On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Microorganisms
2.2. Experimental Approach
2.3. Data Treatment
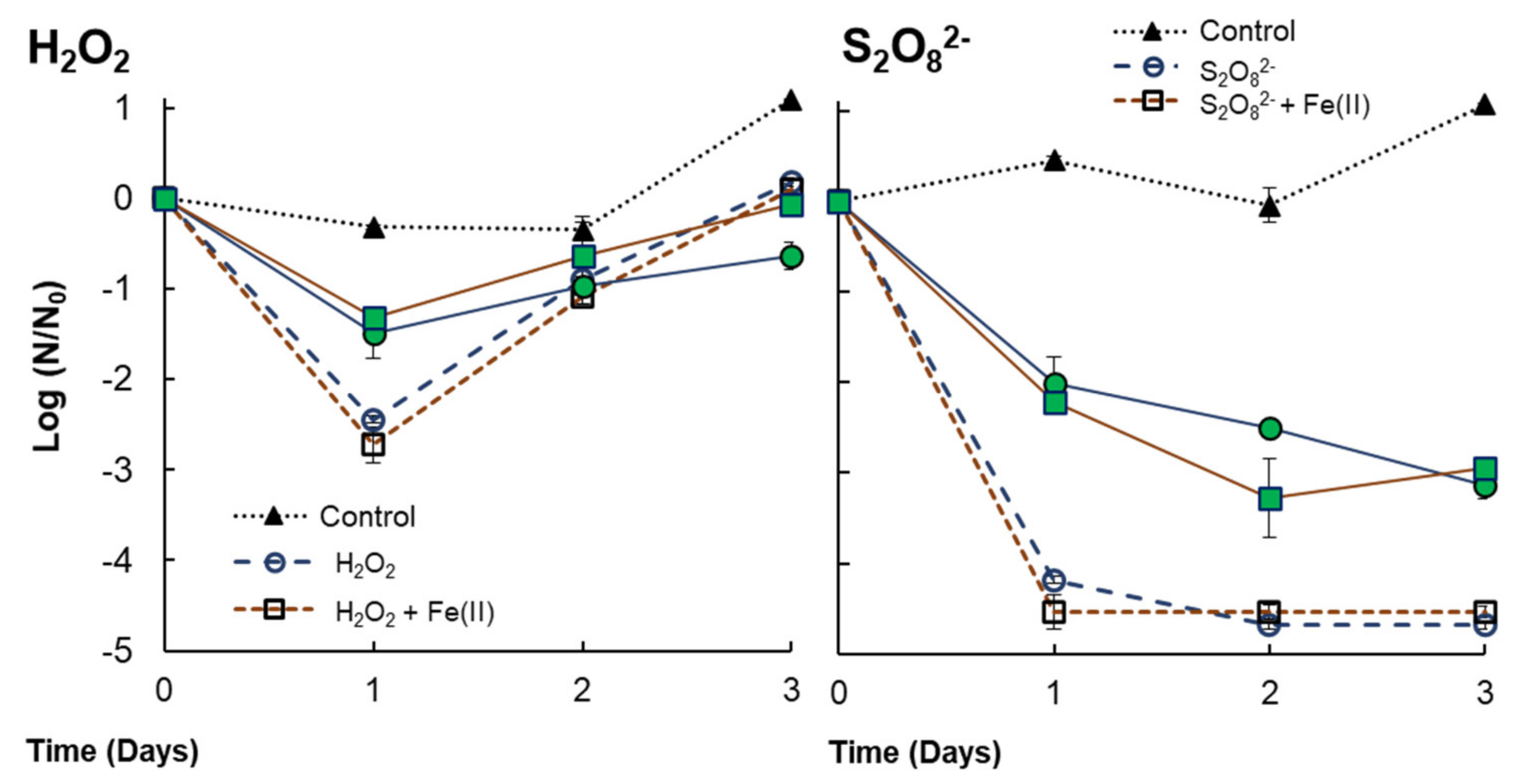
3. Results and Discussion
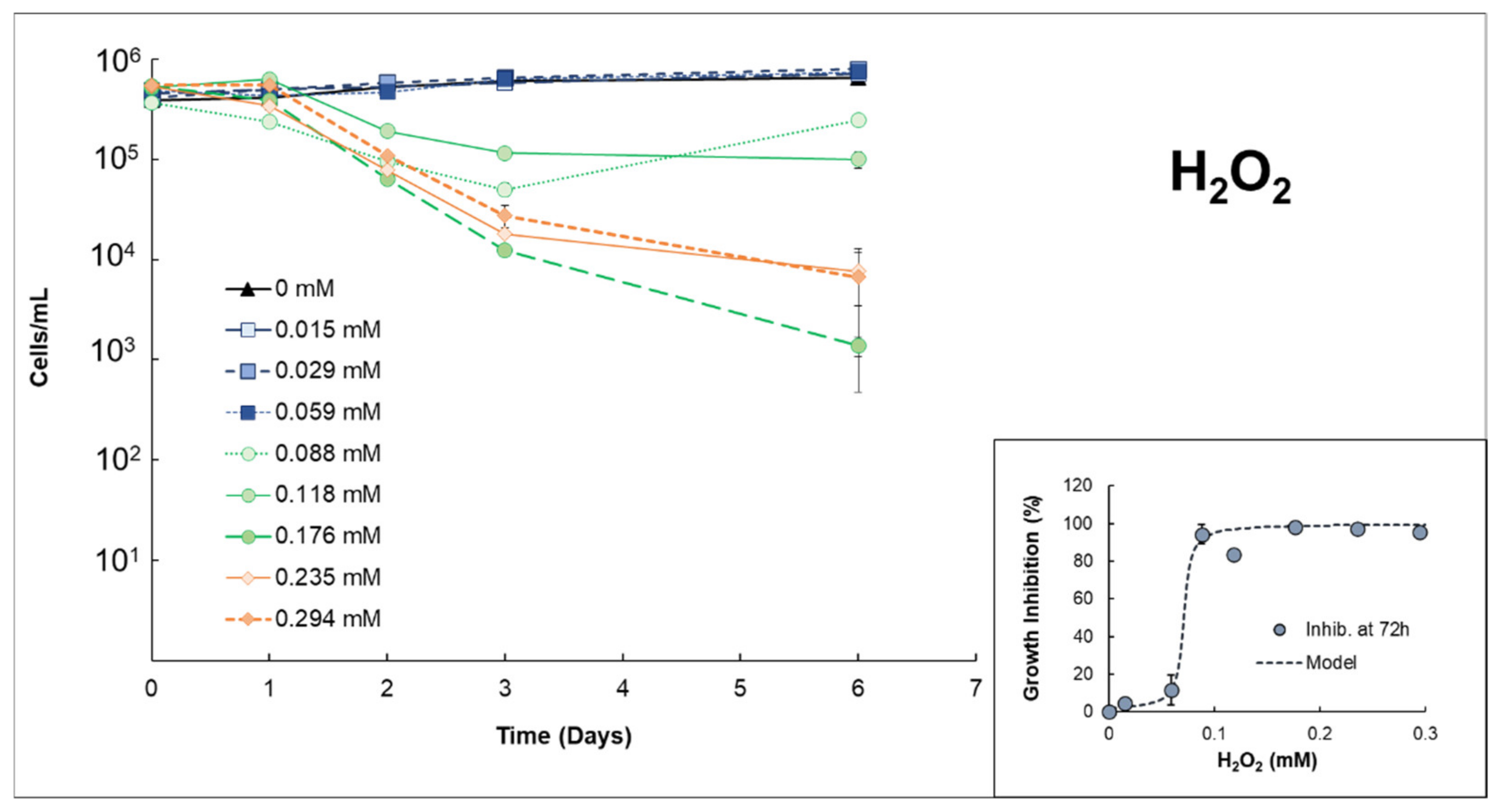
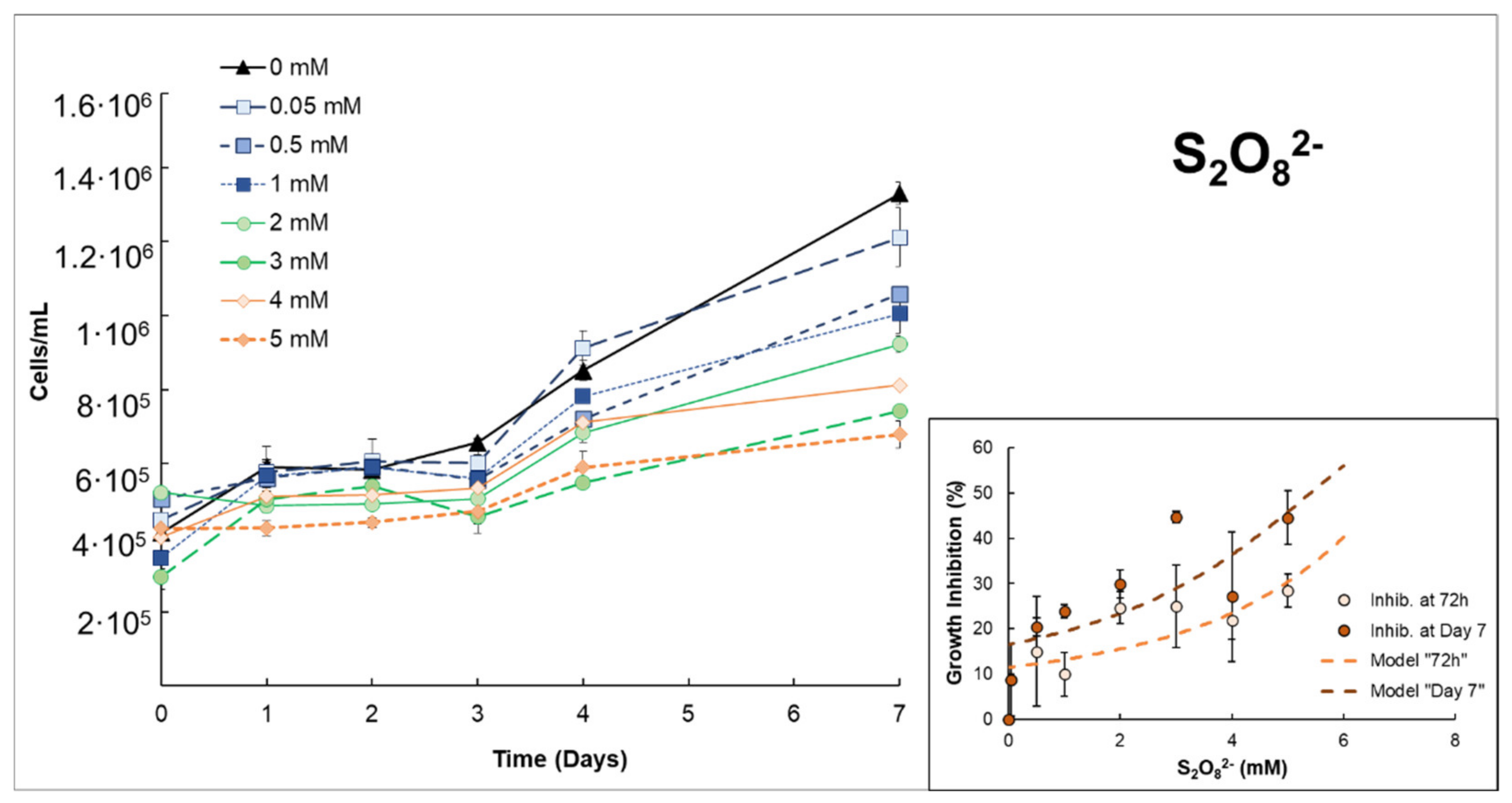
3.1. Effects of H2O2 or S2O82− on Anabaena sp.
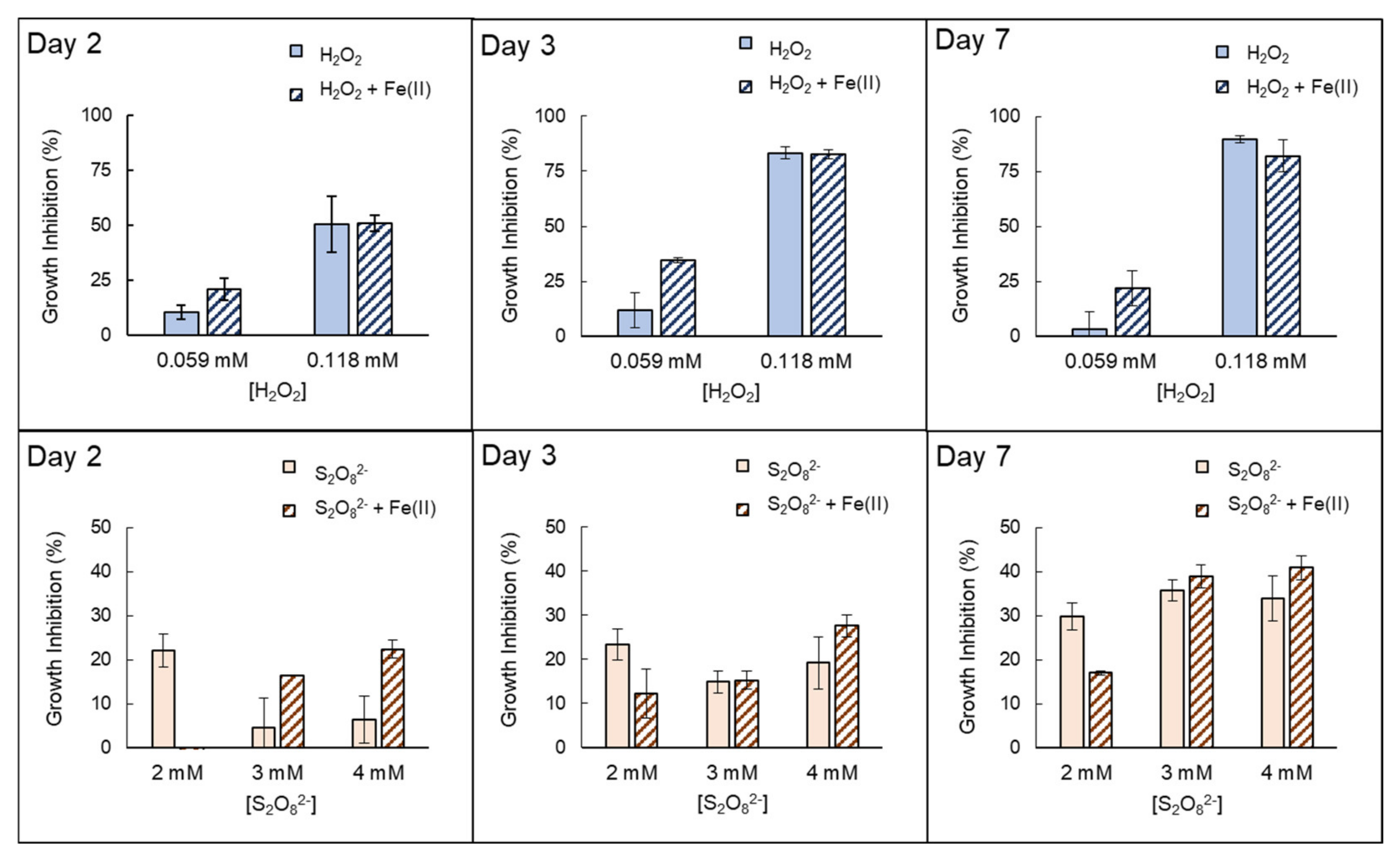
3.2. Inactivation by the Presence of Fe (II)
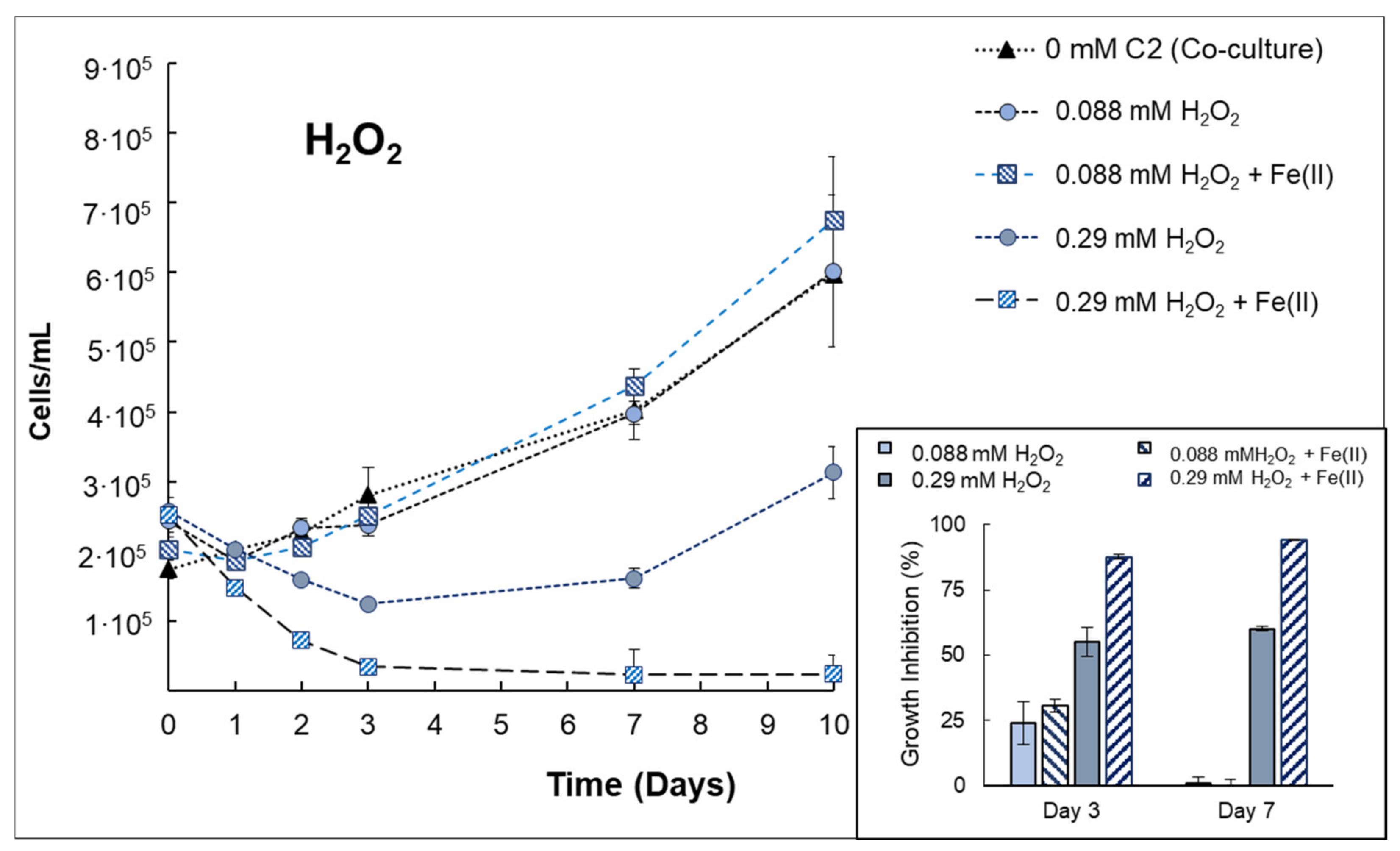
3.3. Behavior of Anabaena sp. Inactivation in Consortium with V. alginolyticus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in Understanding Harmful Algal Blooms: Paradigm Shifts and New Technologies for Research, Monitoring, and Management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munoz, M.; Cirés, S.; de Pedro, Z.M.; Colina, J.Á.; Velásquez-Figueroa, Y.; Carmona-Jiménez, J.; Caro-Borrero, A.; Salazar, A.; Santa María Fuster, M.C.; Contreras, D.; et al. Overview of toxic cyanobacteria and cyanotoxins in Ibero-American freshwaters: Challenges for risk management and opportunities for removal by advanced technologies. Sci. Total Environ. 2021, 761, 143197. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part I-chemical control methods. Harmful Algae 2020, 109, 102099. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Costa, S.T.; Braga, A.C.; Rodrigues, S.M.; Vale, P. Relevance and challenges in monitoring marine biotoxins in non-bivalve vectors. Food Control 2017, 76, 24–33. [Google Scholar] [CrossRef]
