Abstract
Rhizosphere colonization by phytobeneficial Pseudomonas spp. is pivotal in triggering their positive effects on plant health. Many Pseudomonas spp. Determinants, involved in rhizosphere colonization, have already been deciphered. However, few studies have explored the role played by specific plant genes in rhizosphere colonization by these bacteria. Using isogenic Arabidopsis thaliana mutants, we studied the effect of 20 distinct plant genes on rhizosphere colonization by two phenazine-producing P. chlororaphis strains of biocontrol interest, differing in their colonization abilities: DTR133, a strong rhizosphere colonizer and ToZa7, which displays lower rhizocompetence. The investigated plant mutations were related to root exudation, immunity, and root system architecture. Mutations in smb and shv3, both involved in root architecture, were shown to positively affect rhizosphere colonization by ToZa7, but not DTR133. While these strains were not promoting plant growth in wild-type plants, increased plant biomass was measured in inoculated plants lacking fez, wrky70, cbp60g, pft1 and rlp30, genes mostly involved in plant immunity. These results point to an interplay between plant genotype, plant growth and rhizosphere colonization by phytobeneficial Pseudomonas spp. Some of the studied genes could become targets for plant breeding programs to improve plant-beneficial Pseudomonas rhizocompetence and biocontrol efficiency in the field.
Keywords:
Pseudomonas; phenazine; PGPR; rhizosphere; colonization; rhizocompetence; Arabidopsis; SALK; root architecture; immunity 1. Introduction
Plants play a major role in underground bacterial composition, especially in the rhizosphere, the soil portion under the influence of roots [1,2]. This soil compartment receives rhizodeposits, i.e., nutrients available to the rhizosphere-inhabiting microorganisms, including rhizobacteria, affecting their ability to survive and thrive. These nutrients sustain densities around 109 bacteria per gram of rhizosphere soil, which is 10 to 1000 higher than in bulk soil [3,4]. Depending on the plant species, up to 30% of the carbon fixed by the plant through photosynthesis is released into the rhizosphere [5].
Rhizodeposition relies on numerous factors, such as soil type, plant growth, its physiological state, and root system architecture [6,7,8,9,10]. Rhizodeposits include lysates, volatile compounds, sloughed-off root cells and tissues, as well as root exudates [11]. The latter are mainly released by young tissues surrounding root tips and root hairs, while other rhizodeposits, such as lysates, can be found at sites of lateral root emergence [7,12,13,14]. Root hairs are especially interesting because of their interfacing function between the plant and the rhizosphere: they account for about 77% of the total root surface of crop species and play numerous crucial roles for the plant, such as anchorage, water and nutrient uptake, and exudation, which, in turn, affect the rhizosphere microbiome [7,15,16].
Root exudates are particularly studied because of their diversity and their dynamic release patterns [14]. Different types of exudates are released into the rhizosphere, such as organic acids, sugars, amino acids, or phenolics, influencing the bacterial composition of this habitat [6,17,18]. Exudation is mainly mediated by exocytosis for high-molecular-weight compounds and by passive diffusion through the plasma membrane or active secretion by membrane transporters for lighter metabolites [19]. Different transporters involved in the latter have been identified, belonging to the Major Facilitator Superfamily (MFS), the Multidrug and Toxic Compound Extrusion (MATE) family, the Aluminium-Activated Malate Transporters (ALMT) family, and the ATP-binding cassette (ABC) families [13,20]. Mutations in specific ABC transporters in Arabidopsis thaliana have been shown to decrease organic acids exudation or increase amino acids exudation [21]. In tomato, the knockdown of ABC transporters altered the composition of root exudates, which reduced the attraction of a Bacillus strain towards exudates [22].
Plant defense signalling can also impact root exudation and bacterial composition in the rhizosphere. Exogenous application of plant immunity signalling molecules on A. thaliana, such as salicylic acid, jasmonic acid, and nitric oxide, result in an increase in exudation [23]. It also affects the expression profile of transporters belonging to the MATE and ABC families, as well as to the MFS, highlighting the influence of immunity on root exudation. Other studies focusing on the effect of plant defense signalling mutations affecting the rhizosphere and root bacterial community showed that an altered plant immune system could also affect bacterial colonization [24,25,26].
