Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Ethics
2.3. Participants
2.4. Variables
2.5. Outcomes
2.6. Microbiological Studies
2.7. Definitions
2.8. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Treatment Characteristics
3.3. Outcomes
3.4. Risk Factors for Mortality in Patients with Bacteremic PA Pneumonia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, C.; Antoniazzi, F.; Casari, S.; Ravizzola, G.; Gelmi, M.; Pagani, C.; D’Adda, M.; Morello, E.; Re, A.; Borlenghi, E.; et al. P. aeruginosa bloodstream infections among hematological patients: An old or new question? Ann. Hematol. 2012, 91, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Tofas, P.; Samarkos, M.; Piperaki, E.T.; Kosmidis, C.; Triantafyllopoulou, I.D.; Kotsopoulou, M.; Pantazatou, A.; Perlorentzou, S.; Poulli, A.; Vagia, M.; et al. Pseudomonas aeruginosa bacteraemia in patients with hematologic malignancies: Risk factors, treatment and outcome. Diagn. Microbiol. Infect. Dis. 2017, 88, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Nadal, G.; Puerta-Alcalde, P.; Gudiol, C.; Cardozo, C.; Albasanz-Puig, A.; Marco, F.; Laporte-Amargós, J.; Moreno-García, E.; Domingo-Doménech, E.; Chumbita, M.; et al. Inappropriate Empirical Antibiotic Treatment in High-risk Neutropenic Patients with Bacteremia in the Era of Multidrug Resistance. Clin. Infect. Dis. 2020, 70, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jun, Y.H.; Kim, Y.R.; Park, K.G.; Park, Y.J.; Kang, J.Y.; Kim, S.I. Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect. Dis. 2014, 14, 1–7. [Google Scholar] [CrossRef]
- Recio, R.; Viedma, E.; González-Bodí, S.; Villa, J.; Orellana, M.Á.; Mancheño-Losa, M.; Lora-Tamayo, J.; Chaves, F. Clinical and bacterial characteristics of Pseudomonas aeruginosa affecting the outcome of patients with bacteraemic pneumonia. Int. J. Antimicrob. Agents 2021, 58, 106450. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Tumbarello, M.; Caira, M.; Candoni, A.; Cattaneo, C.; Pastore, D.; Fanci, R.; Nosari, A.; Vianelli, N.; Busca, A.; et al. Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica 2011, 96, 32–34. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, H.J.; Moon, S.M.; Park, K.H.; Chong, Y.P.; Kim, M.N.; Kim, S.H.; Lee, S.O.; Kim, Y.S.; Woo, J.H.; et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect. Dis. 2012, 12, 1. [Google Scholar] [CrossRef]
- Peña, C.; Suarez, C.; Ocampo-Sosa, A.; Murillas, J.; Almirante, B.; Pomar, V.; Aguilar, M.; Granados, A.; Calbo, E.; Rodríguez-Baño, J.; et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in pseudomonas aeruginosa bloodstream infections: A post hoc analysis of a prospective cohort. Clin. Infect. Dis. 2013, 57, 208–216. [Google Scholar] [CrossRef]
- Bowers, D.R.; Liew, Y.X.; Lye, D.C.; Kwa, A.L.; Hsu, L.Y.; Tam, V.H. Outcomes of appropriate empiric combination versus monotherapy for pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1270–1274. [Google Scholar] [CrossRef][Green Version]
- Paul, M.; Leibovici, L. Combination therapy for pseudomonas aeruginosa bacteremia: Where do we stand? Clin. Infect. Dis. 2013, 57, 217–220. [Google Scholar] [CrossRef]
- Paul, M.; Dickstein, Y.; Schlesinger, A.; Grozinsky-Glasberg, S.; Soares-Weiser, K.; Leibovici, L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database Syst. Rev. 2013, 2013, CD003038. [Google Scholar] [CrossRef]
- Babich, T.; Naucler, P.; Valik, J.K.; Giske, C.G.; Benito, N.; Cardona, R.; Rivera, A.; Pulcini, C.; Fattah, M.A.; Haquin, J.; et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: A retrospective multicentre study. Int. J. Antimicrob. Agents 2020, 55, 105847. [Google Scholar] [CrossRef]