- Bellés-Garulera, J.; Vila, M.; Borrull, E.; Riobó, P.; Franco, J.M.; Sala, M.M. Variability of planktonic and epiphytic vibrios in a coastal environment affected by Ostreopsis blooms. Sci. Mar. 2016, 80, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, D.I.; Gooch Moore, J.; Stewart, J.R.; Hilborn, E.D.; George, B.J.; Li, Q.; Dickerson, J.; Keppler, C.K.; Sandifer, P.A. Temporal and Environmental Factors Driving Vibrio Vulnificus and V. Parahaemolyticus Populations and Their Associations With Harmful Algal Blooms in South Carolina Detention Ponds and Receiving Tidal Creeks. GeoHealth 2017, 1, 306–317. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Trinanes, J.; Gonzalez-Escalona, N.; Martinez-Urtaza, J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. 2017, 25, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Grimes, D.J. The Vibrios: Scavengers, Symbionts, and Pathogens from the Sea. Microb. Ecol. 2020, 80, 501–506. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef]
- Villacorte, L.O.; Assiyeh, S.; Tabatabai, A.; Anderson, D.M.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. Seawater reverse osmosis desalination and (harmful) algal blooms. Desalination 2015, 360, 61–80. [Google Scholar] [CrossRef]
- Xu, H.; Brookes, J.; Hobson, P.; Pei, H. Impact of copper sulphate, potassium permanganate, and hydrogen peroxide on Pseudanabaena galeata cell integrity, release and degradation of 2-methylisoborneol. Water Res. 2019, 157, 64–73. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Lusty, M.W.; Gobler, C.J. The Efficacy of Hydrogen Peroxide in Mitigating Cyanobacterial Blooms and Altering Microbial Communities across Four Lakes in NY, USA. Toxins 2020, 12, 428. [Google Scholar] [CrossRef]
- Barone, M.E.; Parkes, R.; Herbert, H.; McDonnell, A.; Conlon, T.; Aranyos, A.; Fierli, D.; Fleming, G.T.A.; Touzet, N. Comparative Response of Marine Microalgae to H2O2-Induced Oxidative Stress. Appl. Biochem. Biotechnol. 2021, 193, 4052–4067. [Google Scholar] [CrossRef]
