Viral and Bacterial Zoonotic Agents in Dromedary Camels from Southern Tunisia: A Seroprevalence Study
Abstract
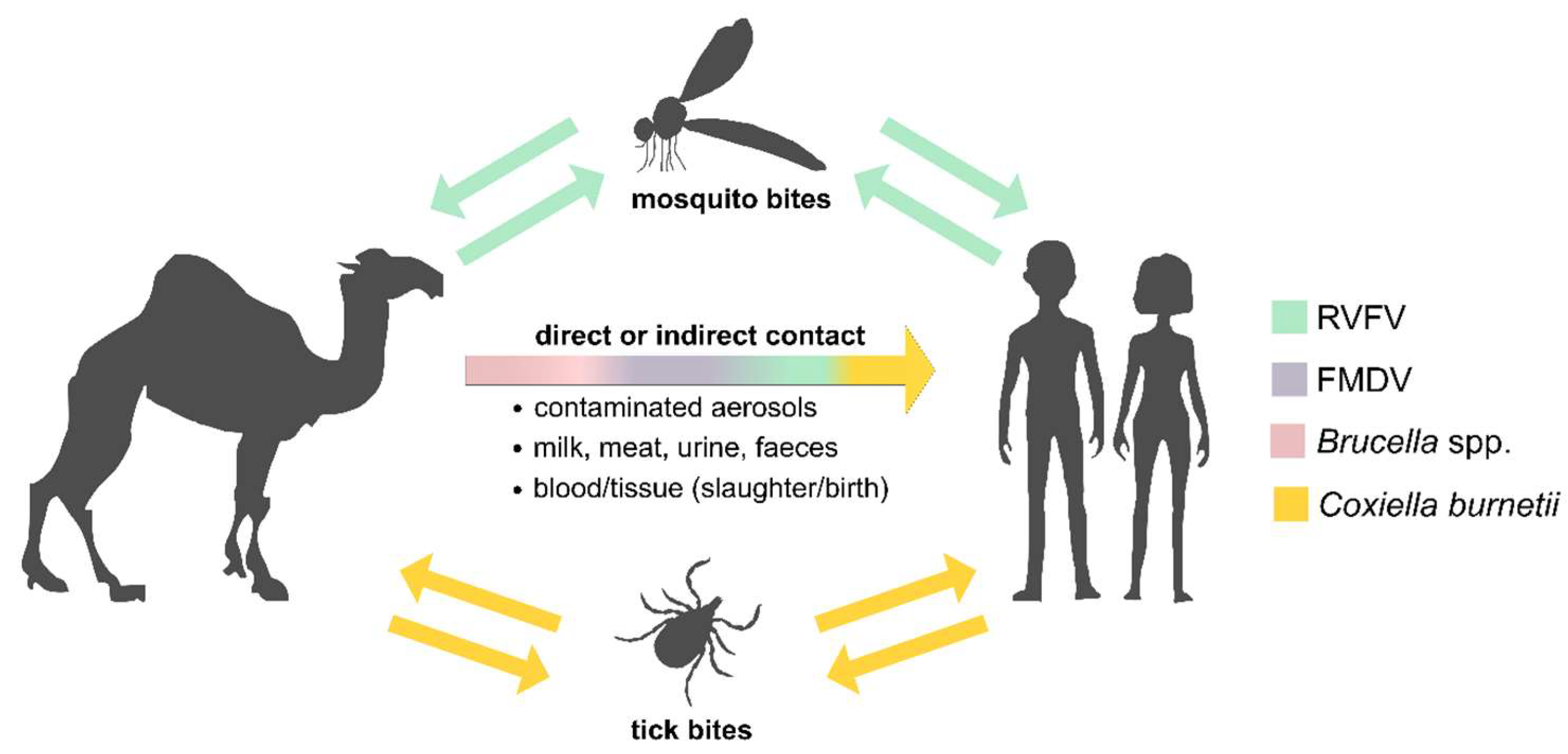
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serological Testing
2.3. Statistical Analysis
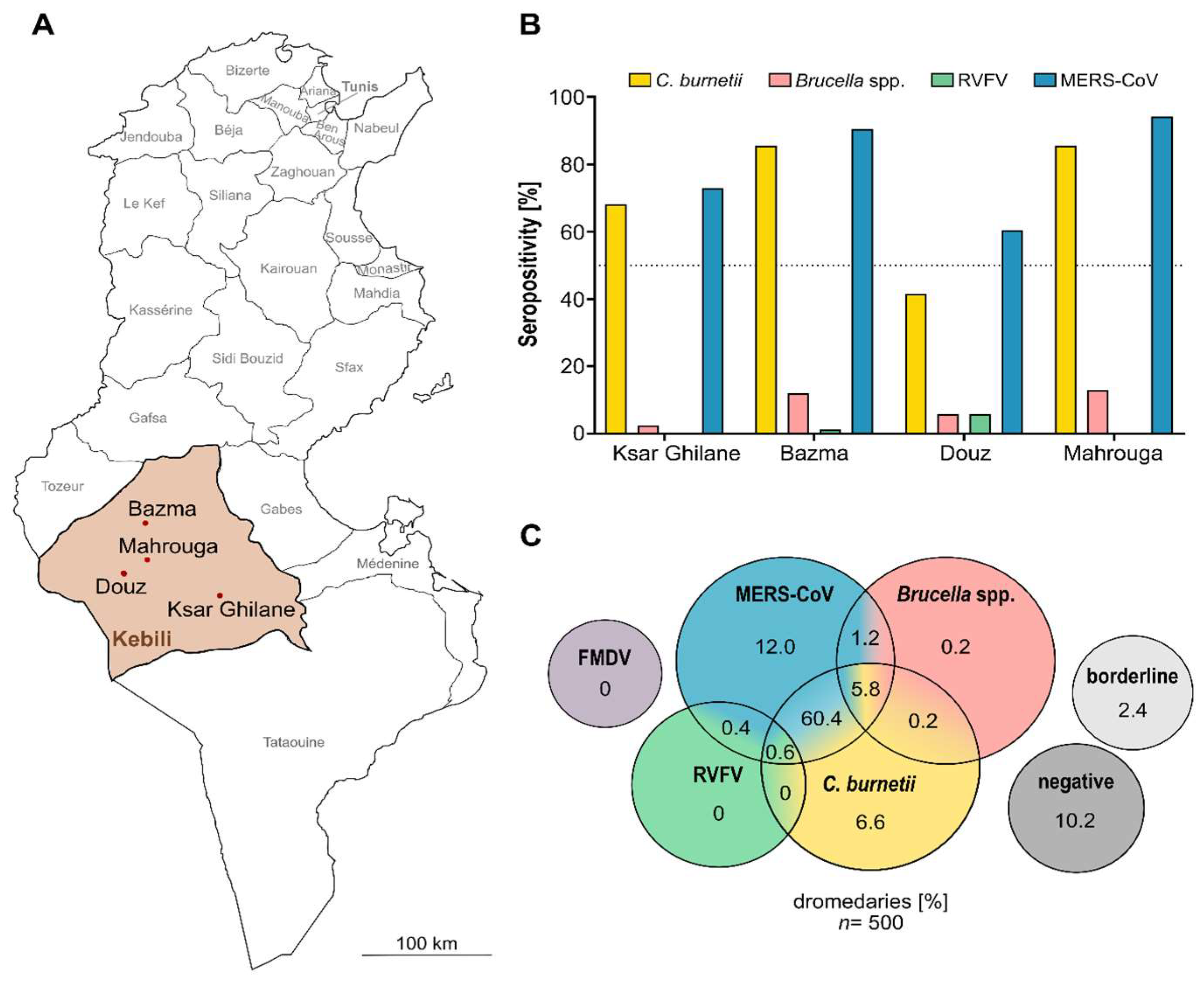
3. Results and Discussion
3.1. Majority of Camels Seropositive for Anti-C. burnetii IgG
3.2. Low Brucella spp. Seropositivity
3.3. Sporadic Serologic Evidence of Rift Valley Fever Infections
3.4. No Evidence of Foot-and-Mouth Disease
3.5. High Serological Evidence for Various Zoonotic Agents in Dromedary Camels of Southern Tunisia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ELISA | Result | Formula | ||
|---|---|---|---|---|
| Negative | Borderline | Positive | ||
| Anti-Brucella ELISA camel | Ratio < 0.8 | Ratio ≥ 0.8– < 1.1 | Ratio ≥ 1.1 | |
| ID Screen® Q Fever Indirect | S/P% ≤ 40% | S/P% > 40%– ≤ 50% | S/P% > 50% | |
| ID Screen® RVF Competition | S/N% > 50% | S/N% < 40%– ≤ 50% | S/N% ≤ 40% | |
| PrioCHECK® FMDV NS | PI = < 50% | / | PI = ≥ 50% | |
References
- Jemli, M.H.; Boulaifene, H.; Azaouzi, Z.; Ben Salem, W.; Khaldi, S. Camel breeding development project Tunisia. Rev. Maroc. Sci. Agron. Vét. 2018, 6, 256–259. [Google Scholar]
- Zhu, S.; Zimmerman, D.; Deem, S.L. A Review of Zoonotic Pathogens of Dromedary Camels. EcoHealth 2019, 16, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B. The tranfer of Brucella abortus antibodies from dam to calf. Vet. Rec. 1977, 100, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Q fever. Vet. Microbiol. 2010, 140, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef]
- Godfroid, J.; Scholz, H.; Barbier, T.; Nicolas, C.; Wattiau, P.; Fretin, D.; Whatmore, A.; Cloeckaert, A.; Blasco, J.; Moriyon, I.; et al. Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev. Vet. Med. 2011, 102, 118–131. [Google Scholar] [CrossRef]
- Anderson, A.; Bijlmer, H.; Fournier, P.E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H.; et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2013, 62, 1–29. [Google Scholar]
