Abstract
In recent years, syngas fermentation has emerged as a promising means for the production of fuels and platform chemicals, with a variety of acetogens efficiently converting CO-rich gases to ethanol. However, the feasibility of syngas fermentation processes is related to the occurrence of syngas impurities such as NH3, H2S, and NOX. Therefore, the effects of defined additions of NH4+, H2S, and NO3− were studied in autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei while applying continuously gassed stirred-tank bioreactors. Any initial addition of ammonium and nitrate curbed the cell growth of the Clostridia being studied and reduced the final alcohol concentrations. C. ljungdahlii showed the highest tolerance to ammonium and nitrate, whereas C. ragsdalei was even positively influenced by the presence of 0.1 g L−1 H2S. Quantitative goals for the purification of syngas were identified for each of the acetogens studied in the used experimental setup. Syngas purification should in particular focus on the NOX impurities that caused the highest inhibiting effect and maintain the concentrations of NH3 and H2S within an acceptable range (e.g., NH3 < 4560 ppm and H2S < 108 ppm) in order to avoid inhibition through the accumulation of these impurities in the bioreactor.
1. Introduction
Syngas (synthesis gas) fermentation is the microbiological conversion of CO-, H2-, and CO2-rich gases to short chain fatty acids or alcohols. If renewable resources are the feedstock for the gasification to syngas, then the microbial production of commercially relevant chemicals demonstrates higher sustainability than fossil feedstock. The biochemical conversion of syngas represents an alternative to the thermochemical conversion to, e.g., ethanol, given the advantages of milder temperatures and pressures, flexibility to H2/CO ratios, and higher product selectivity [1].
Some members of the genus Clostridium are able to convert CO or CO2 and H2 to acetate and ethanol, along with other strain-specific products such as 2,3-butanediol, butanol, and hexanol [2]. These acetogens use the reductive acetyl-CoA pathway for autotrophic carbon fixation. Energy in the form of ATP is conserved by a membrane-bound ATP-synthase, which uses either H+ or Na+ gradients across the membrane [3].
C. autoethanogenum, C. ljungdahlii, and C. ragsdalei belong to the same clade [4], having a sequence similarity of more than 99% [5] and producing acetate, ethanol, and 2,3-butanediol from CO or CO2 and H2 [6]. The growth optima are at 37 °C with a pH optimum of pH 5.5–6.0 [7,8]. All three strains favor the use of CO over H2 and CO2, as theoretically proposed [9] and shown experimentally for C. ljungdahlii [10]. The batch process performances of C. autoethanogenum and C. ljungdahlii were studied in anaerobic flasks with 50 kPa, 45 kPa, and 5 kPa of CO, H2, and CO2, respectively. C. autoethanogenum and C. ljungdahlii achieved similar optical densities and acetate concentrations, whereas the highest ethanol and 2,3-butanediol concentrations were measured with C. autoethanogenum [7]. C. ragsdalei achieved lower optical densities, but it was able to produce more ethanol than acetate.
When generated from biogenic materials, syngas contains (along with the main gas components CO, CO2, H2, and N2) a variety of impurities, depending on the gasification process and feedstock. These might include H2S, NH3, COS, HCN, and NOX [11]. The formation of these impurities can to some extent be controlled by the choice of the gasification conditions. Increasing the equivalence ratio (mass ratio between air and fuel in the gasification process) has been shown to increase the conversion of fuel bound nitrogen to ammonia and N2 but had no influence on the formation of HCN during the gasification of switchgrass [12].
It has already been demonstrated that syngas impurities accumulated in the aqueous fermentation broth may, depending on their concentrations, have an impact on the performance of the fermentation processes [13]. The addition of up to 29.4 mM sulfide, which corresponds to 1.0 g L−1 H2S, promoted autotrophic growth and alcohol formation in batch processes with Clostridium carboxidivorans in a continuously CO/CO2 gassed stirred-tank reactor [14]. However, even the addition of 1.9 mM sulfide (0.065 g L−1 H2S) slightly decreased cell dry weight (CDW) concentration and promoted ethanol production in anaerobic flasks with C. ragsdalei using artificial syngas as a substrate [15]. Sulfide inhibits anaerobic bacteria above a certain threshold and is associated with the undissociated H2S solved in water, which is membrane-permeable [16]. Once inside the cell, it can cause DNA damage and protein denaturation [17]. As a result, there are large differences between the effects of H2S on different anaerobic strains.
The addition of 93.5 mM NH4+ (5.00 g L−1 NH4Cl) promoted the autotrophic growth and alcohol formation of C. carboxidivorans in a continuously CO/CO2 gassed stirred-tank reactor [14], but the addition of 93.4 mM NH4+ (1.68 g L−1) showed no effect on the growth or product formation of C. ragsdalei in anaerobic flasks with a CO/CO2 atmosphere [18]. Ammonium has been shown to decrease the activity of hydrogenases and alcohol-dehydrogenases in acetogens [19], which catalyze the reactions for the supply of reduction equivalents from H2 or CO and for alcohol formation, respectively. Therefore, NH4+ concentrations above a certain threshold could be an obstacle in syngas fermentation processes with the concomitant supply of CO and H2/CO2.
Concentrations above 40 ppm nitric oxide in the syngas inhibited the autotrophic growth of C. carboxidivorans with H2/CO2, but this inhibition was reversible, and a complete inhibition of the hydrogenase activity was determined at 150 ppm NO [20].
Other nitrogen species also affect syngas fermentations with Clostridia. The addition of 0.1 g L−1 NaNO3 increased the lag phase of C. carboxidivorans to 30 h, but higher final CDW concentrations and a strong increase in butyrate concentrations was observed with artificial syngas in a stirred-tank bioreactor [14]. A total of 15 mM NaNO3 (1.275 g L−1) promoted the biomass growth of C. ljungdahlii in anaerobic flasks with H2 and CO2 as the gas phase, but growth was inhibited with CO and CO2 [21]. Moreover, the addition of nitrate reduced ethanol formation and promoted formate production. Substituting ammonium with nitrate on a molar basis led to higher optical densities in a chemostat at mean hydraulic residence times between 2 and 3.5 d using C. ljungdahlii with H2 and CO2 as the gas phase, but substituting ammonium with nitrate was associated with “stochastic metabolic crashes” [22]. C. ljungdahlii is able to reduce nitrate to ammonium, resulting in more than a doubling of the net ATP gain from H2 when compared to the production of acetate from H2 [21].