- Romero-Martínez, L.; Rivas-Zaballos, I.; Moreno-Andrés, J.; Moreno-Garrido, I.; Acevedo-Merino, A.; Nebot, E. Improving the microalgae inactivating efficacy of ultraviolet ballast water treatment in combination with hydrogen peroxide or peroxymonosulfate salt. Mar. Pollut. Bull. 2021, 162, 111886. [Google Scholar] [CrossRef]
- Mathijs, G.D.; Robbert, G.; Mark, A.J. Time and Concentration Dependency in the Potentially Affected Fraction of Species: The case of hydrogen peroxide treatment of ballast water. Environ. Toxicol. Chem. 2008, 27, 746–753. [Google Scholar]
- Ahn, S.; Peterson, T.D.; Righter, J.; Miles, D.M.; Tratnyek, P.G. Disinfection of ballast water with iron activated persulfate. Environ. Sci. Technol. 2013, 47, 11717–11725. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Farinango, G.; Romero-Martínez, L.; Acevedo-Merino, A.; Nebot, E. Application of persulfate salts for enhancing UV disinfection in marine waters. Water Res. 2019, 163, 114866. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Giannakis, S.; Marjanovic, M.; Kohantorabi, M.; Gholami, M.R.; Grandjean, D.; de Alencastro, L.F.; Pulgarín, C. Solar-assisted bacterial disinfection and removal of contaminants of emerging concern by Fe2+-activated HSO5− vs. S2O82− in drinking water. Appl. Catal. B Environ. 2019, 248, 62–72. [Google Scholar] [CrossRef]
- Song, Q.; Niu, X.; Zhang, D.; Song, X.; Li, Y.; Ma, J.; Lai, S.; Yang, Z.; Zhou, S. The behaviors of Microcystis aeruginosa and microcystins during the Fe2+/persulfate (PS) preoxidation-coagulation and flocs storage period. Environ. Res. 2020, 186, 109549. [Google Scholar] [CrossRef]
