Clinical Impact of Aspergillus fumigatus in Children with Cystic Fibrosis
Abstract
1. Introduction
2. Methods
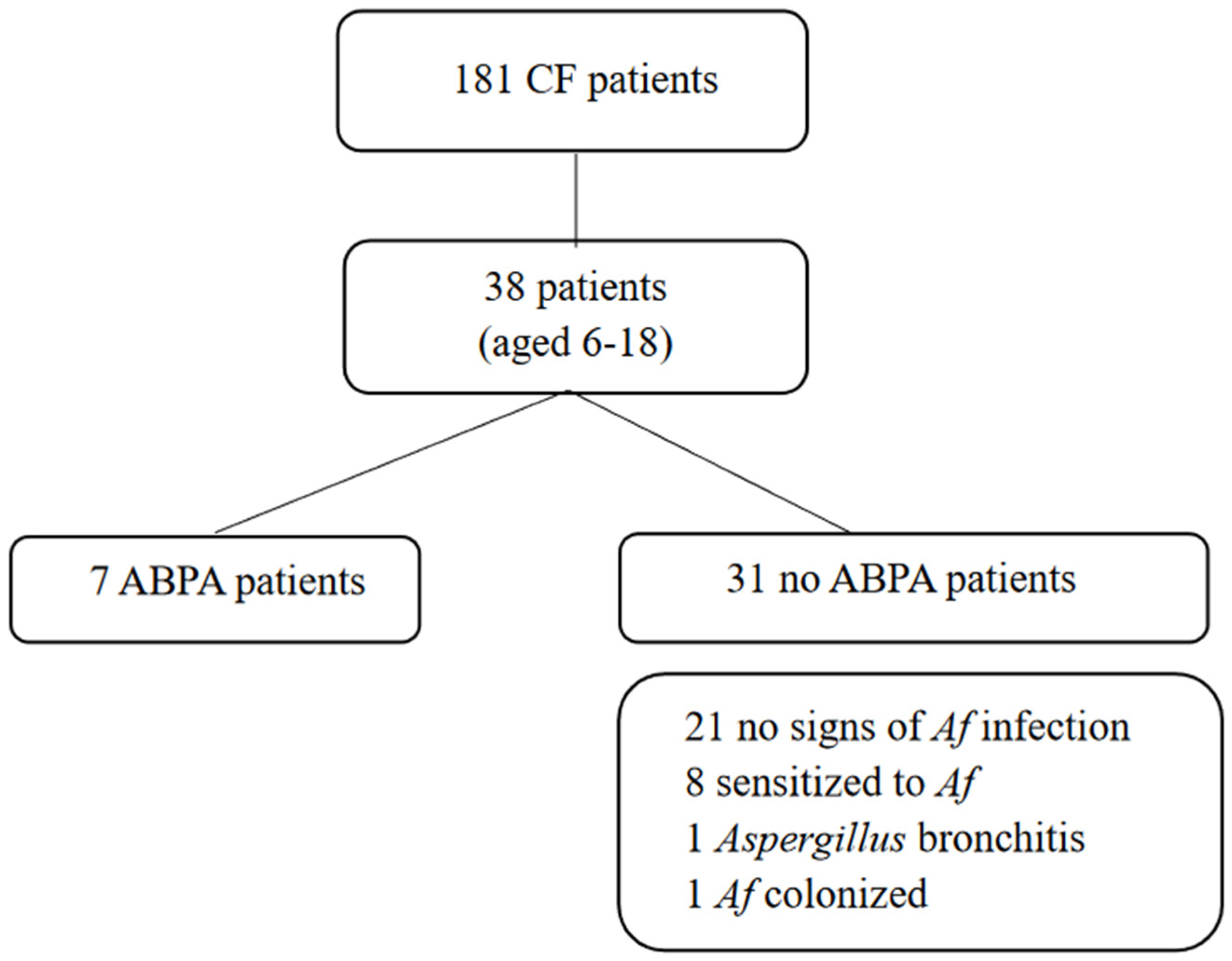
2.1. Study Population
2.2. Data Collection and Definitions
- Stage 1 (acute phase): typical findings are present (serum-specific IgE for Aspergillus, Aspergillus-specific precipitating antibody, radiological abnormalities, peripheral blood eosinophilia).
- Stage 2 (remission phase): asymptomatic patient, no new radiological infiltrates and no increase in total serum IgE for at least 6 months.
- Stage 3 (exacerbation): new pulmonary infiltrates with eosinophilia and doubled IgE levels with respect to remission phase.
- Stage 4: steroid-dependent disease.