- Recio, R.; Mancheño, M.; Viedma, E.; Villa, J.; Orellana, M.Á.; Lora-Tamayo, J.; Chaves, F. Predictors of Mortality in Bloodstream Infections Caused by Pseudomonas aeruginosa and Impact of Antimicrobial Resistance and Bacterial Virulence. Antimicrob. Agents Chemother. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Joo, E.J.; Kang, C.I.; Ha, Y.E.; Park, S.Y.; Kang, S.J.; Wi, Y.M.; Lee, N.Y.; Chung, D.R.; Peck, K.R.; Song, J.H. Impact of inappropriate empiric antimicrobial therapy on outcome in Pseudomonas aeruginosa bacteraemia: A stratified analysis according to sites of infection. Infection 2011, 39, 309–318. [Google Scholar] [CrossRef]
- Lanoix, J.P.; Schmit, J.L.; Douadi, Y. Bacterial lung sepsis in patients with febrile neutropenia. Curr. Opin. Pulm. Med. 2012, 18, 175–180. [Google Scholar] [CrossRef]
- Specchia, G.; Pastore, D.; Carluccio, P.; Mele, G.; Montagna, M.T.; Liso, A.; Rizzi, R.; Stabile Ianora, A.; Liso, V. Pneumonia in acute leukemia patients during induction therapy: Experience in a single institution. Leuk. Lymphoma 2003, 44, 97–101. [Google Scholar] [CrossRef]
- Aguilar-Guisado, M.; Jiménez-Jambrina, M.; Espigado, I.; Rovira, M.; Martino, R.; Oriol, A.; Borrell, N.; Ruiz, I.; Martín-Dávila, P.; de la Cámara, R.; et al. Pneumonia in allogeneic stem cell transplantation recipients: A multicenter prospective study. Clin. Transplant. 2011, 25, 629–638. [Google Scholar] [CrossRef]
- Hakki, M.; Limaye, A.P.; Kim, H.W.; Kirby, K.A.; Corey, L.; Boeckh, M. Invasive Pseudomonas aeruginosa infections: High rate of recurrence and mortality after hematopoietic cell transplantation. Bone Marrow Transplant. 2007, 39, 687–693. [Google Scholar] [CrossRef]
- Rabello, L.S.C.F.; Silva, J.R.L.; Azevedo, L.C.P.; Souza, I.; Torres, V.B.L.; Rosolem, M.M.; Lisboa, T.; Soares, M.; Salluh, J.I.F. Clinical outcomes and microbiological characteristics of severe pneumonia in cancer patients: A prospective cohort study. PLoS ONE 2015, 10, e0120544. [Google Scholar] [CrossRef]
- Guarana, M.; Nucci, M.; Nouér, S.A. Shock and early death in hematologic patients with febrile neutropenia. Antimicrob. Agents Chemother. 2019, 63, 1–25. [Google Scholar] [CrossRef]
- Gudiol, C.; Royo-Cebrecos, C.; Laporte, J.; Ardanuy, C.; Garcia-Vidal, C.; Antonio, M.; Arnan, M.; Carratalà, J. Clinical features, aetiology and outcome of bacteraemic pneumonia in neutropenic cancer patients. Respirology 2016, 21, 1411–1418. [Google Scholar] [CrossRef]
- Carratalà, J.; Rosón, B.; Fernández-Sevilla, A.; Alcaide, F.; Gudiol, F. Bacteremic Pneumonia in Neutropenic Patients with Cancer. Arch. Intern. Med. 1998, 158, 868. [Google Scholar] [CrossRef]
- Gruson, D.; Vargas, F.; Hilbert, G.; Bui, N.; Maillot, T.; Mayet, T.; Pillet, O.; Chene, G.; Gbikpi-Benissan, G. Predictive factors of intensive care unit admission in patients with haematological malignancies and pneumonia. Intensive Care Med. 2004, 30, 965–971. [Google Scholar] [CrossRef]
- Vuotto, F.; Berthon, C.; Lemaitre, N.; Duhamel, A.; Balkaran, S.; Le Ray, E.; Micol, J.B.; Faure, K.; Alfandari, S. Risk factors, clinical features, and outcome of Pseudomonas aeruginosa bacteremia in patients with hematologic malignancies: A case-control study. Am. J. Infect. Control 2013, 41, 527–530. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Executive Summary: Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; Engelhard, D. European guidelines for emperical antibacterial therapy for febrile neutropenic patients in the era of growing resistance. Haematologica 2013, 98, 1826. [Google Scholar] [CrossRef]
- Gudiol, C.; Albasanz-Puig, A.; Laporte-Amargós, J.; Pallarès, N.; Mussetti, A.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; et al. Clinical Predictive Model of Multidrug Resistance in Neutropenic Cancer Patients with Bloodstream Infection Due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.H.; Wingard, J.R. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 23 February 2022).