- Pulgarin, A.; Giannakis, S.; Pulgarin, C.; Ludwig, C.; Refardt, D. A novel proposition for a citrate-modified photo-Fenton process against bacterial contamination of microalgae cultures. Appl. Catal. B Environ. 2020, 265, 118615. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Tang, T.; Xiong, Y.; Dai, R. Removal of cyanobacteria and control of algal organic matter by simultaneous oxidation and coagulation—Comparing the H2O2/Fe(II) and H2O2/Fe(III) processes. Sci. Total Environ. 2020, 720, 137653. [Google Scholar] [CrossRef]
- Wert, E.C.; Dong, M.M.; Rosario-Ortiz, F.L. Using digital flow cytometry to assess the degradation of three cyanobacteria species after oxidation processes. Water Res. 2013, 47, 3752–3761. [Google Scholar] [CrossRef]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Eiler, A.; Gonzalez-Rey, C.; Allen, S.; Bertilsson, S. Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water. FEMS Microbiol. Ecol. 2007, 60, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Andrés, J.; Morillo-Ponce, J.; Ibáñez-López, M.E.; Acevedo-Merino, A.; García-Morales, J.L. Disinfection enhancement of single ozonation by combination with peroxymonosulfate salt. J. Environ. Chem. Eng. 2020, 8, 104335. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Acevedo-Merino, A.; Nebot, E. Study of marine bacteria inactivation by photochemical processes: Disinfection kinetics and growth modeling after treatment. Environ. Sci. Pollut. Res. 2018, 25, 27693–27703. [Google Scholar] [CrossRef]
- Spuhler, D.; Andres Rengifo-Herrera, J.; Pulgarin, C. The effect of Fe2+, Fe3+, H2O2 and the photo-Fenton reagent at near neutral pH on the solar disinfection (SODIS) at low temperatures of water containing Escherichia coli K12. Appl. Catal. B Environ. 2010, 96, 126–141. [Google Scholar] [CrossRef]