Rhizosphere colonization is crucial for numerous groups of microorganisms, including plant growth-promoting rhizobacteria (PGPR), involved in plant growth promotion and/or biocontrol of plant pathogens [27,28,29]. Their ability to competitively colonize the rhizosphere and persist in this dynamic environment is defined as rhizocompetence [19,30]. PGPR are able to improve plant nutrition and plant immunity, while producing an array of antimicrobial compounds, directly hampering the growth of plant pathogens and disease development [4]. Among PGPR of interest, Pseudomonas spp. have been extensively studied, and a high number of species and strains, displaying strong biocontrol activity, have been described [28,31,32]. Pseudomonas spp. are ubiquitous rod-shaped motile Gram-negative bacteria [33], which are found in multiple habitats, thanks to their metabolic versatility [34]. Many of these bacteria can produce antibiotics, including 2,4-diacetylphloroglucinol, pyrrolnitrin, pyoluteorin, and phenazines [29]. Phenazines are heterocyclic and redox-active molecules, capable of efficiently inhibiting a large spectrum of plant pathogens, including several bacteria, fungi and oomycetes [35,36,37]. Some phytobeneficial Pseudomonas spp. can produce phenazine-1-carboxylic acid (PCA), phenazine-1-carboxamide (PCN) or 2-hydroxyphenazine (2-OH-PHZ), which have been shown to be involved in the biocontrol of important plant pathogens [38,39]. Phenazines are also involved in biofilm development [40,41] and iron reduction [42,43], facilitating bacterial survival in the rhizosphere [44].
Many rhizocompetence determinants have already been discovered in phytobeneficial Pseudomonas spp. [45,46,47], and some studies have highlighted the role played by plant genotypes in rhizosphere colonization [48,49,50]. However, little is known about the role played by specific plant genes in the rhizocompetence of Pseudomonas spp. [51,52]. To develop efficient biocontrol strategies, involving promising Pseudomonas strains, we need to better understand how the plant genotype can affect the recruitment and the rhizocompetence of phytobeneficial Pseudomonas spp. This could lead to new plant breeding targets, enabling phytobeneficial Pseudomonas spp. to better colonize the rhizosphere, which, in turn, may benefit plant growth, yield, and protection against pathogens.
The objective of this study was to assess the impact of specific mutations in A. thaliana on the rhizosphere colonization capabilities of two distinct phenazine-producing P. chlororaphis subsp. piscium strains of biocontrol interest: DTR133, a strong rhizosphere colonizer, and ToZa7, which displays a lower rhizocompetence [53]. These strains, both isolated from the tomato rhizosphere, were chosen because of their promising traits for biocontrol against different pathogens [54,55,56,57,58], and their distinct rhizosphere colonization levels on A. thaliana ecotype Columbia. The studied plant mutants were affected in root exudation, immunity, and root system architecture. The impact of P. chlororaphis subsp. piscium strains DTR133 and ToZa7 inoculation on plant biomass was also investigated in the selected plant mutants, to better understand the interplay between rhizosphere colonization and plant physiology. This work points to the involvement of several plant genes in rhizosphere colonization and in plant–bacteria interactions.