- Wootton, M. Laboratory Methods for Antimicrobial Susceptibility Testing. In Antimicrobial/Anti-Infective Materials; CRC Press: Boca Raton, FL, USA, 1999; Volume 44, pp. 308–332. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Garner, J.S.; Jarvis, W.R.; Emori, T.G.; Horan, T.C.; Hughes, J.M. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 1988, 16, 128–140. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model, Statistics for Biology and Health, 1st ed.; Springer: New York, NY, USA, 2000; ISBN 978-1-4419-3161-0. [Google Scholar]
- Righi, E.; Peri, A.M.; Harris, P.N.A.; Wailan, A.M.; Liborio, M.; Lane, S.W.; Paterson, D.L. Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2017, 72, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, L.; Meybeck, A.; Patoz, P.; Van Grunderbeeck, N.; Boussekey, N.; Chiche, A.; Delannoy, P.Y.; Georges, H.; Leroy, O. Impact of combination therapy and early de-escalation on outcome of ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Infect. Dis. 2017, 49, 396–404. [Google Scholar] [CrossRef]
- Suárez, C.; Peña, C.; Gavaldà, L.; Tubau, F.; Manzur, A.; Dominguez, M.A.; Pujol, M.; Gudiol, F.; Ariza, J. Influence of carbapenem resistance on mortality and the dynamics of mortality in Pseudomonas aeruginosa bloodstream infection. Int. J. Infect. Dis. 2010, 14 (Suppl. 3), e73–e78. [Google Scholar] [CrossRef]
- Albasanz-Puig, A.; Gudiol, C.; Puerta-Alcalde, P.; Ayaz, C.M.; Machado, M.; Herrera, F.; Martín-Dávila, P.; Laporte-Amargós, J.; Cardozo, C.; Akova, M.; et al. Impact of the Inclusion of an Aminoglycoside to the Initial Empirical Antibiotic Therapy for Gram-Negative Bloodstream Infections in Hematological Neutropenic Patients: A Propensity-Matched Cohort Study (AMINOLACTAM Study). Antimicrob. Agents Chemother. 