- Eisenberg, G.M. Colorimetric Determination of Hydrogen Peroxide. Ind. Eng. Chem. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Qin, Y.; Li, H. Removal of Microcystis aeruginosa and control of algal organic matter by Fe(II)/peroxymonosulfate pre-oxidation enhanced coagulation. Chem. Eng. J. 2021, 403, 126381. [Google Scholar] [CrossRef]
- Hampel, M.; Moreno-Garrido, I.; Sobrino, C.; Lubián, L.M.; Blasco, J. Acute toxicity of LAS homologues in marine microalgae: Esterase activity and inhibition growth as endpoints of toxicity. Ecotoxicol. Environ. Saf. 2001, 48, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Drábková, M.; Matthijs, H.C.P.; Admiraal, W.; Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Drabkova, M.; Admiraal, W.; Marsalek, B. Combined Exposure to Hydrogen Peroxide and Light-Selective Effects on Cyanobacteria, Green Algae, and Diatoms. Environ. Sci. Technol. 2006, 41, 309–314. [Google Scholar] [CrossRef]
- Sandrinia, G.; Piela, T.; Xua, T.; White, E.; Qina, H.; Slota, P.C.; Huismana, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [Green Version]
- Farinelli, G.; Giagnorio, M.; Ricceri, F.; Giannakis, S.; Tiraferri, A. Evaluation of the Effectiveness, Safety, and Feasibility of 9 Potential Biocides to Disinfect Acidic Landfill Leachate from Algae and Bacteria. Water Res. 2021, 191, 116801. [Google Scholar] [CrossRef]