- Wernery, U. Camelid brucellosis: A review. J. Bacteriol. Mycol. 2016, 3, 1019. [Google Scholar] [CrossRef]
- Dalrymple-Champneys, W. The Future of Brucella Infection in Animals and Man. R. Soc. Health J. 1960, 80, 366–368. [Google Scholar] [CrossRef]
- Spink, W.W. Brucellosis; epidemiology, clinical manifestations, diagnosis. Semin. Int. 1956, 5, 15–17. [Google Scholar]
- Maurin, M.; Raoult, D. Q fever. Clin. Microbiol. Rev. 1999, 12, 518–553. [Google Scholar] [CrossRef] [PubMed]
- Kaabia, N.; Letaief, A. Q Fever in Tunisia. Pathol. Biol. 2009, 57, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Vanderburg, S.; Rubach, M.; Halliday, J.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef] [PubMed]
- Daubney, R.; Hudson, J.R.; Garnham, P.C. Enzootic hepatitis or rift valley fever. An undescribed virus disease of sheep cattle and man from east africa. J. Pathol. Bacteriol. 1931, 34, 545–579. [Google Scholar] [CrossRef]
- Dar, O.; McIntyre, S.; Hogarth, S.; Heymann, D. Rift Valley Fever and a New Paradigm of Research and Development for Zoonotic Disease Control. Emerg. Infect. Dis. 2013, 19, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.; Cope, S.; Botros, B.; Hibbs, R.; Imam, I.; El-Sharkawy, M.; Oun, S.; Morrill, J.; Shope, R.; Darwish, M. Recurrence of Rift Valley fever in Egypt. Lancet 1993, 342, 1149–1150. [Google Scholar] [CrossRef]
- Bukbuk, D.N.; Fukushi, S.; Tani, H.; Yoshikawa, T.; Taniguchi, S.; Iha, K.; Fukuma, A.; Shimojima, M.; Morikawa, S.; Saijo, M.; et al. Development and validation of serological assays for viral hemorrhagic fevers and determination of the prevalence of Rift Valley fever in Borno State, Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 768–773. [Google Scholar] [CrossRef]
- Durand, J.P.; Bouloy, M.; Richecoeur, L.; Peyrefitte, C.N.; Tolou, H. Rift Valley Fever Virus Infection among French Troops in Chad. Emerg. Infect. Dis. 2003, 9, 751–752. [Google Scholar] [CrossRef]