Information on the effect of syngas impurities on syngas fermentation processes is sparse, and the comparison between different strains is obscured by varying process designs and conditions [13]. Many of the published results are based on simple batch studies applying non-controlled anaerobic flasks with low gas amounts, low gas-liquid mass transfer rates, and low power input, but these conditions do not reflect scalable continuously gassed syngas fermentation processes and lack the quantitative analysis of gas consumption rates and, therefore, carbon balances. Our report represents an extensive comparison of fully controlled syngas fermentation processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei, respectively, in stirred-tank bioreactors at defined power input, gassing rates, and identical medium compositions for studying the strain-specific effects of defined additions of ammonium, sulfide, and nitrate on batch process performance. The process performance of these Clostridia were compared to published results using C. carboxidivorans [14]. Since the syngas components investigated may be present as impurities in syngas, the quantitative criteria for syngas purification processes were identified. This study also provides insights into the choice of preferred strains respecting their individual capabilities of converting CO-rich syngas.
2. Materials and Methods
2.1. Microorganisms and Cultivation Media
C. autoethanogenum (DSM 10061), C. ljungdahlii (DSM 13528), and C. ragsdalei (DSM 15248) were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). Precultures were prepared in 500 mL flasks with a butyl rubber septum and a previously published medium [23]. The detailed medium composition is listed in the Supplementary Material (Table S4). The medium was anaerobized through boiling and subsequent gassing with N2. Cysteine hydrochloride was added prior to inoculation from a previously anaerobized and sterilized stock solution. Precultures were incubated at a temperature of 37 °C and an agitation rate of 100 RPM (WiseCube WIS-20, Witeg Labortechnik GmbH, Wertheim, Germany). C. autoethanogenum precultures were prepared with 5 g L−1 xylose as a carbon source. Precultures of C. ljungdahlii and C. ragsdalei were prepared autotrophically with 1.2 bar CO, 0.4 bar CO2, and 0.4 bar H2.
2.2. Batch Processes in Stirred-Tank Bioreactors
Continuously gassed batch processes were performed in stirred-tank bioreactors with two six-blade Rushton turbines, temperature and pH control, and a working volume of 1 L (C. autoethanogenum; KLF2000, Bioengineering AG, Wald, Switzerland; C. ljungdahlii and C. ragsdalei: Labfors 2, Infors HT, Bottmingen, Switzerland). The reactors were sterilized with 1 L of demineralized water. The medium was autoclaved separately in closed 1 L flasks with butyl rubber septa and transferred through a sterilized silicone tube to the reactor. The medium was anaerobized with a gas mixture of either 60% CO, 20% CO2, and 20% H2 (C. autoethanogenum and C. ragsdalei) or 20% CO, 20% CO2, 20% H2, and 40% N2 (C. ljungdahlii) with 5 NL h−1 (under standard conditions by ISO 10,780 [24]) for at least 12 h at 37 °C. Stirrer speeds were 800 min−1 (C. ljungdahlii and C. ragsdalei) and 1200 min−1 (C. autoethanogenum), corresponding to a volumetric power input of 3.5 W L−1 and 15.1 W L−1, respectively. Inoculation was performed using resuspended cells in an anaerobic phosphate saline buffer to achieve an initial optical density OD600 of 0.1 in the stirred-tank bioreactor. Batch processes were performed at a temperature of 37 °C, and the pH level was kept constant at pH 6 with the addition of 3 M NaOH or 2 M H2SO4. Mass flow controllers (C. autoethanogenum: Bronkhorst F-101D-RAD-33-V, Wagner Mess- und Regeltechnik, Offenbach, Germany; C. ljungdahlii and C. ragsdalei: WMR 4000, Brooks Instrument GmbH, Dresden, Germany) ensured a constant total gas flow rate of 5 NL h−1 (ISO 10,780 [24]) at either 200 mbar CO2, 200 mbar H2, and 600 mbar CO (C. autoethanogenum and C. ragsdalei) or 200 mbar CO2, 200 mbar H2, 200 mbar CO, and 400 mbar N2 (C. ljungdahlii) at a total pressure of 1 bar.
2.3. Supply of Defined Syngas Impurities
The effect of syngas impurities was studied via the addition of the desired component to the reaction medium prior to inoculation according to previously reported methods [14]. Ammonium was added as NH4Cl (1 g L−1, 3 g L−1, and 6 g L−1, respectively). Nitrate was added as NaNO3 (0.1 g L−1, 0.2 g L−1, and 0.5 g L−1, respectively). Hydrogen sulfide was added as thioacetamide (TAA) (0.1 g L−1, 0.2 g L−1, and 0.5 g L−1, respectively).
2.4. Analytical Methods
2.4.1. Liquid Product Analysis
Samples from the processes with C. autoethanogenum were collected through a sampling valve at the bottom of the bioreactor and analyzed by HPLC (LC-2030C Plus, Shimadzu, Kyoto, Japan). Samples from the processes with C. ljungdahlii and C. ragsdalei were taken using a syringe through a diaphragm at the top of the reactor and analyzed by HPLC (Finnigan Surveyor Plus, Thermo Scientific, Waltham, MA, USA). All samples were analyzed for the main products acetate, ethanol, and 2,3-butanediol, as well as the sugars and metabolites xylose, fructose, and formate. Both HPLC instruments were equipped with a cation exchange column (HPX-87H, Bio-Rad, Munich, Germany) at a column temperature of 60 °C. The elution conditions were isocratic, with 5 mM H2SO4 as the mobile phase and a constant flow rate of 0.6 mL min−1 (C. autoethanogenum) and 0.5 mL min−1 (C. ljungdahlii and C. ragsdalei). Both instruments used a refractive index detector (RID) and were combined with a standard series of defined concentrations between 0.05 g L−1 and 5.00 g L−1 of every measured substance with each measurement. The optical density OD600 of the samples was measured using a spectrophotometer (Genesys 10S UV–Vis, Thermo Scientific, Neuss, Germany) at 600 nm, and previously identified individual correlation factors were used to estimate the cell dry weight concentrations (C. autoethanogenum: 0.38 ± 0.02 g L−1; C. ljungdahlii: 0.41 ± 0.01 g L−1; C. ragsdalei: 0.42 ± 0.03 g L−1). Samples for optical density were measured in triplicate for optical densities higher than 0.3.