- Stage 5 (pulmonary fibrosis): chest X-ray or CT scan shows irreversible fibrosis and chronic cavitation.
2.3. HRCT Evaluation and Scoring System
2.4. Statistical Analysis
3. Results
3.1. Prevalence of Af-Related Disease
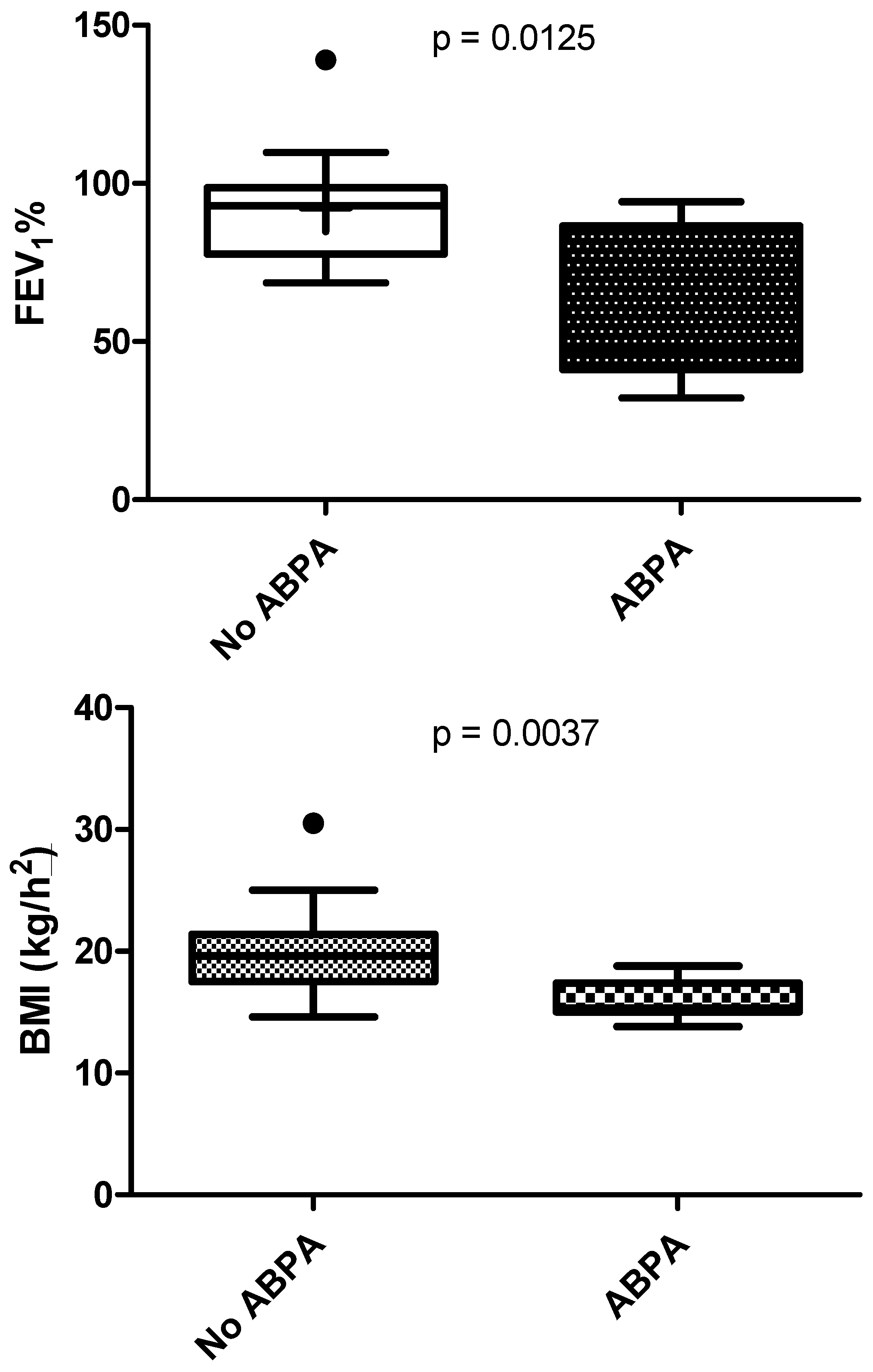
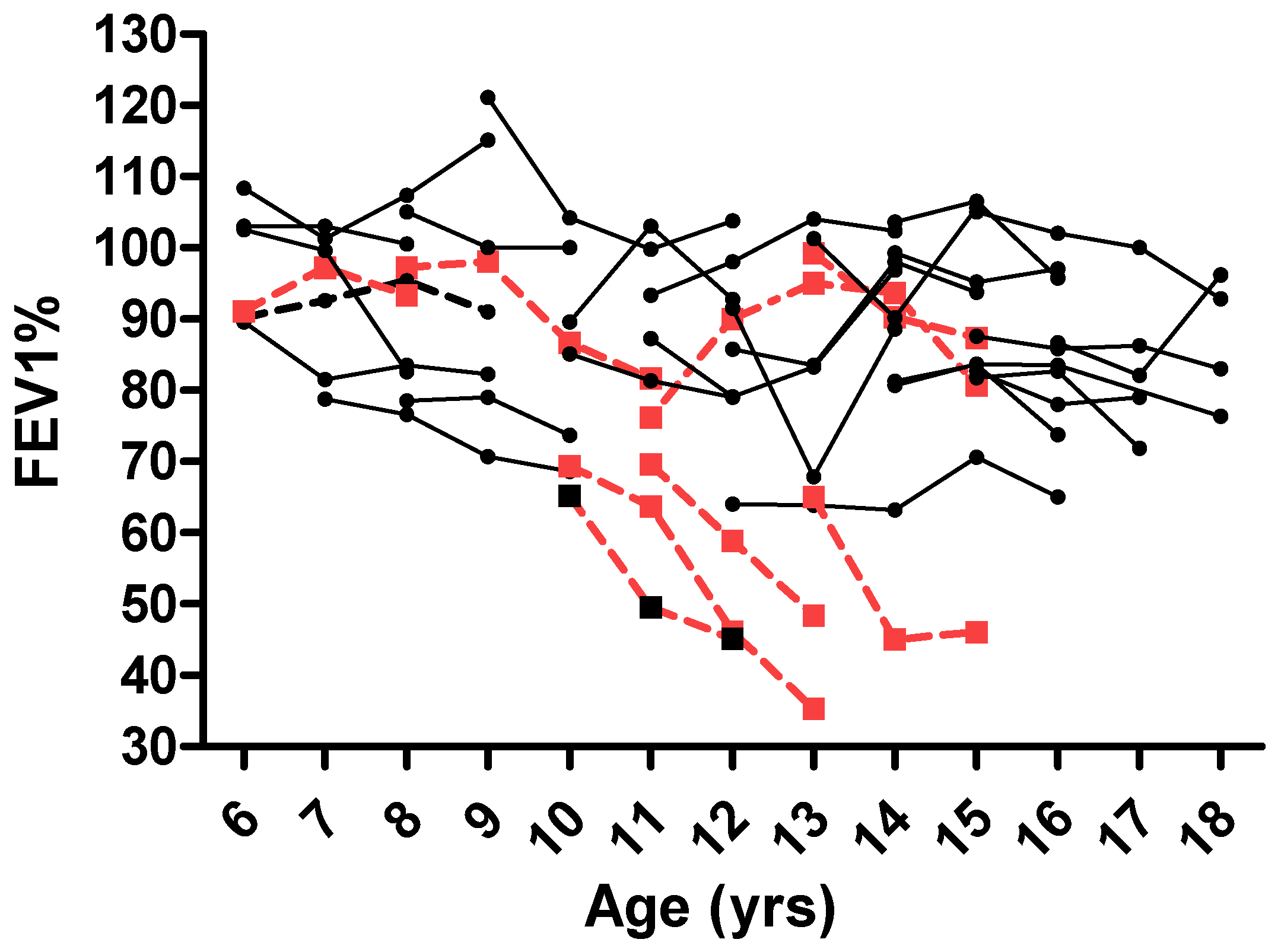
3.2. ABPA Effect on Lung Function
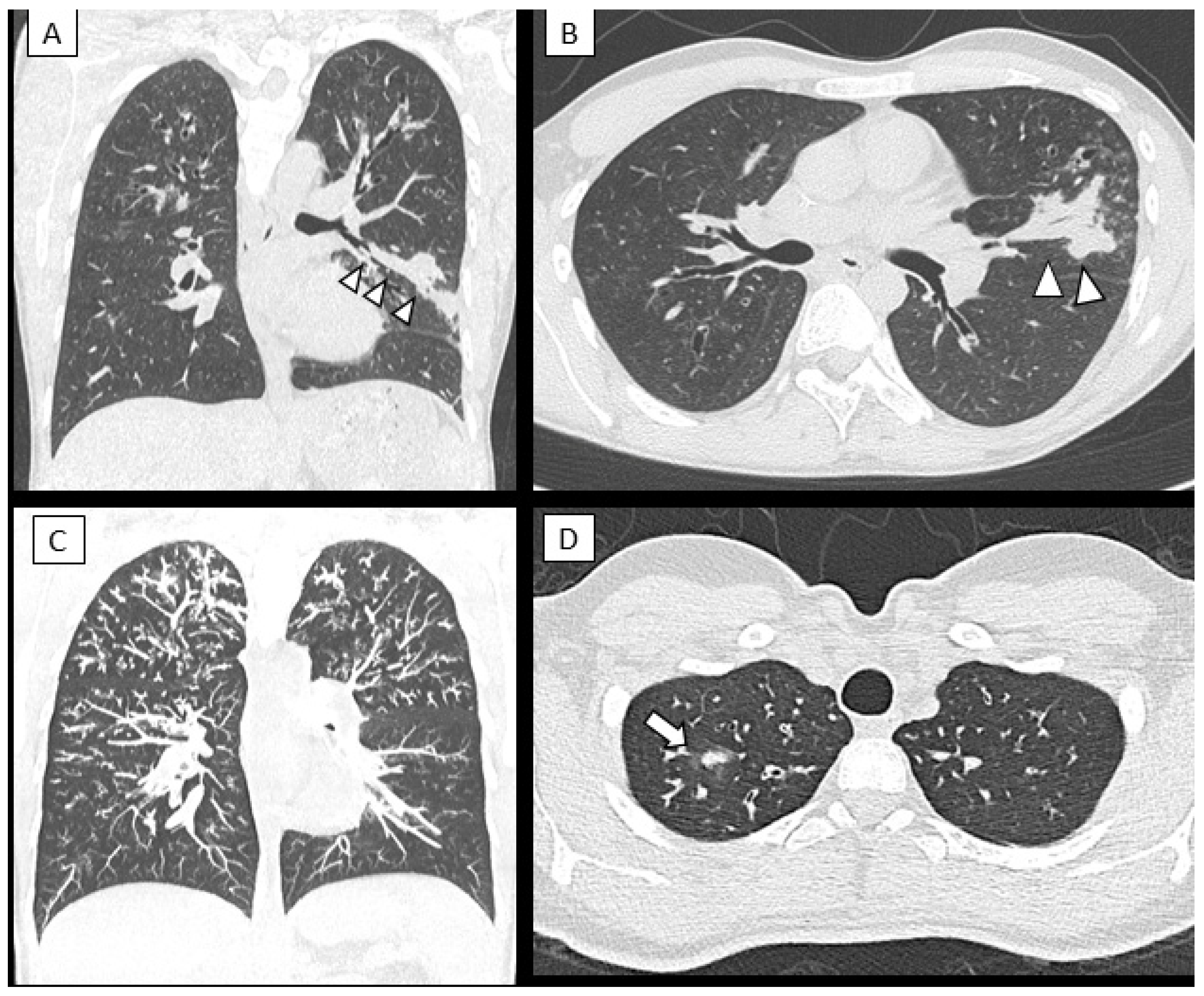
3.3. Changes in Lung Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Foster, K.; Alton, H. Chronic lung infection in children. Paediatr. Respir Rev. 2003, 4, 225–229. [Google Scholar] [CrossRef]
- Stevens, D.A.; Moss, R.B.; Kurup, V.P.; Knutsen, A.P.; Greenberger, P.; Judson, M.A.; Denning, D.W.; Crameri, R.; Brody, A.S.; Light, M.; et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis: State of the art. Cystic Fibrosis Foundation Consensus Conference. Clin. Infect. Dis. 2003, 37, S225–S264. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Brunel, S.F.; Warris, A. Aspergillus infections in cystic fibrosis. J. Infect. 2016, 72, S50–S55. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. Republished: The clinical spectrum of pulmonary aspergillosis. Postgrad. Med. J. 2015, 91, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Armstead, J.; Morris, J.; Denning, D.W. Multi-Country Estimate of Different Manifestations of Aspergillosis in Cystic Fibrosis. PLoS ONE 2014, 9, e98502. [Google Scholar]
- Valletta, E.; Braggion, C.; Mastella, G. Sensitization to Aspergillus and allergic bronchopulmonary aspergillosis in a cystic fibrosis population. Pediatr. Asthma Allergy Immunol. 1993, 7, 43–49. [Google Scholar] [CrossRef]
- Mroueh, S.; Spock, A. Allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Chest 1994, 105, 32–36. [Google Scholar] [CrossRef]
- Geller, D.E.; Kaplowitz, H.; Light, M.J.; Colin, A. Allergic bronchopulmonary aspergillosis in cystic fibrosis. Reported prevalence, regional distribution, and patient characteristics. Chest 1999, 116, 639–646. [Google Scholar] [CrossRef]
- Becker, J.W.; Burke, W.; McDonald, G.; Greenberger, P.A.; Henderson, W.R.; Aitken, M.L. Prevalence of allergic bronchopulmonary aspergillosis and atopy in adult patients with cystic fibrosis. Chest 1996, 109, 1536–1540. [Google Scholar] [CrossRef]