2021, 65, e00045-21. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Tansarli, G.S.; Bliziotis, I.A.; Falagas, M.E. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: A meta-analysis. Int. J. Antimicrob. Agents 2013, 41, 301–310. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Cherian, J.D.; Avent, M.L.; Paterson, D.L. Combination antibiotic therapy for Pseudomonas aeruginosa bacteremia in febrile neutropenic patients? The question still remains. Infect. Dis. 2018, 50, 403–406. [Google Scholar] [CrossRef]
- Giamarellou, H. Aminoglycosides plus beta-lactams against gram-negative organisms: Evaluation of in vitro synergy and chemical interactions. Am. J. Med. 1986, 80, 126–137. [Google Scholar] [CrossRef]
- Chumbita, M.; Puerta-Alcalde, P.; Gudiol, C.; Garcia-Pouton, N.; Laporte-Amargós, J.; Ladino, A.; Albasanz-Puig, A.; Helguera, C.; Bergas, A.; Grafia, I.; et al. Impact of empirical antibiotic regimens on mortality in neutropenic patients with bloodstream infection presenting with septic shock. Antimicrob. Agents Chemother. 2021, 66, AAC-01744. [Google Scholar] [CrossRef]
- Criscuolo, M.; Trecarichi, E.M. Ceftazidime/avibactam and ceftolozane/tazobactam for multidrug-resistant gram negatives in patients with hematological malignancies: Current experiences. Antibiotics 2020, 9, 58. [Google Scholar] [CrossRef]
- Fernández-Cruz, A.; Alba, N.; Semiglia-Chong, M.A.; Padilla, B.; Rodríguez-Macías, G.; Kwon, M.; Cercenado, E.; Chamorro-de-Vega, E.; Machado, M.; Pérez-Lago, L.; et al. A case-control study of real-life experience with ceftolozane-tazobactam in patients with hematologic malignancy and pseudomonas aeruginosa infection. Antimicrob. Agents Chemother. 2019, 63, e02340-18. [Google Scholar] [CrossRef]
| No Antibiotic Treatment (Death < 48 h) | 3/294 (1) |