2.4.2. Online Exhaust Gas Analysis
The exhaust gas was cooled to 2 °C with a reflux condenser prior to analysis. The gas flow rate was measured online by a mass flow meter (Wagner Mess- und Regeltechnik GmbH, Offenbach, Germany), and micro gas chromatography (micro GC 490, Agilent Technologies, Waldbronn, Germany) was applied to measure the concentrations of CO, CO2, and H2 online in the exhaust gas. The µGC was equipped with three channels with individual separation columns (channel 1: molecular sieve, carrier gas argon, 80 °C, 250 kPa, for the separation of H2, N2, and CO; channel 2: PlotPQ, carrier gas helium, 80 °C, 150 kPa, for the separation of CO2, NH3, and NOx; channel 3: CP-Sil 5, carrier gas helium, 45 °C, 100 kPa, for the separation of CO2, and H2S). Every channel uses an individual thermal conductivity detector (TCD). The online data were used to calculate the volumetric uptake or production rates of CO, CO2, and H2. The individual flow rate of each gas component in the exhaust gas was calculated by multiplying of the individual gas partial pressure of each component measured online by µGC with the total exhaust gas flowrate measured online with mass flow meters. The total consumption of each component was estimated by numerical integration of the individual gas flow rate with a step time of 10 min.
3. Results and Discussion
3.1. Autotrophic Reference Batch Processes in Continuously Gassed Stirred-Tank Bioreactors
All of the strains were first cultivated without the addition of any syngas impurities as autotrophic reference batch processes (Figure 1). It has to be noted that for the microorganism C. ljungdahlii, a reduced CO partial pressure of 200 mbar was chosen as opposed to 600 mbar for C. autoethanogenum and C. ragsdalei. This reduction was chosen because preliminary studies showed increased growth and ethanol formation for this strain at reduced CO partial pressure (data not shown), and this study aimed to compare the three strains at their best performance. The highest CDW concentrations were observed with C. ragsdalei (0.56 g L−1), followed by C. autoethanogenum (0.52 g L−1) and C. ljungdahlii (0.23 g L−1). C. ragsdalei produced considerably more acetate, with a final concentration of 4.61 g L−1, whereas C. autoethanogenum produced the highest ethanol and 2,3-butanediol concentrations (2.51 g L−1 ethanol and 0.53 g L−1 2,3-butanediol, respectively). No 2,3-butanediol formation was observed with C. ljungdahlii. CO was the only gaseous substrate to be consumed by all strains. No H2 uptake was observed in any of the batch processes. Part of the CO was converted to CO2 for providing the necessary reducing equivalents. Further details on total CO consumption and CO2 production are given in Tables S1–S3 in the Supplementary Materials. The maximal CO uptake rates varied considerably at ≈16 mmol L−1 h−1 (C. ragsdalei), ≈12 mmol L−1 h−1 (C. autoethanogenum), and ≈6 mmol L−1 h−1 (C. ljungdahlii). Compared to the high volumetric flow rate of the syngas, the CO uptake rates are relatively low, resulting in maximum CO conversions of 11.9% (C. ragsdalei), 9.0% (C. autoethanogenum), and 13.4% (C. ljungdahlii), which does not support any kind of limitation by the carbon input.
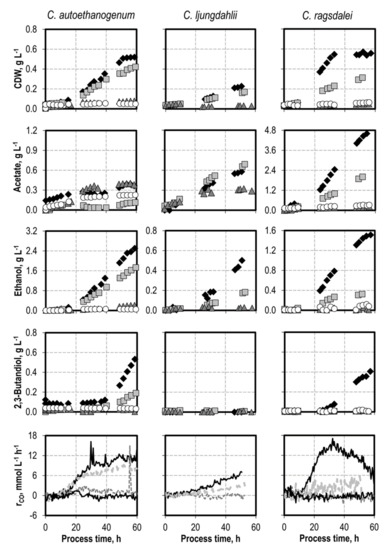
Figure 1.
Autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei at varying ammonium chloride concentrations: C. autoethanogenum, reference batch process (black diamond, black top line), +1.0 g L−1 NH4Cl (light grey square, light grey line), +3.0 g L−1 NH4Cl (dark grey triangle, dark grey line), +5.0 g L−1 NH4Cl (white circle, black bottom line); C. ljungdahlii and C. ragsdalei, reference batch process (black diamond, black top line), +3.0 g L−1 NH4Cl (light grey square, light grey line), +6.0 g L−1 NH4Cl (dark grey triangle, dark grey line), +9.0 g L−1 NH4Cl (white circle, black bottom line). The processes were performed in a stirred-tank reactor with continuous gassing (C. autoethanogenum and C. ragsdalei: 600 mbar CO, 200 mbar CO2, and 200 mbar H2; C. ljungdahlii: 400 mbar N2, 200 mbar CO, 200 mbar H2, and 200 mbar CO2), pH 6 controlled with 3 M NaOH and 2 M H2SO4, 37 °C, volumetric power input of 15.1 W L−1 (C. autoethanogenum) and 3.5 W L−1 (C. ljungdahlii and C. ragsdalei). rCO represents the CO uptake rate.
The final alcohol to acetate ratio achieved in the autotrophic reference batch processes exhibited a distinct maximum using C. autoethanogenum (7.60 g g−1), as compared to 0.86 g g−1 with C. ljungdahlii or 0.41 g g−1 with C. ragsdalei. The high CO uptake rates observed with C. ragsdalei mainly resulted in high acetate production. High organic acid production rates and concentrations have been associated with a failure to trigger alcohol production [25,26], as well as with higher ATP maintenance costs [27].