- Hutcheson, P.S.; Knudson, A.P.; Rejent, A.J.; Slavin, R.G. A 12-year longitudinal study of Aspergillus sensitivity in patients with cystic fibrosis. Chest 1996, 110, 363–366. [Google Scholar] [CrossRef]
- Mastella, G.; Rainisio, M.; Harms, H.K.; Hodson, M.E.; Koch, C.; Navarro, J.; Strandvik, B.; McKenzie, S.G. Allergic bronchopulmonary aspergillosis in cystic fibrosis. A European epidemiological study. epidemiologic registry of cystic fibrosis. Eur. Respir. J. 2000, 16, 464–471. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Miligkos, M.; Bossi, A.; Colombo, C.; Hatziagorou, E.; Kashirskaya, N.; De Monestrol, I.; Thomas, M.; Mei-Zahav, M.; Chrousos, G.; et al. Effect of allergic bronchopulmonary aspergillosis on FEV1in children and adolescents with cystic fibrosis: A European Cystic Fibrosis Society Patient Registry analysis. Arch. Dis. Child. 2017, 102, 742–747. [Google Scholar] [CrossRef]
- Maturu, V.N.; Agarwal, R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: Systematic review and meta-analysis. Clin. Exp. Allergy 2015, 45, 1765–1778. [Google Scholar] [CrossRef]
- Nikolaizik, W.H.; Crameri, R.; Blaser, K.; Schöni, M.H. Skin test reactivity to recombinant Aspergillus fumigatus allergen I/a in patients with cystic fibrosis. Int. Arch. Allergy Immunol. 1996, 111, 403–408. [Google Scholar] [CrossRef]
- Wojnarowski, C.; Eichler, I.; Gärtner, C.; Götz, M.; Renner, S.; Koller, D.Y.; Frischer, T. Sensitization to Aspergillus fumigatus and lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1997, 155, 1902–1907. [Google Scholar] [CrossRef]
- Liu, J.C.; Modha, D.E.; Gaillard, E.A. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis? J. Cyst. Fibros. 2013, 12, 187–193. [Google Scholar] [CrossRef]
- Breuer, O.; Schultz, A.; Garratt, L.W.; Turkovic, L.; Rosenow, T.; Murray, C.P.; Karpievitch, Y.V.; Akesson, L.; Dalton, S.; Sly, P.D.; et al. Aspergillus infections and progression of structural lung disease in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 688–696. [Google Scholar] [CrossRef]
- Kraemer, R.; Delosea, N.; Ballinari, P.; Gallati, S.; Crameri, R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2006, 174, 1211–1220. [Google Scholar]
- Saunders, R.V.; Modha, D.E.; Claydon, A.; Gaillard, E.A. Chronic Aspergillus fumigatus colonization of the pediatric cystic fibrosis airway is common and may be associated with a more rapid decline in lung function. Med. Mycol. 2006, 54, 537–543. [Google Scholar] [CrossRef]