| Initial empirical monotherapy | 173/294 (58.9) |
| Piperacillin/tazobactam | 84 (48.6) |
| Antipseudomonal carbapenems (imipenem, meropenem) | 54 (31.2) |
| Antipseudomonal cephalosporins | 24 (13.9) |
| Polymyxins (Colistin/Polymyxin B) | 2 (1.1) |
| Fluoroquinolones | 1 (0.6) |
| Aminoglycoside | 1 (0.6) |
| Others a | 7 (4) |
| Initial empirical combination therapy | 118/294 (40.1) |
| β-lactam + AG | 87 (73.7) |
| β-lactam + non-AG | 27 (22.9) |
| Non-β-lactam combination | 4 (3.4) |
| Appropriate empirical treatment | 226/294 (76.9) |
| Monotherapy b | 147/226 (65) |
| Piperacillin/tazobactam | 73 (49.7) |
| Anti-pseudomonal carbapenems (imipenem, meropenem) | 37 (25.2) |
| Anti-pseudomonal cephalosporins | 23 (15.7) |
| Polymyxins (Colistin, Polymyxin B) | 12 (8.2) |
| Fluoroquinolone (levofloxacin/ciprofloxacin) | 2 (1.4) |
| Combined therapy | 79/226 (35) |
| β-lactam + AG | 59 (74.7) |
| β-lactam + non-AG | 20 (25.3) |
| Inappropriate empirical treatment | 68/294 (23.1) |
| No antibiotic treatment (Death < 48 h) | 3/68 (4.4) |
| Monotherapy | 41/68 (60.3) |
| Anti-pseudomonal carbapenems (imipenem, meropenem) | 16 (39) |
| Piperacillin/tazobactam | 15 (36.6) |
| Anti-pseudomonal cephalosporins | 2 (4.9) |
| Aminoglycosides | 1 (2.4) |
| Others c | 7 (17) |
| Combination therapy | 24/68 (35.3) |
| β-lactam + AG d | 21 (87.5) |
| β-lactam + non-AG | 1 (4.2) |
| Non-β-lactam combination | 2 (8.3) |
| PA Pneumonia n = 294 (%) | PA BSI n = 723 (%) | p-Value | |
|---|---|---|---|
| 30-day case-fatality rate | 162 (55.1) | 227 (31.4) | <0.001 |
| Persistent BSI (48h from BSI onset) | 41 (14.5) | 71 (9.9) | 0.048 |
| ICU admission | 126 (42.9) | 186 (25.7) | <0.001 |
| Need for mechanical ventilation | 83 (28.2) | 115 (15.9) | <0.001 |