- Faye, O.; Bâ, H.; Ba, Y.; Freire, C.C.; Faye, O.; Ndiaye, O.; Elgady, I.O.; Zanotto, P.M.; Diallo, M.; Sall, A.A. Reemergence of Rift Valley Fever, Mauritania, 2010. Emerg. Infect. Dis. 2014, 20, 300–303. [Google Scholar] [CrossRef]
- Hassan, O.A.; Ahlm, C.; Evander, M. A need for One Health approach—Lessons learned from outbreaks of Rift Valley fever in Saudi Arabia and Sudan. Infect. Ecol. Epidemiol. 2014, 4, 20710. [Google Scholar] [CrossRef]
- Bird, B.H.; Nichol, S.T. Breaking the chain: Rift Valley fever virus control via livestock vaccination. Curr. Opin. Virol. 2012, 2, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chengula, A.A.; Mdegela, R.H.; Kasanga, C.J. Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania. SpringerPlus 2013, 2, 549. [Google Scholar] [CrossRef] [PubMed]
- Arsevska, E.; Hellal, J.; Mejri, S.; Hammami, S.; Marianneau, P.; Calavas, D.; Hénaux, V. Identifying Areas Suitable for the Occurrence of Rift Valley Fever in North Africa: Implications for Surveillance. Transbound. Emerg. Dis. 2015, 63, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Baudin, M.; Jumaa, A.M.; Jomma, H.J.E.; Karsany, M.S.; Bucht, G.; Näslund, J.; Ahlm, C.; Evander, M.; Mohamed, N. Association of Rift Valley fever virus infection with miscarriage in Sudanese women: A cross-sectional study. Lancet Glob. Health 2016, 4, e864–e871. [Google Scholar] [CrossRef]
- Terrestrial Animal Health Code 2021; OIE, World Organization for Animal Health: Paris, France, 2021.
- Coetzer, J.A. The pathology of Rift Valley fever. II. Lesions occurring in field cases in adult cattle, calves and aborted foetuses. Onderstepoort J. Vet. Res. 1982, 49, 11–17. [Google Scholar]
- Selmi, R.; Mamlouk, A.; Ben Said, M.; Ben Yahia, H.; Abdelaali, H.; Ben Chehida, F.; Daaloul-Jedidi, M.; Gritli, A.; Messadi, L. First serological evidence of the Rift Valley fever Phlebovirus in Tunisian camels. Acta Trop. 2020, 207, 105462. [Google Scholar] [CrossRef]
- Gladue, D.P.; O’Donnell, V.; Baker-Branstetter, R.; Holinka, L.G.; Pacheco, J.M.; Fernández Sainz, I.; Lu, Z.; Ambroggio, X.; Rodriguez, L.; Borca, M.V. Foot-and-mouth disease virus modulates cellular vimentin for virus survival. J. Virol. 2013, 87, 6794–6803. [Google Scholar] [CrossRef]