- Berruti, I.; Oller, I.; Polo-López, M.I. Direct oxidation of peroxymonosulfate under natural solar radiation: Accelerating the simultaneous removal of organic contaminants and pathogens from water. Chemosphere 2021, 279, 130555. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, K.; Bai, L.; Minakata, D.; Seo, Y.; Kaya Göktaş, R.; Dionysiou, D.D.; Tang, C.-J.; Wei, Z.; Spinney, R. Inactivation of pathogenic microorganisms by sulfate radical: Present and future. Chem. Eng. J. 2019, 371, 222–232. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, M.; Zhu, X.; Yu, H.; Otte, M.L. Interactions between Fe and light strongly affect phytoplankton communities in a eutrophic lake. Ecol. Indic. 2021, 126, 107664. [Google Scholar] [CrossRef]
- Hunnestad, A.V.; Vogel, A.I.M.; Armstrong, E.; Digernes, M.G.; Van Ardelan, M.; Hohmann-Marriott, M.F. From the Ocean to the Lab—Assessing Iron Limitation in Cyanobacteria: An Interface Paper. Microorganisms 2020, 8, 1889. [Google Scholar] [CrossRef]
- Hunnestad, A.V.; Vogel, A.I.M.; Digernes, M.G.; Van Ardelan, M.; Hohmann-Marriott, M.F. Iron Speciation and Physiological Analysis Indicate that Synechococcus sp. PCC 7002 Reduces Amorphous and Crystalline Iron Forms in Synthetic Seawater Medium. J. Mar. Sci. Eng. 2020, 8, 996. [Google Scholar] [CrossRef]
- Stevanovic, M.; Hahn, A.; Nicolaisen, K.; Mirus, O.; Schleiff, E. The components of the putative iron transport system in the cyanobacterium Anabaena sp. PCC 7120. Environ. Microbiol. 2012, 14, 1655–1670. [Google Scholar] [CrossRef]
- Moreno-Andrés, J.; Vallés, I.; García-Negueroles, P.; Santos-Juanes, L.; Arques, A. Enhancement of Iron-Based Photo-Driven Processes by the Presence of Catechol Moieties. Catalysts 2021, 11, 372. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Matthijs, H.C.P.; Schuurmans, J.M.; Piel, T.; van Herk, M.J.; Sigon, C.A.M.; Visser, P.M.; Huisman, J. Interspecific protection against oxidative stress: Green algae protect harmful cyanobacteria against hydrogen peroxide. Environ. Microbiol. 2021, 23, 2404–2419. [Google Scholar] [CrossRef]