- Amin, R.; Dupuis, A.; Aaron, S.; Ratjen, F. The Effect of Chronic Infection with Aspergillus fumigatus on Lung Function and Hospitalization in Patients with Cystic Fibrosis. Chest 2010, 137, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Arleth, S.; Spaeth, A.; Bertele-Harms, R.-M.; Harms, H.K. Correlation of IgE antibody titer to Aspergillus fumigatus with decreased lung function in cystic fibrosis. Pediatr. Pulmonol. 1990, 8, 12–15. [Google Scholar] [CrossRef]
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W.; ABPA Complicating Asthma ISHAM Working Group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev. Respir. Med. 2016, 10, 1317–1334. [Google Scholar] [CrossRef]
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. Diagnosing Allergic Bronchopulmonary Aspergillosis: A Review. Cureus 2012, 11, e4550. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Horsley, A.; Denning, D.W. What is the importance of classifying Aspergillus disease in cystic fibrosis patients? Expert Rev. Respir. Med. 2014, 8, 389–392. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brandt, C.; Roehmel, J.; Rickerts, V.; Melichar, V.; Niemann, N.; Schwarz, C. Aspergillus Bronchitis in Patients with Cystic Fibrosis. Mycopathologia 2017, 183, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kidd, T.J.; Canton, R.; Ekkelenkamp, M.; Johansen, H.K.; Gilligan, P.; LiPuma, J.J.; Bell, S.C.; Elborn, J.S.; Flume, P.A.; VanDevanter, D.R.; et al. Antimicrobial Resistance in Cystic Fibrosis International Working Group. Defining antimicrobial resistance in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 696–704. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Bhalla, M.; Turcios, N.; Aponte, V.; Jenkins, M.; Leitman, B.; McCauley, D.I.; Naidich, D. Cystic fibrosis: Scoring system with thin-section CT. Radiology 1991, 179, 783–788. [Google Scholar] [CrossRef]
- Gothe, F.; Kappler, M.; Griese, M. Increasing Total Serum IgE, Allergic Bronchopulmonary Aspergillosis, and Lung Function in Cystic Fibrosis. J. Allergy Clin. Immunol. 2017, 5, 1591–1598.e6. [Google Scholar] [CrossRef]