2. Materials and Methods
2.1. Plant Cultivation
Seeds of A. thaliana ecotype Columbia and 20 isogenic mutants were obtained from the Arabidopsis Biological Resource Center (Columbus, OH, USA) (Table 1). The seeds were sterilized in a bleach and Tween 20 solution for 2 min, then transferred into ethanol 95% for another 2 min, and washed four times in sterile distilled water. They were placed in a 0.1% agar solution and kept at 4 °C for 4 d to enhance and synchronize germination. Sowing was performed in peat-based substrate (Pro-Mix, Premier tech, Rivière-du-Loup, Canada) and in washed and sterilized sand (All-purpose sand, Quickrete, Atlanta, GA, USA) in 10 cm diameter plastic pots. Plants grown in peat-based substrate were used to assess rhizosphere colonization by two Pseudomonas strains. Uninoculated sand-grown plants were also used in parallel to phenotypically characterize the impact of the mutations under study. Growth chambers (PGR15, Conviron, Winnipeg, Canada) were used to grow all plants under 60% relative humidity and a 16:8 photoperiod (day:night) at 21 °C, 200 µmol m−2 s−1, and 20 °C respectively, in a randomized configuration. Plants grown in peat-based substrate were fertilized once, 2 w after sowing, with a 1 g·L−1 solution of 20-20-20 fertilizer (All Purpose Fertilizer Water Soluble 20-20-20, Plant-Prod, Brampton, Canada), while those grown in sand received 10% of the same fertilization dose every two days.

Table 1.
Genotypes of A. thaliana used in this study. All mutant strains derive from Col-0. Additional characteristics are available on The Arabidopsis Information Resource website (https://www.arabidopsis.org/, accessed on 13 March 2018).
2.2. Mutant Screening
The A. thaliana isogenic mutants where chosen based on their phenotypic profiles in relation to root architecture, immunity, or exudation. All investigated mutants were originally generated by T-DNA insertion from A. thaliana ecotype Columbia [63]. To confirm the presence of the insertion, one leaf was sampled on each plant one day before inoculation of plants grown in peat-based substrate. Plant DNA was extracted following Springer’s protocol [76]. For each DNA sample, two PCR reactions were performed: one targeting the inserted T-DNA, and the other targeting the native gene. Each PCR mixture contained 1 µL of template DNA and was prepared with 2.5 µL of ThermoPol reaction buffer 10× (New England Biolabs, Ipswich, MA, USA), 3 µL of reverse and forward primers and probe (final concentration at 0.6 µmol.L−1), and 0.625 units of Taq DNA polymerase (New England Biolabs). Sterile dH2O was added for a total reaction volume of 25 µL. The cycling conditions were 95 °C for 5 min followed by 35 cycles of 95 °C for 30 s, annealing for 30 s (for the annealing temperature specific for each gene, see Table A1) and elongation at 72 °C for 2 min. The reaction ended with a final elongation of 10 min at 72 °C. Annealing temperatures were retrieved from the reagent’s manufacturer calculator (https://tmcalculator.neb.com/#!/main, accessed on 23 July 2018). Amplified fragments were verified by electrophoresis on a 1.5% agarose gel. This allowed us to discriminate potential wild-type plants from mutants and heterozygous from homozygous plants. Primer sequences (Table A1) were retrieved from the T-DNA Express website (http://signal.salk.edu/cgi-bin/tdnaexpress, accessed on 13 March 2018) through the iSect tool [77], except for the pgp1, the wrky70 and the cbp60g primers, which were designed using Geneious Prime v.2019.2.1 (Biomatters Ltd., Auckland, New Zealand). LBb1.3 was used as left border primer to detect the inserted T-DNA. The primers were custom synthesized by Integrated DNA Technologies (Coralville, IA, USA). Only homozygous plants for a given mutation were further considered.
2.3. Bacterial Inoculation in the Rhizosphere
Two strains originally isolated from the tomato rhizosphere were used in this study: P. chlororaphis subsp. piscium DTR133 (a strong rhizosphere colonizer on A. thaliana) and ToZa7 (a weaker rhizosphere colonizer) [57,58]. They were grown in King’s B broth medium [78] at 120 RPM and 25 °C for 24 h from cryopreserved cultures. Bacterial concentration was assessed with a spectrophotometer at 600 nm (Novaspec II, Pharmacia Biotech, Piscataway, NJ, USA) and adjusted to 2 × 108 CFU ml−1. Inoculation was performed on the 20 isogenic mutants and on the wild type. Three-week-old A. thaliana plants grown in peat-based substrate were inoculated by pipetting 5 mL of the adjusted bacterial suspensions at the base of the stems. Each plant was inoculated with one strain only (or water for the control plants). For each bacterial strain and plant genotype, five replicates were generated for a total of 110 experimental units. This experiment was replicated a second time. Plants grown in sand were not inoculated, as they were used to phenotypically characterize the impact of the mutations under study.