- Capella, G.L. Foot and mouth disease in human beings. Lancet 2001, 358, 1374. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.D.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- Eckstein, S.; Ehmann, R.; Gritli, A.; Ben Yahia, H.; Diehl, M.; Wölfel, R.; Ben Rhaiem, M.; Stoecker, K.; Handrick, S.; Ben Moussa, M. Prevalence of Middle East Respiratory Syndrome Coronavirus in Dromedary Camels, Tunisia. Emerg. Infect. Dis. 2021, 27, 1964–1968. [Google Scholar] [CrossRef]
- Faye, B. Camel Farming Sustainability: The Challenges of the Camel Farming System in the XXIth Century. J. Sustain. Dev. 2013, 6, 74. [Google Scholar] [CrossRef]
- Rissmann, M.; Eiden, M.; EL Mamy, B.O.; Isselmou, K.; Doumbia, B.; Ziegler, U.; Homeier-Bachmann, T.; Yahya, B.; Groschup, M.H. Serological and genomic evidence of Rift Valley fever virus during inter-epidemic periods in Mauritania. Epidemiology Infect. 2017, 145, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Selmi, R.; Mamlouk, A.; Ben Yahia, H.; Abdelaali, H.; Ben Said, M.; Sellami, K.; Daaloul-Jedidi, M.; Jemli, M.H.; Messadi, L. Coxiella burnetii in Tunisian dromedary camels (Camelus dromedarius): Seroprevalence, associated risk factors and seasonal dynamics. Acta Trop. 2018, 188, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Mazloum, K.; Alnakhli, H. Serological evidence of natural exposure of camels Camelus dromedaries to foot and mouth disease virus. Vet. World 2012, 5, 197–200. [Google Scholar] [CrossRef]
- Kaabia, N.; Rolain, J.-M.; Khalifa, M.; Jazia, E.B.; Bahri, F.; Raoult, D.; Letaief, A. Serologic Study of Rickettsioses among Acute Febrile Patients in Central Tunisia. Ann. N. Y. Acad. Sci. 2006, 1078, 176–179. [Google Scholar] [CrossRef]
- Bellazreg, F.; Kaabia, N.; Hachfi, W.; Khalifa, M.; Ben Jazia, E.; Ghanouchi, N.; Brahem, A.; Bahri, F.; Letaief, A. Acute Q fever in hospitalised patients in Central Tunisia: Report of 21 cases. Clin. Microbiol. Infect. 2009, 15, 138–139. [Google Scholar] [CrossRef][Green Version]