- Jubin, V.; Ranque, S.; Le Bel, N.S.; Sarles, J.; Dubus, J.-C. Risk Factors for Aspergillus Colonization and Allergic Bronchopulmonary Aspergillosis in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2010, 45, 764–771. [Google Scholar] [CrossRef]
- Noni, M.; Katelari, A.; Dimopoulos, G.; Doudounakis, S.E.; Tzoumaka-Bakoula, C.; Spoulou, V. Aspergillus fumigatus chronic colonization and lung function decline in cystic fibrosis may have a two-way relationship. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2235–2241. [Google Scholar] [CrossRef]
- Harun, S.N.; Wainwright, C.; Grimwood, K.; Hennig, S. Aspergillus and progression of lung disease in children with cystic fibrosis. Thorax 2018, 74, 125–131. [Google Scholar] [CrossRef]
- Frauchiger, B.S.; Binggeli, S.; Yammine, S.; Spycher, B.; Krüger, L.; Ramsey, K.A.; Latzin, P. Longitudinal course of clinical lung clearance index in children with cystic fibrosis. Eur. Respir. J. 2021, 58, 2002686. [Google Scholar] [CrossRef]
- Al Shakirchi, M.; Sorjonen, K.; Klingspor, L.; Bergman, P.; Hjelte, L.; de Monestrol, I. The Effects of Aspergillus fumigatus Colonization on Lung Function in Patients with Cystic Fibrosis. J. Fungi 2021, 7, 944. [Google Scholar] [CrossRef]
- Hong, G.; Psoter, K.J.; Jennings, M.T.; Merlo, C.A.; Boyle, M.P.; Hadjiliadis, D.; Kawut, S.M.; Lechtzin, N. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 624–630. [Google Scholar] [CrossRef]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: An Irish registry analysis. BMC Pulm. Med. 2017, 17, 70. [Google Scholar] [CrossRef]
- McCashney, A.; Robinson, P. Structural lung disease following allergic bronchopulmonary aspergillosis complicating pediatric cystic fibrosis. Pediatr. Pulmonol. 2021, 56, 3737–3744. [Google Scholar] [CrossRef]
- De Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef]
- McMahon, M.A.; Chotirmall, S.H.; McCullagh, B.; Branagan, P.; McElvaney, N.; Logan, P. Radiological abnormalities associated with Aspergillus colonization in a cystic fibrosis population. Eur. J. Radiol. 2012, 81, e197–e202. [Google Scholar] [CrossRef] [PubMed]
- Tiddens, H.A.; Rosenow, T. What did we learn from two decades of chest computed tomography in cystic fibrosis? Pediatr. Radiol. 2014, 44, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.S. Scoring systems for CT in cystic fibrosis: Who cares? Radiology 2004, 231, 296–298. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.A.; Lindblad, A.; Rubin, L.; Hop, W.C.; de Jongste, J.C.; Brink, M.; Tiddens, H.A. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006, 61, 80–85. [Google Scholar] [CrossRef]