The observed maximum specific growth rate (0.050 h−1) and maximum CO uptake rate (6 mmol L−1 h−1) of C. ljungdahlii were in accordance with the published data [28]. The reported maximum specific growth rates of C. ragsdalei varied considerably, e.g., 0.175 h−1 [8] or 0.065 h −1 [29]. The growth rate observed in this study was in line with the reported data (0.116 h−1). The measured maximum specific growth rate of C. autoethanogenum (0.065 h−1) exceeded that of the data from the literature, e.g., 0.042 h−1 achieved with a gas mixture of 2% CO, 23% CO2, and 65% H2 [30].
3.2. Defined Addition of Impurities: Ammonium
Ammonium was supplemented as NH4Cl before inoculation with C. autoethanogenum (+ 1.0 g L−1, + 3.0 g L−1, and + 5.0 g L−1 NH4Cl), C. ljungdahlii (+ 3.0 g L−1, and + 6.0 g L−1 NH4Cl), and C. ragsdalei (+ 3.0 g L−1, + 6.0 g L−1, and + 9.0 g L−1 NH4Cl) (see Figure 1). The initial addition of 3.0 g L−1 NH4Cl reduced the final CDW concentration of C. autoethanogenum by 85%, of C. ljungdahlii by 26%, and of C. ragsdalei by 45%. Growth inhibition was observed for C. autoethanogenum after the addition of 5.0 g L−1 NH4Cl and for C. ljungdahlii and C. ragsdalei after the addition of 6.0 g L−1 NH4Cl. The inhibitory effect of NH4Cl addition was also reflected in the observed decrease in the CO uptake rates in all batch processes after supplementation with ammonium. Alcohol production was reduced after any initial addition of NH4Cl. The supplementation with 3.0 g L−1 NH4Cl prevented any formation of 2,3-butanediol with C. autoethanogenum and C. ragsdalei.
3.3. Defined Addition of Impurities: Nitrate
Nitrate was added as NaNO3 before inoculation with C. autoethanogenum (0.1 g L−1, 0.2 g L−1, 0.5 g L−1, and 1.0 g L−1 NaNO3), C. ljungdahlii (0.1 g L−1, and 0.5 g L−1 NaNO3), and C. ragsdalei (0.1 g L−1, 0.2 g L−1, and 0.5 g L−1 NaNO3) (see Figure 2). Adding nitrate slowed the growth of C. autoethanogenum and C. ragsdalei in all of the nitrate concentrations studied. The complete growth inhibition of C. ragsdalei was observed after adding 0.5 g L−1 NaNO3. C. ljungdahlii exhibited the highest tolerance after nitrate additions, with little difference in the final cell dry weight concentrations in all cases, but a clearly shorter exponential growth phase.
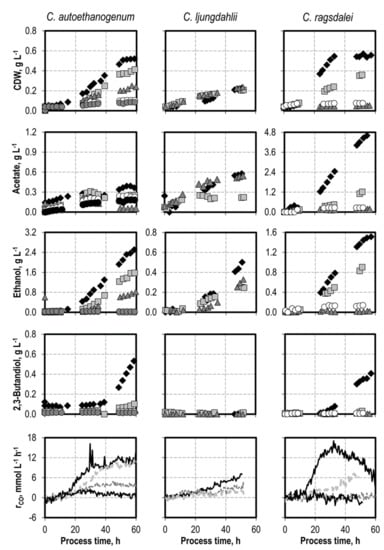
Figure 2.
Autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei at varying NaNO3 concentrations: C. autoethanogenum, reference batch process (black diamond, black top line), +0.1 g L−1 NaNO3 (light grey square, light grey line), +0.2 g L−1 NaNO3 (dark grey triangle, dark grey line), +0.5 g L−1 NaNO3 (white dircle, black bottom line), +1.0 g L−1 NaNO3 (dark grey circle); C. ljungdahlii and C. ragsdalei, reference batch process (black diamond, black top line), +0.1 g L−1 NH4Cl (light grey square, light grey line), +0.5 g L−1 NaNO3 (dark grey triangle, dark grey line), +1.0 g L−1 NaNO3 (white circle, black bottom line). The batch processes were performed in a stirred-tank reactor with continuous gassing (C. autoethanogenum and C. ragsdalei: 600 mbar CO, 200 mbar CO2, and 200 mbar H2; C. ljungdahlii: 400 mbar N2, 200 mbar CO, 200 mbar H2, and 200 mbar CO2), pH 6 controlled with 3 M NaOH and 2 M H2SO4, 37 °C, volumetric power input of 15.1 W L−1 (C. autoethanogenum) and 3.5 W L−1 (C. ljungdahlii and C. ragsdalei). rCO represents the CO uptake rate.
No net production of acetate was observed with C. ljungdahlii in the final 30 h of the batch processes with 0.1 g L−1 NaNO3 and 0.5 g L−1 NaNO3, respectively. A strong reduction of acetate production occurred in the batch processes with C. ragsdalei, which was independent of the initial NaNO3 concentration. Nitrate is known to increase the ATP/ADP ratio in C. ljungdahlii [21]. More ATP can thus be provided through nitrate reduction with less acetate production for biomass formation.
Ethanol production was reduced in all of the batch processes with the addition of NaNO3. C. ljungdahlii showed the lowest reduction of final ethanol concentrations. 2,3-Butanediol formation was strongly inhibited by nitrate, and no 2,3-butanediol was detected with C. ljungdahlii and C. ragsdalei. A reduced formation of 0.10 g L−1 2,3-butanediol was observed with C. autoethanogenum at 0.1 g L−1 NaNO3.
3.4. Defined Addition of Impurities: Hydrogen Sulfide
Hydrogen sulfide was added as thioacetamide (TAA) before inoculation with C. autoethanogenum (0.1 g L−1 H2S, 0.3 g L−1, and 0.5 g L−1), as well as with C. ljungdahlii and C. ragsdalei (0.1 g L−1, and 0.5 g L−1 H2S) (see Figure 3).