- Muturi, M.; Akoko, J.; Nthiwa, D.; Chege, B.; Nyamota, R.; Mutiiria, M.; Maina, J.; Thumbi, S.M.; Nyamai, M.; Kahariri, S.; et al. Serological evidence of single and mixed infections of Rift Valley fever virus, Brucella spp. and Coxiella burnetii in dromedary camels in Kenya. PLoS Negl. Trop. Dis. 2021, 15, e0009275. [Google Scholar] [CrossRef]
- El Nabi, G.; Bakhiet, A.O.; Alshaikh, M.A.; Aljumaah, R.S.; Mohammed, O.B.; Hussein, M.F. Prevalence of Antibodies to Coxiella burnetii in Camel Milk in Riyadh Region, Saudi Arabia: A Comparison with Serum. J. Anim. Res. 2015, 5, 431. [Google Scholar] [CrossRef]
- Klemmer, J.; Njeru, J.; Emam, A.; El-Sayed, A.; Moawad, A.; Henning, K.; Elbeskawy, M.A.; Sauter-Louis, C.; Straubinger, R.; Neubauer, H.; et al. Q fever in Egypt: Epidemiological survey of Coxiella burnetii specific antibodies in cattle, buffaloes, sheep, goats and camels. PLoS ONE 2018, 13, e0192188. [Google Scholar] [CrossRef]
- Kersh, G.J.; Fitzpatrick, K.A.; Self, J.S.; Priestley, R.A.; Kelly, A.J.; Lash, R.R.; Marsden-Haug, N.; Nett, R.J.; Bjork, A.; Massung, R.F.; et al. Presence and Persistence of Coxiella burnetii in the Environments of Goat Farms Associated with a Q Fever Outbreak. Appl. Environ. Microbiol. 2013, 79, 1697–1703. [Google Scholar] [CrossRef]
- Wittwer, M.; Hammer, P.; Runge, M.; Valentin-Weigand, P.; Neubauer, H.; Henning, K.; Mertens-Scholz, K. Inactivation Kinetics of Coxiella burnetii During High-Temperature Short-Time Pasteurization of Milk. Front. Microbiol. 2022, 12, 753871. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Osman, I.O.; Million, M.; Raoult, D. Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East? Front. Vet. Sci. 2020, 7, 558481. [Google Scholar] [CrossRef] [PubMed]
- OiE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021. Vol. Chapter 3.1.16., Q Fever. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.16_Q_FEVER.pdf (accessed on 14 December 2021).
