regulation in Plant Metabolism. Front. Microbiol. 2019, 10, 1208. [Google Scholar] [CrossRef]
- Chen, X.-L.; Sun, M.-C.; Chong, S.-L.; Si, J.-P.; Wu, L.-S. Transcriptomic and Metabolomic Approaches Deepen Our Knowledge of Plant–Endophyte Interactions. Front. Plant Sci. 2022, 12, 700200. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bhardwaj, P.; Sharma, S. Metabolomic Insights into Endophyte-Derived Bioactive Compounds. Front. Microbiol. 2022, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, G. Analysis of Secondary Metabolites from Plant Endophytic Fungi. In Plant Pathogenic Fungi and Oomycetes: Methods and Protocols; Ma, W., Wolpert, T., Eds.; Springer: New York, NY, USA, 2018; pp. 25–38. [Google Scholar]
- Segers, K.; Declerck, S.; Mangelings, D.; Heyden, Y.V.; Eeckhaut, A.V. Analytical techniques for metabolomic studies: A review. Bioanalysis 2019, 11, 2297–2318. [Google Scholar] [CrossRef] [PubMed]
- Cavill, R.; Jennen, D.; Kleinjans, J.; Briedé, J.J. Transcriptomic and metabolomic data integration. Brief. Bioinform. 2015, 17, 891–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noor, E.; Cherkaoui, S.; Sauer, U. Biological insights through omics data integration. Curr. Opin. Syst. Biol. 2019, 15, 39–47. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Zhang, Z. Comparative transcriptome combined with metabolomic and physiological analyses revealed ROS-mediated redox signaling affecting rice growth and cellular iron homeostasis under varying pH conditions. Plant Soil 2018, 434, 343–361. [Google Scholar] [CrossRef]
- Locke, A.M.; Barding, G.A., Jr.; Sathnur, S.; Larive, C.K.; Bailey-Serres, J. Rice SUB1A constrains remodelling of the transcriptome and metabolome during submergence to facilitate post-submergence recovery. Plant Cell Environ. 2018, 41, 721–736. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Differential Regulation of Carbon and Nitrogen Metabolism in Response to Nitrogen Availability. Int. J. Mol. Sci. 2019, 20, 2349. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Cho, K.-S.; Sohn, H.-B.; Ha, I.J.; Hong, S.-Y.; Lee, H.; Kim, Y.-M.; Nam, M.H. Network analysis of the metabolome and transcriptome reveals novel regulation of potato pigmentation. J. Exp. Bot. 2016, 67, 1519–1533. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct. Plant Biol. 2019, 46, 30–43. [Google Scholar] [CrossRef]
- Sade, D.; Shriki, O.; Cuadros-Inostroza, A.; Tohge, T.; Semel, Y.; Haviv, Y.; Willmitzer, L.; Fernie, A.R.; Czosnek, H.; Brotman, Y. Comparative metabolomics and transcriptomics of plant response to Tomato yellow leaf curl virus infection in resistant and susceptible tomato cultivars. Metabolomics 2014, 11, 81–97. [Google Scholar] [CrossRef]
- Yang, C.; Wu, P.; Yao, X.; Sheng, Y.; Zhang, C.; Lin, P.; Wang, K. Integrated Transcriptome and Metabolome Analysis Reveals Key Metabolites Involved in Camellia oleifera Defense against Anthracnose. Int. J. Mol. Sci. 2022, 23, 536. [Google Scholar] [CrossRef]
- Huang, S.; Chaudhary, K.; Garmire, L.X. More Is Better: Recent Progress in Multi-Omics Data Integration Methods. Front. Genet. 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).