- Giannakis, S.; Voumard, M.; Rtimi, S.; Pulgarin, C. Bacterial disinfection by the photo-Fenton process: Extracellular oxidation or intracellular photo-catalysis? Appl. Catal. B Environ. 2018, 227, 285–295. [Google Scholar] [CrossRef]

| Target Organism | Treatment | [H2O2] (mmol/L) | [S2O82−] (mmol/L) |
|---|---|---|---|
| Anabaena sp. | Single oxidant | 0.015–0.29 | 0.05–5 |
| + Fe(II) [Fe(II)] = 18 µmol/L | 0.059, 0.118 1 | 1, 3, 5 1 | |
| Anabaena sp. + V. alginolyticus | Single oxidant | 0.088, 0.29 2 | 3 2 |
| + Fe(II) [Fe(II)] = 18 µmol/L | 0.088, 0.29 2 | 3 2 | |
| V. alginolyticus | Single Oxidant | 0.29 | 3 |
| + Fe (II) [Fe(II)] = 18 µmol/L | 0.29 | 3 |
| Target Organism | Treatment | Key Findings |
|---|---|---|
| Anabaenasp. | H2O2 | High growth inhibition (EC50% 72h = 0.0712 mmol H2O2/L ± 0.007). The strong effects of H2O2 itself inhibit properly quantifying possible extra damage caused by the presence of Fe(II). |
| S2O82− | Low–moderate growth inhibition ((EC50% 72 h = 6.80 mmol S2O82−/L ± 1.33)). The addition of Fe(II) notably increases growth inhibition in the first 48–72 h. However, this improvement decreased with longer exposure times, e.g., on day 7. | |
| Anabaenasp. (+V. alginolyticus) | H2O2 | The presence of bacteria implies increasing the H2O2 concentration up to 0.29 mmol H2O2/L to obtain a growth inhibition (at 72 h) of 55% ± 5.73, indicating that the presence of bacteria can protect cyanobacteria from H2O2 exposure. The effect of H2O2 + Fe(II) was evidenced in co-cultures, increasing the growth inhibition by 58.4% compared to single H2O2. |