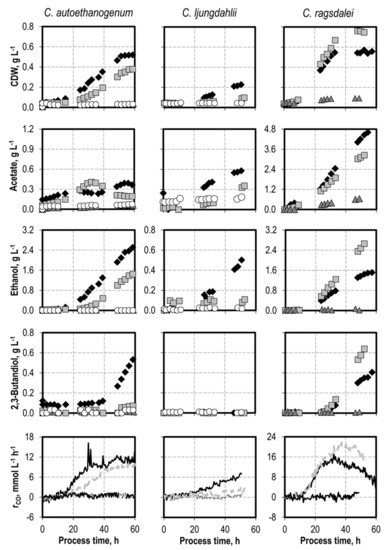
Figure 3.
Autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei at varying initial H2S concentrations supplied by addition of thioacetamide: C. autoethanogenum, reference batch process (black diamond, black top line), +0.1 g L−1 H2S (light grey square, light grey line), +0.3 g L−1 H2S (dark grey triangle, dark grey line), +0.5 g L−1 H2S (white circle, black bottom line); C. ljungdahlii and C. ragsdalei, reference batch process (black diamond, black top line), +0.1 g L−1 H2S (light grey square, light grey line), +0.2 g L−1 NH4Cl (dark grey triangle, dark grey line), +0.5 g L−1 NH4Cl (white circle, black bottom line). The batch processes were performed in a stirred-tank reactor with continuous gassing (C. autoethanogenum and C. ragsdalei: 600 mbar CO, 200 mbar CO2, and 200 mbar H2; C. ljungdahlii: 400 mbar N2, 200 mbar CO, 200 mbar H2, and 200 mbar CO2), pH 6 controlled with 3 M NaOH and 2 M H2SO4, 37 °C, volumetric power input of 15.1 W L−1 (C. autoethanogenum) and 3.5 W L−1 (C. ljungdahlii and C. ragsdalei). rCO represents the CO uptake rate.
All of the TAA concentrations investigated decreased the cell dry weight concentrations; the final product concentrations; and, correspondingly, the CO uptake rates of C. autoethanogenum and C. ljungdahlii. Adding 0.3 g L−1 H2S completely inhibited the growth and CO uptake of C. autoethanogenum. No growth of C. ljungdahlii and C. ragsdalei was observed with 0.5 g L−1 H2S. A concentration of 0.1 g L−1 H2S induced a lag phase of 20 h with C. autoethanogenum, but it increased acetate production in the first 30 h. The acetate concentration later decreased until the end of the process. 2,3-Butanediol formation of C. autoethanogenum was strongly inhibited in the batch process with 0.1 g L−1 H2S. The addition of 0.1 g L−1 H2S resulted in less biomass formation with C. ljungdahlii, a delayed acetate production, and no alcohol formation.
The addition of 0.1 g L−1 H2S increased the final cell dry weight concentration of C. ragsdalei by 34%. Higher CO uptake rates were also observed, with maximal CO uptake rates reaching approximately 21 mmol L−1 h−1. Product formation shifted from acetate to alcohol production, with not only higher final concentrations of ethanol and 2,3-butanediol when compared with the reference batch process for each strain, but also higher biomass-related yields of both alcohols. This effect might occur due to the additional presence of a sulfur source or to the reducing effect of H2S, which may lead to a more reduced redox potential in the cultivation medium and, thus, an increase in growth and reduction of acetate to ethanol.
3.5. Comparison of Clostridial Strains
The results with the acetogens C. autoethanogenum, C. ljungdahlii, and C. ragsdalei of this study were compared to previously published reference data with C. carboxidivorans [14], which have been measured in batch operated stirred-tank bioreactors at comparable reaction conditions. An overall comparison of the autotrophic reference batch process performances of the three strains studied, including the published results for C. carboxidivorans [14], shows that C. autoethanogenum was able to produce the highest amounts of biomass and alcohols while also maintaining low acid concentrations (Figure 4). C. ragsdalei achieved 91% (w/w) of the maximum cell dry weight concentration measured with C. autoethanogenum and further produced the highest amounts of organic acids. C. ljungdahlii showed the lowest production of biomass, organic acids, and alcohols.
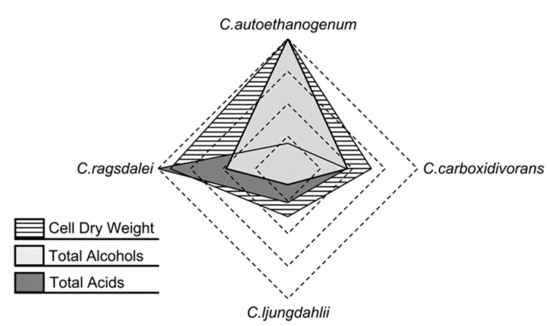
Figure 4.
Comparison of the autotrophic reference batch processes with C. autoethanogenum, C. ljungdahlii, C. ragsdalei, and C. carboxidivorans (data for C. carboxidivorans extracted from previously published results in [14]) with respect to maximum CDW concentrations, maximum total concentrations of alcohols on a C mol basis, and maximum total concentration of acids on a C mol basis.
The addition of ammonium favored biomass and alcohol formation in the autotrophic batch processes with C. carboxidivorans [14] while reducing the productivity of the other three Clostridial strains (Figure 5). C. autoethanogenum demonstrated the lowest tolerance to NH4Cl, with growth inhibition already occurring with the supplementation of 3.0 g L−1 NH4Cl, whereas C. ljungdahlii and C. ragsdalei showed inhibition with the supplementation of 6.0 g L−1 NH4Cl.
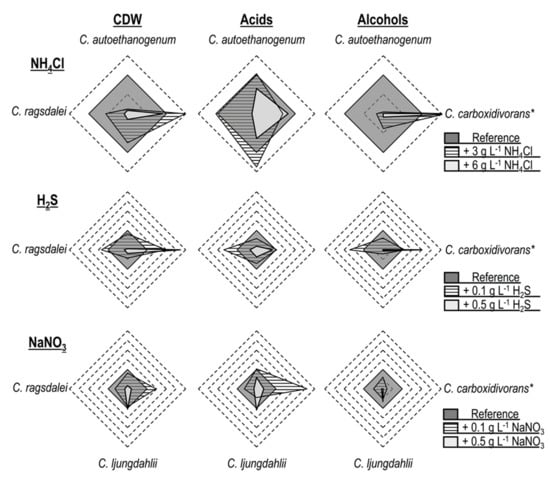
Figure 5.