- Liszewski, M.C.; Ciet, P.; Lee, E.Y. MR imaging of lungs and airways in children: Past and present. Magn. Reson. Imaging Clin. N. Am. 2019, 27, 201–225. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Gupta, P.; Shrivastav, A.; Saxena, A.K.; Mathew, J.L.; Singh, M.; Agarwal, R. Evaluation of 3 T lung magnetic resonance imaging in children with allergic bronchopulmonary aspergillosis: Pilot study. Eur. J. Radiol. 2019, 111, 88–92. [Google Scholar] [CrossRef]
- Breuer, O.; Schultz, A.; Turkovic, L.; De Klerk, N.; Keil, A.D.; Brennan, S.; Harrison, J.; Robertson, C.; Robinson, P.J.; Sly, P.D.; et al. Changing prevalence of lower airway infections in young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 590–599. [Google Scholar] [CrossRef]
- Poore, T.S.; Meier, M.; Towler, E.; Martiniano, S.L.; Brinton, J.T. DeBoer, E.M.; Sagel, S.D.; Wagner, B.D.; Zemanick, E.T. Clinical characteristics of people with cystic fibrosis and frequent fungal infection. Pediatr. Pulmonol. 2022, 57, 152–161. [Google Scholar] [CrossRef]
- Ritz, N.; Ammann, R.A.; Aebischer, C.C.; Schoeni-Affolter, F.; Schoeni, M.H. Risk factors for allergic bronchopulmonary aspergillosis and sensitisation to Aspergillus fumigatus in patients with cystic fibrosis. Eur. J. Pediatr. 2005, 164, 577–582. [Google Scholar] [CrossRef][Green Version]
- Nguyen, L.D.; Viscogliosi, E.; Delhaes, L. The lung mycobiome: An emerging field of the human respiratory microbiome. Front. Microbiol. 2015, 6, 89. [Google Scholar] [CrossRef]
- De Baets, F.; De Keyzer, L.; Van daele, S.; Schelstraete, P.; Van Biervliet, S.; Van Braeckel, E.; Thomas, M.; Wanyama, S.S. Risk factors and impact of allergic bronchopulmonary aspergillosis in Pseudomonas aeruginosa-negative CF patients. Pediatr. Allergy Immunol. 2018, 29, 726–731. [Google Scholar] [CrossRef]
- Lv, Q.; Elders, B.B.L.J.; Warris, A.; Caudri, D.; Ciet, P.; Tiddens, H.A.W.M. Aspergillus-related lung disease in people with cystic fibrosis: Can imaging help us to diagnose disease? Eur. Respir. Rev. 2021, 30, 210103. [Google Scholar] [CrossRef]