| S2O82− | Similar growth inhibition percentages were observed when S2O82− was tested in monocultures (44.29–47.73% at day 7). This suggests that similar damage was caused by S2O82− in the presence of bacteria. | |
| V. alginolyticus (+ Anabaena sp.) | H2O2 | H2O2 exposure (0.29 mM) implies 2.44 LRV at 24 h. It is reduced to 1.48 LRV with the presence of Anabaena sp. The consumption of H2O2 in the first 24 h would imply bacterial regrowth after this time. The addition of Fe(II) does not reflect an improvement in bacteria inactivation. |
| S2O82− | S2O82− exposures (3 mM) implies 4.18 LRV at 24 h. It is reduced to 2.01 LRV with the presence of Anabaena sp. No differences were detected by PDS or PDS/Fe(II). Residual S2O82− might avoid bacterial regrowth. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Andrés, J.; Rivas-Zaballos, I.; Acevedo-Merino, A.; Nebot, E. On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water. Microorganisms 2022, 10, 735. https://doi.org/10.3390/microorganisms10040735
Moreno-Andrés J, Rivas-Zaballos I, Acevedo-Merino A, Nebot E. On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water. Microorganisms. 2022; 10(4):735. https://doi.org/10.3390/microorganisms10040735
Chicago/Turabian StyleMoreno-Andrés, Javier, Ignacio Rivas-Zaballos, Asunción Acevedo-Merino, and Enrique Nebot. 2022. "On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water" Microorganisms 10, no. 4: 735. https://doi.org/10.3390/microorganisms10040735
APA StyleMoreno-Andrés, J., Rivas-Zaballos, I., Acevedo-Merino, A., & Nebot, E. (2022). On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water. Microorganisms, 10(4), 735. https://doi.org/10.3390/microorganisms10040735