- Vaccins. Ceva Tunisian Online Representation. Available online: https://www.ceva.tn/Produits/Ovins-Caprins/Vaccins (accessed on 7 February 2022).
- De Cremoux, R.; Rousset, E.; Touratier, A.; Audusseau, G.; Nicollet, P.; Ribaud, D.; David, V.; Le Pape, M. Assessment of vaccination by a phase I Coxiella burnetii-inactivated vaccine in goat herds in clinical Q fever situation: 1. FEMS Immunol. Med. Microbiol. 2012, 64, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Guatteo, R.; Seegers, H.; Joly, A.; Beaudeau, F. Prevention of Coxiella burnetii shedding in infected dairy herds using a phase I C. burnetii inactivated vaccine. Vaccine 2008, 26, 4320–4328. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Wolfe, D.N. Vaccination against Q Fever for Biodefense and Public Health Indications. Front. Microbiol. 2014, 5, 726. Available online: http://journal.frontiersin.org/article/10.3389/fmicb.2014.00726/abstract (accessed on 30 August 2021). [CrossRef] [PubMed]
- Sellens, E.; Bosward, K.L.; Willis, S.; Heller, J.; Cobbold, R.; Comeau, J.L.; Norris, J.M.; Dhand, N.K.; Wood, N. Frequency of Adverse Events Following Q Fever Immunisation in Young Adults. Vaccines 2018, 6, 83. [Google Scholar] [CrossRef]
- Corbel, M.J. Brucellosis in Humans and Animals; World Health Organization, FAO, OIE—World Organisation for Animal Health, Eds.; World Health Organization: Geneva, Switzerland, 2006; 89p.
- Godfroid, J. Brucellosis in livestock and wildlife: Zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health 2017, 75, 34. [Google Scholar] [CrossRef]
- Refai, M. Incidence and control of brucellosis in the Near East region. Vet. Microbiol. 2002, 90, 81–110. [Google Scholar] [CrossRef]
- Gideon Informatics, I.; Berger, S. Infectious Diseases of Tunisia. Los Angeles: Gideon Informatics, Incorporated. 2020. Available online: https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=6131180 (accessed on 11 August 2021).