| Variables | Alive n = 132 | Dead n = 162 | HR | CI 95% | p-Value |
|---|---|---|---|---|---|
| Age (y), mean (SD) | 61.6 (14.8) | 61.1 (13.6) | 1.00 | 0.99–1.01 | 0.781 |
| Gender (female) | 41 (31.1) | 51 (31.5) | 1.13 | 0.81–1.58 | 0.534 |
| Acute leukemia | 39 (29.5) | 56 (34.6) | 1.12 | 0.81–1.55 | 0.448 |
| Refractory disease | 48 (36.4) | 71 (43.8) | 1.29 | 0.95–1.76 | 0.122 |
| HSCT | 20 (15.2) | 35 (21.6) | 1.17 | 0.81–1.71 | 0.344 |
| GVHD | 7 (43.8) | 14 (48.3) | 1.09 | 0.53–2.27 | 0.806 |
| Comorbidities a | 77 (62.1) | 95 (60.1) | 0.97 | 0.71–1.34 | 0.810 |
| BSI acquisition (hospital-acquired) | 58 (43.9) | 88 (54.3) | 1.18 | 0.87–1.61 | 0.223 |
| Prior corticosteroid treatment (1 month) | 71 (53.8) | 94 (58.8) | 1.13 | 0.83–1.55 | 0.417 |
| Severe neutropenia | 68 (54) | 96 (59.6) | 1.26 | 0.92–1.73 | 0.165 |
| Septic shock | 34 (26) | 107 (66) | 3.56 | 2.56–4.94 | <0.001 |
| Multidrug-resistant strain | 11 (8.3) | 41 (25.3) | 2.05 | 1.43–2.93 | <0.001 |
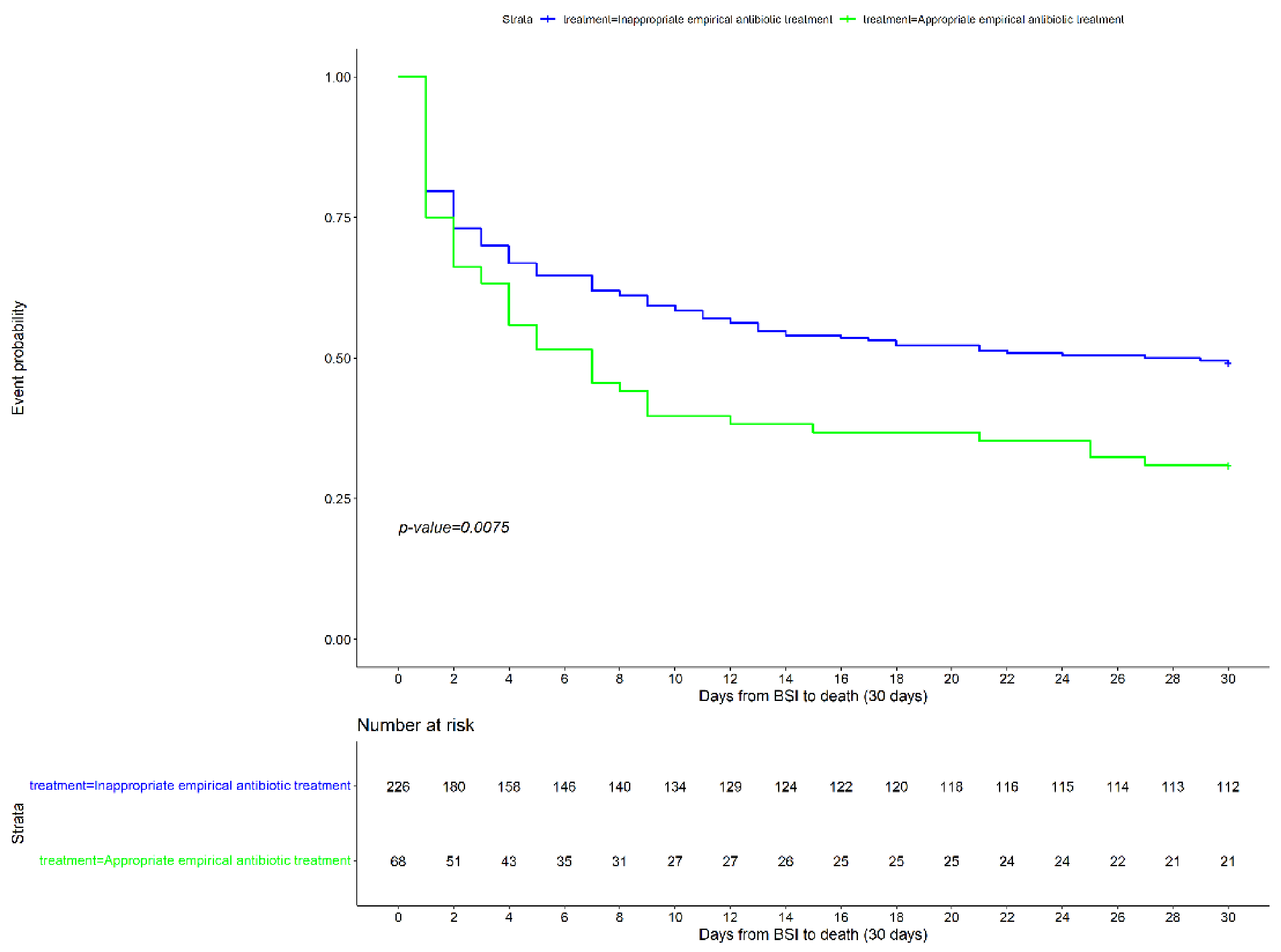
| Inappropriate empirical antibiotic treatment | 21 (15.9) | 47 (29.0) | 1.57 | 1.21–2.21 | 0.009 |
| Empirical treatment adequacy | 0.016 | ||||
| Inappropriate empirical antibiotic treatment | 21 (15.9) | 47 (29) | Ref | Ref | Ref |
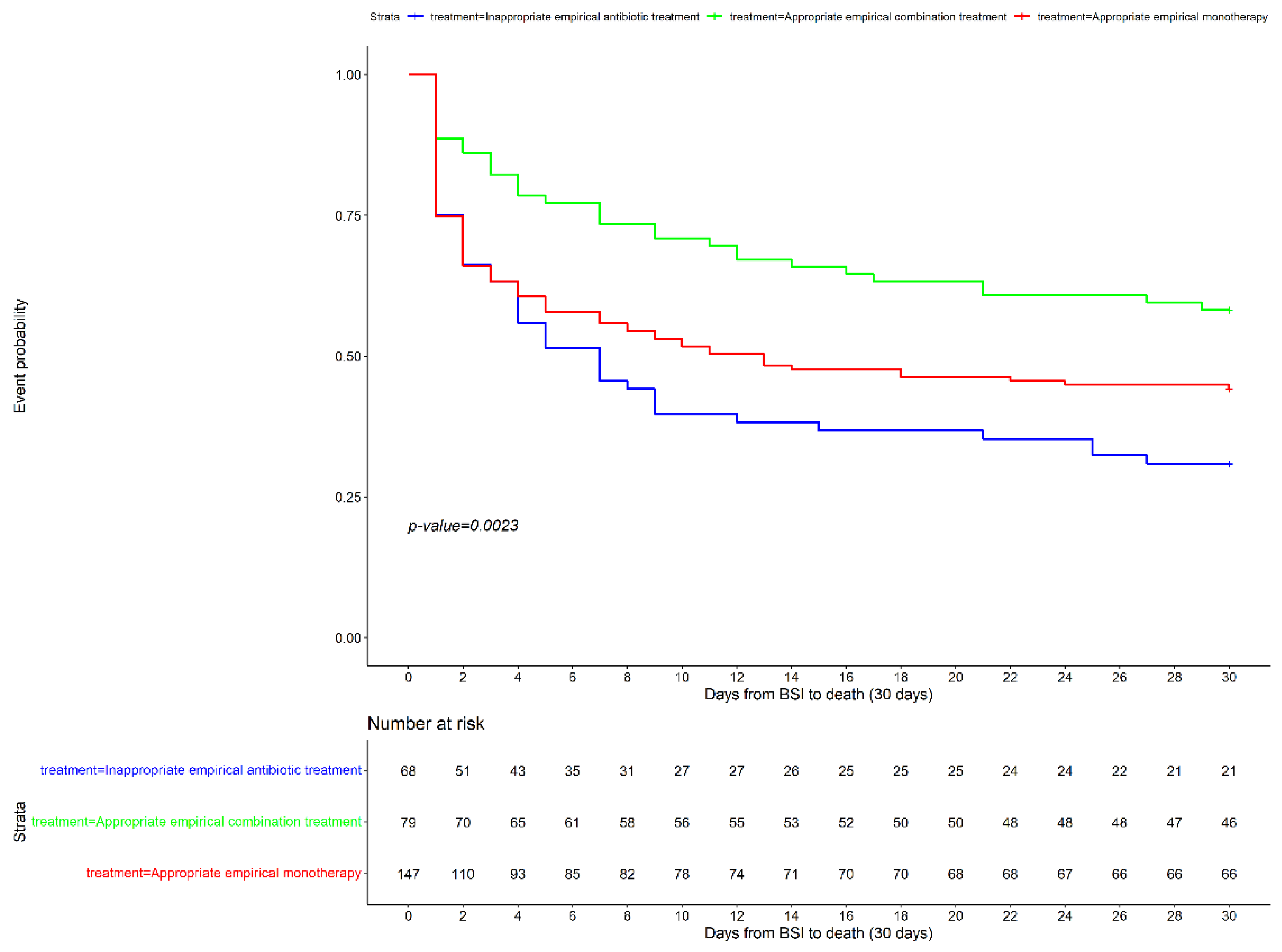
| Appropriate empirical treatment (monotherapy) | 65 (49.2) | 82 (50.6) | 0.75 | 0.52–1.07 | 0.115 |
| Appropriate empirical treatment (combination treatment) | 46 (34.8) | 33 (20.4) | 0.46 | 0.29–0.72 | 0.001 |
| Predictors | 30-Day Case-Fatality Rate | |||
|---|---|---|---|---|
| aHR | Std. Error | CI 95% | p-Value | |
| Age | 1.00 | 0.01 | 0.99–1.01 | 0.616 |
| Gender | 1.17 | 0.20 | 0.83–1.64 | 0.376 |
| Inappropriate empirical antibiotic treatment | 1.44 | 0.26 | 1.01–2.03 | 0.042 |
| Septic shock | ||||