Comparison of autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, C. ragsdalei, and C. carboxidivorans*. All values in the radar plots were calculated relative to the individual maximum concentration observed with the indicated microorganism (reference process = 1.0). * Data of C. carboxidivorans extracted from previously published results in [14], with concentrations of +5.0 g L−1 NH4Cl, +0.1 g L−1 H2S, and +0.1 g L−1 NaNO3  , or +7.5 g L−1 NH4Cl, +0.5 g L−1 H2S, and +1.0 g L−1 NaNO3
, or +7.5 g L−1 NH4Cl, +0.5 g L−1 H2S, and +1.0 g L−1 NaNO3  .
.
 , or +7.5 g L−1 NH4Cl, +0.5 g L−1 H2S, and +1.0 g L−1 NaNO3
, or +7.5 g L−1 NH4Cl, +0.5 g L−1 H2S, and +1.0 g L−1 NaNO3  .
.
H2S addition promoted biomass, acid, and alcohol formation in the autotrophic batch processes with C. ragsdalei at a low initial concentration (0.1 g L−1 H2S). C. carboxidivorans biomass production was increased with both added sulfide concentrations, whereby an increase in the formation of alcohols was solely observed with 0.5 g L−1 H2S [14]. The other strains exhibited reduced productivities after H2S addition, with C. ljungdahlii showing a strong inhibition to sulfide. The addition of 0.5 g L−1 H2S resulted in strong inhibitions of C. autoethanogenum, C. ljungdahlii, and C. ragsdalei and thus represents a critical impurity in the syngas fermentation with these strains.
Nitrate slowed or inhibited the biomass formation of C. autoethanogenum and C. ragsdalei, with the effect increasing at higher initial nitrate concentrations. C. ljungdahlii was, in turn, less influenced by nitrate, leading to equivalent cell dry weight concentrations with and without nitrate, as well as showing a higher tolerance to this impurity. The alcohol formation in all of the strains studied was suppressed by nitrate, including C. carboxidivorans [14], but C. carboxidivorans was stimulated by the addition of 0.1 g L−1 NaNO3 and produced more biomass and organic acids [14].
Overall, the three Clostridial strains studied exhibited similar responses to the added impurities. These responses were, in turn, very different from those published with C. carboxidivorans [14], which showed more robustness to the effects of the syngas impurities NH3 and H2S. Since C. carboxidivorans is genetically not as closely related to the other three studied strains [4,5], it is not surprising that its response to the defined impurities should differ. C. autoethanogenum, C. ljungdahlii, and C. ragsdalei were generally inhibited by the addition of H2S (as TAA), nitrate, and ammonium, with the exception of the addition of 0.1 g L−1 H2S to the autotrophic batch process with C. ragsdalei. As a consequence, the accumulation of 0.1 g L−1 H2S, 0.073 g L−1 NO3−1 (equivalent to 0.1 g L−1 NaNO3), and 2.14 g L−1 NH4+ (equivalent to 6.3 g L−1 NH4Cl) in fermentation processes with real syngas should be avoided.
Further details on total CO consumption, total CO2 production, carbon balance recoveries, specific growth rates, and the maximum concentrations of CDW and products are provided in the Supplementary Materials (Tables S1–S3) for all the batch processes described in this work using the individual microorganisms (C. autoethanogenum, C. ljungdahlii, and C. ragsdalei, respectively).
The identified inhibiting concentrations of impurities in the liquid phase were used to estimate the corresponding concentrations in the gas phase and, therefore, provide a quantitative goal respecting the quality requirements of real syngases. The typical orders of magnitude for trace impurities in real biogenic syngas from entrained flow gasification of biogenic residues are 4500 ppm NH3, 500 ppm H2S, and 200 ppm NOx [31,32]. The corresponding concentrations in a syngas were estimated given the assumption of complete absorption of the syngas trace component in the liquid phase within a process time of 60 h, as previously described [14]. Typical solubilities of the investigated gas impurities in pure water at 25 °C and 1013.25 mbar partial pressure are 0.1876 mol NH3 mol−1 H2O, 1.830∙10−3 mol H2S mol−1 H2O, 3.477∙10−5 mol NO mol−1 H2O, and 1.488∙10−4 mol NO2 mol−1 H2O [33]. These concentrations correspond to 199.7 g L−1 NH3, 3.90 g L−1 H2S, 0.065 g L−1 NO, and 1.28 g L−1 NO2, respectively. However, it has to be noted that these concentrations do not take any chemical reaction into account [33]. All investigated concentrations of NH4+, H2S, and NO3− are in the range of these typical solubilities. It should be noted that the impurities threshold identified in this study represents a conservative limit, since in a continuously gassed process, the impurities would gradually accumulate in the cell broth. Thus, the growth phase would occur at lower impurity concentrations than the one in this study, leading presumably to higher CDW and product concentrations and the adaption of the cells.
The initial addition of 3.0 g L−1 NH4Cl in the liquid phase was found to be inhibiting for growth and product formation of C. autoethanogenum, C. ljungdahlii, and C. ragsdalei in the autotrophic batch processes. Under the given assumptions, 3.0 g L−1 NH4Cl would be reached at a concentration of 4560 ppm NH3 in the gas phase within a total batch process time of 60 h. Since the standard medium in all of the autotrophic batch processes already contained 3.3 g L−1 NH4Cl [23], a reduction of the initially supplied NH4Cl could reduce the inhibiting effect of NH3 provided by a typical biogenic syngas (4500 ppm NH3). It has already been shown that a reduction of the initial ammonium concentration in the medium by 50% did not influence biomass growth or product formation of C. ragsdalei [18]. Additionally, with the continuous gassing, the ammonium concentration would increase with process time rather than remaining constant, enabling a possible adaption of the cells. The growth of C. ragsdalei resumed with 6.0 g L−1 and 9.0 g L−1 NH4Cl after 100 h (data not shown), and growth of C. autoethanogenum resumed with 3.0 g L−1 NH4Cl after 78 h (data not shown), indicating that an adaption to increasing ammonium concentrations may be possible.