- Blasco, J. A review of the use of B. melitensis Rev 1 vaccine in adult sheep and goats. Prev. Vet. Med. 1997, 31, 275–283. [Google Scholar] [CrossRef]
- Van den Heever, L.W.; Katz, K.W.; Te Brugge, L.A. On the inactivation of Brucella abortus in naturally contaminated milk by commercial pasteurisation procedures. J. S. Afr. Vet. Assoc. 1982, 53, 233–234. [Google Scholar]
- Bosworth, A.; Ghabbari, T.; Dowall, S.; Varghese, A.; Fares, W.; Hewson, R.; Zhioua, E.; Chakroun, M.; Tiouiri, H.; Ben Jemaa, M.; et al. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zouaghi, K.; Bouattour, A.; Aounallah, H.; Surtees, R.; Krause, E.; Michel, J.; Mamlouk, A.; Nitsche, A.; M’Ghirbi, Y. First Serological Evidence of Crimean-Congo Hemorrhagic Fever Virus and Rift Valley Fever Virus in Ruminants in Tunisia. Pathogens 2021, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Ngoshe, Y.B.; Avenant, A.; Rostal, M.K.; Karesh, W.B.; Paweska, J.T.; Bagge, W.; Van Vuren, P.J.; Kemp, A.; Cordel, C.; Msimang, V.; et al. Patterns of Rift Valley fever virus seropositivity in domestic ruminants in central South Africa four years after a large outbreak. Sci. Rep. 2020, 10, 5489. [Google Scholar] [CrossRef] [PubMed]
- Faburay, B.; LaBeaud, A.D.; McVey, D.S.; Wilson, W.C.; Richt, J.A. Current Status of Rift Valley Fever Vaccine Development. Vaccines 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- FAO. Quarterly Reports of the The European Commission for the Control of Foot-and-Mouth Disease (EuFMD). Available online: http://www.fao.org/eufmd/resources/reports/quarterlyreport/en/ (accessed on 21 September 2021).
- Sana, K.; Ameni, B.S.; Kaouther, G.; Jamel, C.; Samia, M.; Anissa, D.; Naceur, B.M. An Overview of Foot and Mouth Disease Situation in Tunisia (1975–2017). J. Vet. Sci. Technol. 2018, 9, 6. [Google Scholar] [CrossRef]
| Sampling Parameter | No. Dromedaries | No. Seropositive Dromedaries (%) | ||
|---|---|---|---|---|
| Coxiella burnetii | Brucella spp. | Rift Valley Fever Virus | ||
| 500 | 368 (73.6) | 37 (7.4) | 5 (1.0) | |
| Sex | ||||
| Male | 130 | 68 (52.3) | 5 (3.8) | 5 (3.8) |
| Female | 370 | 300 (81.1) | 32 (8.6) | 0 |
| Age | ||||
| Juvenile | 45 | 13 (28.9) | 0 | 0 |
| 0–6 months | 4 | 0 | 0 | 0 |
| 6–24 months | 41 | 13 (31.7) | 0 | 0 |
| Adult | 455 | 355 (78.0) | 37 (8.1) | 5 (1.1) |
| 2–6 years | 80 | 40 (50.0) | 4 (5.0) | 0 |
| 6–12 years | 179 | 142 (79.3) | 18 (10.1) | 4 (2.2) |
| 12–25 years | 190 | 167 (87.9) | 14 (7.4) | 1 (0.5) |
| >25 years | 6 | 6 (100) | 1 (16.7) | 0 |
| Sampling locations | ||||
| Ksar Ghilane (∑ = 6) | 211 | 144 (68.2) | 5 (2.4) | 0 |
| Site 1 | 28 | 9 (32.1) | 0 | 0 |
| Site 2 | 20 | 7 (35.0) | 2 (10.0) | 0 |
| Site 3 | 30 | 22 (73.3) | 0 | 0 |
| Site 4 | 20 | 19 (95.0) | 0 | 0 |
| Site 5 | 73 | 54 (74.0) | 3 (4.1) | 0 |
| Site 6 | 40 | 33 (82.5) | 0 | 0 |
| Bazma (∑ = 7) | 167 | 143 (85.6) | 20 (12.0) | 2 (1.2) |