| Group 1 (0–48 h from BSI onset) | 6.53 | 1.87 | 3.73–11.43 | <0.001 |
| Group 2 (48 h–10 days from BSI onset) | 2.89 | 0.22 | 1.66–5.05 | <0.001 |
| Group 3 (10 days–30 days from BSI onset) | 1.41 | 0.58 | 0.63–3.14 | 0.400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albasanz-Puig, A.; Durà-Miralles, X.; Laporte-Amargós, J.; Mussetti, A.; Ruiz-Camps, I.; Puerta-Alcalde, P.; Abdala, E.; Oltolini, C.; Akova, M.; Montejo, J.M.; et al. Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia. Microorganisms 2022, 10, 733. https://doi.org/10.3390/microorganisms10040733
Albasanz-Puig A, Durà-Miralles X, Laporte-Amargós J, Mussetti A, Ruiz-Camps I, Puerta-Alcalde P, Abdala E, Oltolini C, Akova M, Montejo JM, et al. Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia. Microorganisms. 2022; 10(4):733. https://doi.org/10.3390/microorganisms10040733
Chicago/Turabian StyleAlbasanz-Puig, Adaia, Xavier Durà-Miralles, Júlia Laporte-Amargós, Alberto Mussetti, Isabel Ruiz-Camps, Pedro Puerta-Alcalde, Edson Abdala, Chiara Oltolini, Murat Akova, José Miguel Montejo, and et al. 2022. "Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia" Microorganisms 10, no. 4: 733. https://doi.org/10.3390/microorganisms10040733
APA StyleAlbasanz-Puig, A., Durà-Miralles, X., Laporte-Amargós, J., Mussetti, A., Ruiz-Camps, I., Puerta-Alcalde, P., Abdala, E., Oltolini, C., Akova, M., Montejo, J. M., Mikulska, M., Martín-Dávila, P., Herrera, F., Gasch, O., Drgona, L., Morales, H. M. P., Brunel, A.-S., García, E., Isler, B., ... on behalf of the IRONIC Study Group. (2022). Effect of Combination Antibiotic Empirical Therapy on Mortality in Neutropenic Cancer Patients with Pseudomonas aeruginosa Pneumonia. Microorganisms, 10(4), 733. https://doi.org/10.3390/microorganisms10040733