| No ABPA | ABPA (n = 7) | p-Value | |||||
|---|---|---|---|---|---|---|---|
| No Signs of Af (n = 21) |
Af Sensitization (n = 8) | Af Colonization (n = 1) | Aspergillus Bronchitis (n = 1) | Total No ABPA (n = 31) | |||
| Age, mean ± S D | 12.9 ± 4.3 | 14.6 ± 2.9 | 17.7 | 16.2 | 13.3 ± 3.9 | 13.4 ± 2.5 | ns |
| Sex | |||||||
| Male | 11 (52.4) | 5 (62.5) | 1 (100.0) | 1 (100.0 | 18 (58.1) | 5 (81.4) | ns |
| Female | 10 (47.6) | 3 (37.5) | - | - | 13 (41.9) | 2 (28.6) | |
| BMI (kg/m2), mean ± SD | 20.36 ± 3.61 | 19.79 ± 2.48 | 19.6 | 17.6 | 19.7 ± 3.41 | 15.96 ± 1.68 | <0.005 |
| Pancreatic insufficiency, n (%) | 5 (24) | 5 (62.5) | 1 (100) | 1 (100) | 12 (38.7) | 6 (86) | <0.05 |
| CFTR gene mutation, n (%) | <0.001 | ||||||
| Homo-F508del | 2 (9.5) | 3 (37.5) | 1 (100) | 1 (100) | 7 (22.5) | 2 (28.5) | |
| Hetero-F508del | 15 (71.5) | 3 (37.5) | - | - | 18 (58) | 2 (28.5) | |
| Others | 4 (19) | 2 (25) | - | - | 6 (19.3) | 3 (43) | |
| FEV1, % predicted | 91.3 ± 18.9 | 96.8 ± 22.3 | 71.9 | 95.3 | 92.3 ± 19.3 | 61.5 ± 25.9 | <0.001 |
| FEV1 change over 3 years (%) | −3.7 ± 10.2 | 9.14 ± 14.1 | −14.9 | −39 | 2 ± 14 | −27 ± 19.1 | <0.001 |
| Comorbidities | |||||||
| Diabetes, n (%) | 1 (4.7) | 2 (25) | 0 | 1 | 4 (12.9) | 0 | ns |
| CT score | 22.2 ± 2.8 | 17.1 ± 5.8 | 15 | 12 | 19.7 ± 5 | 14 ± 3.6 | <0.005 |
| Exacerbations in the last 12 months | 0.9 ± 1.26 | 3.50 ± 3.25 | 1 | 6 | 1.74 ± 2.33 | 4.43 ± 2.44 | <0.005 |
| Sputum culture | |||||||
| Pseudomonas aeruginosa, n (%) | 9 (42.9) | 1 (12.5) | 0 (0) | 1 (100) | 10 (32.2) | 3 (42.8) | ns |
| Aspergillus, n (%) | 0 (0) | 2 (25) | 0 (0) | 0 (0) | 2 (6.4) | 0 (0) | ns |
| Pseudomonas aeruginosa AND Aspergillus, n (%) | 0 (0) | 2 (25) | 1 (100) | 0 (0) | 3 (9.6) | 2 (28.5) | ns |
| Multiresistant bacteria, n (%) | 4 (19) | 2 (25) | 1 (100) | 1 (100) | 8 (25.8) | 0 (0) | ns |
| Multiresistant bacteria AND Aspergillus, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (14.2) | ns |
| Treatments | |||||||
| Long-term inhaled antibiotics, n (%) * | 1 (4.7) | 2 (25) | 0 (0) | 1 (100) | 4 (12.9) | 4 (57.2) | <0.05 |
| Oral azithromycin, n (%) | 1 (4.7) | 3 (37.5) | 1 (100) | 1 (100) | 6 (19.3) | 4 (57.2) | 0.007 |
| Nebulized hypertonic saline, n (%) | 1 (4.7) | 0 (0) | 0 (0) | 0 (0) | 1 (3.2) | 1 (14.3) | <0.001 |