| Site 1 | 25 | 21 (84.0) | 0 | 0 |
| Site 2 | 30 | 28 (93.3) | 2 (6.7) | 0 |
| Site 3 | 15 | 14 (93.3) | 5 (33.3) | 0 |
| Site 4 | 15 | 15 (100) | 3 (20.0) | 0 |
| Site 5 | 21 | 18 (85.7) | 2 (9.5) | 0 |
| Site 6 | 16 | 14 (87.5) | 4 (25.0) | 0 |
| Site 7 | 45 | 33 (73.3) | 4 (8.9) | 2 (4.4) |
| Douz (∑ = 5) | 53 | 22 (41.5) | 3 (5.7) | 3 (5.7) |
| Site 1a | 4 | 3 (75.0) | 0 | 1 (25.0) |
| Site 1b | 4 | 1 (25.0) | 0 | 0 |
| Site 2 | 24 | 10 (41.7) | 2 (8.3) | 1 (4.2) |
| Site 3 | 18 | 7 (38.9) | 1 (5.6) | 1 (5.6) |
| Site 4 | 3 | 1 (33.3) | 0 | 0 |
| Mahrouga (∑ = 2) | 69 | 58 (84.1) | 9 (13.0) | 0 |
| Site 1 | 42 | 35 (83.3) | 5 (11.9) | 0 |
| Site 2 | 27 | 23 (85.2) | 4 (14.8) | 0 |
| Animal husbandry | ||||
| Free-roaming | 382 | 306 (80.1) | 28 (7.3) | 0 |
| Kept enclosed | 118 | 62 (52.5) | 9 (7.6) | 5 (4.2) |
| Variable | Univariate | Multivariate c | ||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex | ||||
| Female | 3.91 (2.5–5.94) | <0.0001 (****) a | 0.7846 (0.21–2.50) | 0.6962 (ns) |
| Age | ||||
| Adult (>2 y) | 8.74 (4.50–17.71) | <0.0001 (****) a | 16.06 (7.49–36.92) | <0.0001 (****) |
| Husbandry | ||||
| Free roaming | 3.64 (2.356–5.60) | <0.0001 (****) a | 14.4 (3.85–61.77) | 0.0001 (***) |
| Location Site | <0.0001 (****) b | |||
| Ksar Ghilane | 0.35 (0.20–0.61) | 0.0002 (***) | ||
| Bazma | 3.62 (2.10–6.46) | <0.0001 (****) | ||
| Douz | 0.54 (0.25–1.14) | 0.1067 (ns) | ||
| Mahrouga | 0.93 (0.44–2.10) | 0.8481 (ns) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, S.; Ehmann, R.; Gritli, A.; Ben Rhaiem, M.; Ben Yahia, H.; Diehl, M.; Wölfel, R.; Handrick, S.; Ben Moussa, M.; Stoecker, K. Viral and Bacterial Zoonotic Agents in Dromedary Camels from Southern Tunisia: A Seroprevalence Study. Microorganisms 2022, 10, 727. https://doi.org/10.3390/microorganisms10040727
Eckstein S, Ehmann R, Gritli A, Ben Rhaiem M, Ben Yahia H, Diehl M, Wölfel R, Handrick S, Ben Moussa M, Stoecker K. Viral and Bacterial Zoonotic Agents in Dromedary Camels from Southern Tunisia: A Seroprevalence Study. Microorganisms. 2022; 10(4):727. https://doi.org/10.3390/microorganisms10040727
Chicago/Turabian StyleEckstein, Simone, Rosina Ehmann, Abderraouf Gritli, Mohamed Ben Rhaiem, Houcine Ben Yahia, Manuel Diehl, Roman Wölfel, Susann Handrick, Mohamed Ben Moussa, and Kilian Stoecker. 2022. "Viral and Bacterial Zoonotic Agents in Dromedary Camels from Southern Tunisia: A Seroprevalence Study" Microorganisms 10, no. 4: 727. https://doi.org/10.3390/microorganisms10040727
APA StyleEckstein, S., Ehmann, R., Gritli, A., Ben Rhaiem, M., Ben Yahia, H., Diehl, M., Wölfel, R., Handrick, S., Ben Moussa, M., & Stoecker, K. (2022). Viral and Bacterial Zoonotic Agents in Dromedary Camels from Southern Tunisia: A Seroprevalence Study. Microorganisms, 10(4), 727. https://doi.org/10.3390/microorganisms10040727