The initial addition of 0.1 g L−1 NaNO3 was found to inhibit growth and alcohol production with C. ragsdalei, and C. autoethanogenum. An amount of 0.1 g L−1 NaNO3 would correspond to a gas phase concentration of 118 ppm NOx after a process time of 60 h if the entire amount of nitrogen from the NOx was absorbed in the liquid phase and converted into NO3−. Therefore, the purification of a typical biogenic syngas at 200 ppm NOx would be necessary to ensure a stable process without reduced alcohol production. However, the growth of C. ljungdahlii was not affected, and its alcohol production was only slightly reduced by the addition of NaNO3 concentrations of up to 0.5 g L−1 NaNO3. Under the same assumptions, a concentration of 0.5 g L−1 NaNO3 would be reached after 60 h with a syngas at 588 ppm NOx. Thus, a biogenic syngas at 200 ppm NOx might not need a further purification for this trace component if C. ljungdahlii were applied for syngas fermentation.
An initial concentration of 0.1 g L−1 H2S was found to inhibit growth as well as alcohol production of C. autoethanogenum and C. ljungdahlii. An amount of 0.1 g L−1 H2S corresponds to 108 ppm H2S in the syngas within a batch process time of 60 h. The inhibiting concentration for C. ragsdalei of 0.5 g L−1 H2S corresponds to a concentration of 540 ppm H2S in the gas phase for 60 h. Thus, a biogenic syngas at a typical concentration of 200 ppm H2S would not be critical in autotrophic batch processes with C. ragsdalei. If C. autoethanogenum or C. ljungdahlii were applied for syngas fermentation, H2S separation from the biogenic syngas would be necessary.
4. Conclusions
C. autoethanogenum was shown to produce the highest cell dry weight and alcohol concentrations in continuously gassed batch processes without the addition of syngas impurities, as compared to C. ljungdahlii and C. ragsdalei. Syngas impurities such as NH3, NOx, and H2S critically impacted batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei to varying extents, thus differing from published results with C. carboxidivorans [14]. The results presented herein offer an initial setpoint for the quality requirements of real syngas with respect to the impurities tested. Further investigation of the possible combinatory effects of these impurities are of utmost relevance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms10040681/s1, Table S1: Total consumption of CO and production of CO2, biomass, and products on a C-mol basis and carbon balances, as well as maximum CDW concentrations, maximum product concentrations, and the maximum specific growth rate of the autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei in continuously gassed stirred-tank bioreactors (V = 1 L) with the initial addition of NH4Cl, Table S2: Total consumption of CO and production of CO2, biomass, and products on a C-mol basis and carbon balances, as well as maximum CDW concentrations, maximum product concentrations, and the maximum specific growth rate of the autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei in continuously gassed stirred-tank bioreactors (V = 1 L) with the initial addition of H2S, Table S3: Total consumption of CO and production of CO2, biomass, and products on a C-mol basis and carbon balances, as well as maximum CDW concentrations, maximum product concentrations, and the maximum specific growth rate of the autotrophic batch processes with C. autoethanogenum, C. ljungdahlii, and C. ragsdalei in continuously gassed stirred-tank bioreactors (V = 1 L) with the initial addition of NaNO3, Table S4: Composition of the liquid medium previously described by Doll et al. [23] for precultures in anaerobic shaken bottles and batch processes in stirred-tank bioreactors.
Author Contributions
L.O. and A.R. have contributed equally and share the first authorship; conceptualization, L.O., A.R. and D.W.-B.; methodology and investigation, L.O., A.R., L.N., P.S. and E.H.; data discussion and analysis, L.O., A.R., L.N., P.S., E.H. and D.W.-B.; writing—original draft preparation, L.O. and A.R.; writing—review and editing, D.W.-B.; visualization, L.O. and A.R.; supervision, project administration, and funding acquisition, D.W.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal Ministry of Education and Research (BMBF), Germany (ReGasFerm—Grant number 031B0677A).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors gratefully thank Philipp Johne, Philipp Leuter, Sebastian Fendt, and Hartmut Spliethoff (Institute of Energy Systems, Technical University of Munich) for their ongoing support and many helpful discussions. The support of Luis Oliveira and Anton Rückel from the TUM Graduate School (Technical University of Munich, Germany) is acknowledged as well.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Griffin, D.W.; Schultz, M.A. Fuel and chemical products from biomass syngas: A comparison of gas fermentation to thermochemical conversion routes. Environ. Prog. Sustain. Energy 2012, 31, 219–224. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Biological conversion of carbon monoxide: Rich syngas or waste gases to bioethanol. Biofuels Bioprod. Bioref. 2011, 5, 93–114. [Google Scholar] [CrossRef] [Green Version]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.W.; Chae, C.G.; Kwon, S.J.; Kim, Y.J.; Lee, J.-H.; Lee, H.S. Domestication of the novel alcohologenic acetogen Clostridium sp. AWRP: From isolation to characterization for syngas fermentation. Biotechnol. Biofuels 2019, 12, 228. [Google Scholar] [CrossRef]
- Groher, A.; Weuster-Botz, D. Comparative reaction engineering analysis of different acetogenic bacteria for gas fermentation. J. Biotechnol. 2016, 228, 82–94. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeister, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Weuster-Botz, D.; Dürre, P. Bacterial anaerobic synthesis gas (syngas) and CO2 + H2 fermentation. Adv. Appl. Microbiol. 2018, 103, 143–221. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Poehlein, A.; Linder, S.; Erz, C.; Hummel, T.; Hoffmeister, S.; Daniel, R.; Dürre, P. Industrial acetogenic biocatalysts: A comparative metabolic and genomic analysis. Front. Microbiol. 2016, 7, 1036. [Google Scholar] [CrossRef] [Green Version]
- Huhnke, R.L.; Lewis, R.S.; Tanner, R.S. Isolation and Characterization of Novel Clostridial Species; International Publication Number WO 2008/028055; World Intellectual Property Organization: Geneva, Switzerland, 2008; Available online: https://patentimages.storage.googleapis.com/5f/a1/62/130873153df169/WO2008028055A2.pdf (accessed on 11 March 2022).