| Inhaled corticosteroids, n (%) | 6 (28.6) | 5 (62.5) | 1 (100) | 1 (100) | 13 (41.9) | 6 (85.8) | <0.05 |
| No Signs of Af (n = 21) | Af Sensitization (n = 8) | ABPA (n = 7) | Af Colonization (n = 1) | Aspergillus Bronchitis (n = 1) | |
|---|---|---|---|---|---|
| Eosinophil count | 0.24 ± 0.20 | 0.31 ± 0.17 | 0.55 ± 0.37 | 0.16 ± 0.04 | 0.24 ± 0.09 |
| Total IgE | 91.5 ± 101.7 | 214.6 ± 182.5 | 918.4 ± 509.3 | 27.83 ± 6.62 | 110.10 ± 56.15 |
| Specific IgE for Aspergillus fumigatus | 0 ± 0.01 | 1.10 ± 1.48 | 12.51 ± 6.56 | <0.01 | 0.12 ± 0.11 |
| Aspergillus specific precipitating antibodies | 17.4 ± 140.8 | 42.4 ± 240.2 | 64.9 ± 340.1 | 44.3 ± 4.0 | >100 |
| Af in sputum, n (%) | 0 (0) | 4 (50) | 4 (57) | 1 (100) | 0 (0) |
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| BMI | 1.32 | [−0.98–3.63] | 0.25 |
| CT score | 0.38 | [−1.11–1.87] | 0.60 |
| ABPA | −27.64 | [−47.23–−8.05] | <0.01 |
| Constant | 57.61 | [9.92–105.9] | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fainardi, V.; Sodini, C.; Deolmi, M.; Ciuni, A.; Skenderaj, K.; Stabile, M.B.; Neglia, C.; Zani, E.M.; Spaggiari, C.; Sverzellati, N.; et al. Clinical Impact of Aspergillus fumigatus in Children with Cystic Fibrosis. Microorganisms 2022, 10, 739. https://doi.org/10.3390/microorganisms10040739
Fainardi V, Sodini C, Deolmi M, Ciuni A, Skenderaj K, Stabile MB, Neglia C, Zani EM, Spaggiari C, Sverzellati N, et al. Clinical Impact of Aspergillus fumigatus in Children with Cystic Fibrosis. Microorganisms. 2022; 10(4):739. https://doi.org/10.3390/microorganisms10040739
Chicago/Turabian StyleFainardi, Valentina, Chiara Sodini, Michela Deolmi, Andrea Ciuni, Kaltra Skenderaj, Maria Bice Stabile, Cosimo Neglia, Elena Mariotti Zani, Cinzia Spaggiari, Nicola Sverzellati, and et al. 2022. "Clinical Impact of Aspergillus fumigatus in Children with Cystic Fibrosis" Microorganisms 10, no. 4: 739. https://doi.org/10.3390/microorganisms10040739
APA StyleFainardi, V., Sodini, C., Deolmi, M., Ciuni, A., Skenderaj, K., Stabile, M. B., Neglia, C., Zani, E. M., Spaggiari, C., Sverzellati, N., Esposito, S., & Pisi, G. (2022). Clinical Impact of Aspergillus fumigatus in Children with Cystic Fibrosis. Microorganisms, 10(4), 739. https://doi.org/10.3390/microorganisms10040739