- Hu, P.; Bowen, S.H.; Lewis, R.S. A thermodynamic analysis of electron production during syngas fermentation. Bioresour. Technol. 2011, 102, 8071–8076. [Google Scholar] [CrossRef]
- Younesi, H.; Najafpour, G.; Mohamed, A.R. Ethanol and acetate production from synthesis gas via fermentation processes using anaerobic bacterium, Clostridium ljungdahlii. Biochem. Eng. J. 2005, 27, 110–119. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K. A review of conversion processes for bioethanol production with a focus on syngas fermentation. Biofuel Res. J. 2015, 2, 268–280. [Google Scholar] [CrossRef]
- Broer, K.M.; Brown, R.C. Effect of equivalence ratio on partitioning of nitrogen during biomass gasification. Energy Fuels 2016, 30, 407–413. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of syngas composition on anaerobic fermentation. Reactions 2021, 2, 25. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on syngas fermentation with Clostridium carboxidivorans in stirred-tank reactors with defined gas impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Jacobsen, L.T.; Horton, J.G.; Lewis, R.S. Sulfide assessment in bioreactors with gas replacement. Biochem. Eng. J. 2010, 49, 429–434. [Google Scholar] [CrossRef]
- O’Flaherty, V.; Mahony, T.; O’Kennedy, R.; Colleran, E. Effect of pH on growth kinetics and sulphide toxicity thresholds of a range of methanogenic, syntrophic and sulphate-reducing bacteria. Process Biochem. 1998, 33, 555–569. [Google Scholar] [CrossRef]
- Ntagia, E.; Chatzigiannidou, I.; Williamson, A.J.; Arends, J.B.A.; Rabaey, K. Homoacetogenesis and microbial community composition are shaped by pH and total sulfide concentration. Microb. Biotechnol. 2020, 13, 1026–1038. [Google Scholar] [CrossRef] [Green Version]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbiol. Biotechnol. 2012, 28, 1553–1561. [Google Scholar] [CrossRef]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Ahmed, A.; Lewis, R.S. Fermentation of biomass-generated synthesis gas: Effects of nitric oxide. Biotechnol. Bioeng. 2007, 97, 1080–1086. [Google Scholar] [CrossRef]
- Emerson, D.F.; Woolston, B.M.; Liu, N.; Donnelly, M.; Currie, D.H.; Stephanopoulos, G. Enhancing hydrogen-dependent growth of and carbon dioxide fixation by Clostridium ljungdahlii through nitrate supplementation. Biotechnol. Bioeng. 2019, 116, 294–306. [Google Scholar] [CrossRef]
- Klask, C.-M.; Kliem-Kuster, N.; Molitor, B.; Angenent, L.T. Nitrate feed improves growth and ethanol production of Clostridium ljungdahlii with CO2 and H2, but results in stochastic inhibition events. Front. Microbiol. 2020, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Rückel, A.; Kämpf, P.; Wende, M.; Weuster-Botz, D. Two stirred-tank bioreactors in series enable continuous production of alcohols from carbon monoxide with Clostridium carboxidivorans. Bioprocess Biosyst. Eng. 2018, 41, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- ISO 10780:1994; Stationary Source Emissions—Measurement of Velocity and Volume Flowrate of Gas Streams in Ducts. International Organization for Standardization: Geneva, Switzerland, 2016.
- Wang, S.; Zhang, Y.; Dong, H.; Mao, S.; Zhu, Y.; Wang, R.; Luan, G.; Li, Y. Formic acid triggers the “acid crash” of acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Appl. Environ. Microbiol. 2011, 77, 1674–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddox, I.S.; Steiner, E.; Hirsch, S.; Wessner, S.; Gutierrez, N.A.; Gapes, J.R.; Schuster, K.C. The cause of “acid-crash” and “acidogenic fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. J. Mol. Microbiol. Biotechnol. 2000, 2, 95–100. [Google Scholar] [PubMed]
- Valgepea, K.; de Souza Pinto Lemgruber, R.; Meaghan, K.; Palfreyman, R.W.; Abdalla, T.; Heijstra, B.D.; Behrendorff, J.B.; Tappel, R.; Köpke, M.; Simpson, S.D.; et al. Maintenance of ATP homeostasis triggers metabolic shifts in gas-fermenting acetogens. Cell Syst. 2017, 4, 505–515.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, M.; Teleki, A.; Weitz, S.; Niess, A.; Freund, A.; Bengelsdorf, F.R.; Takors, R. Electron availability in CO2, CO and H2 mixtures constrains flux distribution, energy management and product formation in Clostridium ljungdahlii. Microb. Biotechnol. 2020, 13, 1831–1846. [Google Scholar] [CrossRef]
- Maddipati, P.; Atiyeh, H.K.; Bellmer, D.D.; Huhnke, R.L. Ethanol production from syngas by Clostridium strain P11 using corn steep liquor as a nutrient replacement to yeast extract. Bioresour. Technol. 2011, 102, 6494–6501. [Google Scholar] [CrossRef]
- Heffernan, J.K.; Valgepea, K.; de Souza Pinto Lemgruber, R.; Casini, I.; Plan, M.; Tappel, R.; Simpson, S.D.; Köpke, M.; Nielsen, L.K.; Marcellin, E. Enhancing CO2-valorization using Clostridium autoethanogenum for sustainable fuel and chemicals production. Front. Bioeng. Biotechnol. 2020, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Kremling, M.; Briesemeister, L.; Gaderer, M.; Fendt, S.; Spliethoff, H. Oxygen-blown entrained flow gasification of biomass: Impact of fuel parameters and oxygen stoichiometric ratio. Energy Fuels 2017, 31, 3949–3959. [Google Scholar] [CrossRef]
- Kremling, M.B. Experimentelle Untersuchungen zur Sauerstoffgeblasenen Flugstromvergasung von Staubförmiger Biomasse. Ph.D. Thesis, Technical University of Munich, München, Germany, 2018. ISBN 978-3-8439-3551-7. [Google Scholar]
- Battino, R.; Seybold, P.G.; Campanell, F.C. Correlations Involving the Solubility of Gases in Water at 298.15 K and 101325 Pa. J. Chem. Eng. Data 2011, 56, 727–732. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).