2.4. Rhizosphere Harvest and Sample Processing
Three weeks following inoculation of A. thaliana grown in peat-based substrate, rhizosphere soil was manually harvested by recovering about 30 mL of soil surrounding the roots. The harvested soil was promptly frozen in liquid nitrogen, before being lyophilized (ModulyoD-115, Thermo Electron Corporation, Waltham, MA, USA) and subsequently stored at −80 °C. DNA was then extracted according to Griffiths and colleagues [79], followed by a DNA purification step to remove potential PCR inhibitors (DNeasy PowerClean Cleanup Kit, Qiagen, Hilden, Germany). Aboveground plant parts were also harvested, dried at 70 °C for 10 d and weighed. Roots of plants grown in peat-based substrate could not be harvested and weighed given their thinness, density, and strong adhesion to the substrate.
2.5. Bacterial Quantification by qPCR
To estimate P. chlororaphis subsp. piscium DTR133 and ToZa7 rhizosphere colonization, the copy number of the phzD gene was assessed by quantitative PCR (qPCR) from all rhizosphere soil DNA samples. Encoding an isochorismatase, this gene belongs to the phenazine biosynthetic cluster [37]. A TaqMan probe and a primer pair designed in a previous study targeting both Pseudomonas strains under study were used, specifically generating a 83 bp-amplicon [53]. The TaqMan probe was labeled with a 6-carboxyfluorescein (6-FAM) reporter dye at the 5’ end and a non-fluorescent minor groove-binding quencher at the 3’ end (MGBNFQ, Applied Biosystems, Waltham, MA, USA). The primers were custom synthesized by Integrated DNA Technologies. The sequences used to amplify the phzD 83 bp-amplicon are: probe, GAA TAC GCC GCC AGC; forward primer, GCA AGG MGC AYC ACT GGA T; reverse primer, TCA TAG CAB CAC CTC RTC GG.
To standardize quantification, the 83-bp phzD PCR amplicon was inserted into the 2,976-bp pKRX plasmid (National Institute of Genetics, Mishima, Japan). The resulting plasmid was transferred into E. coli to be replicated before being extracted using a kit (QIAprep Spin Miniprep, Qiagen) and checked on agarose gel for an insert of the correct size following PCR amplification. The amount of plasmid DNA was assessed using a fluorometer (Qubit 3 Fluorometer, Thermo Fisher Scientific, Waltham, MA, USA). The number of plasmid copies was then inferred by considering the molar mass of the modified pKRX plasmid containing the phzD amplicon. Amplicon-containing plasmid DNA was then serially diluted to known concentrations to generate standard curves ranging from 108 to 101 gene copies µL−1.
A qPCR bioassay using the phzD gene as a target was performed with a Bio-Rad CFX Connect real-time PCR detection system and the iTaq universal probe supermix kit (Bio-Rad Laboratories, Hercules, CA, USA). Each qPCR mixture was prepared with 5 µL (1×) of iTaq universal probe supermix, 0.4 µL of reverse and forward primers and probe (final concentration at 0.2 µmol.L−1), 2.8 µL of sterile dH2O, and 2.4 µL of tenfold-diluted template DNA, for a total volume of 10 µL. The cycling conditions were 95 °C for 2 min followed by 40 two-step cycles consisting of 95 °C for 5 s and 60 °C for 30 s. No template controls were included in each qPCR run by adding sterile dH2O instead of DNA. All qPCRs were replicated 3 times. Standard curves were generated for each 96-well qPCR plate. phzD copy numbers were adjusted per gram of rhizosphere soil. The primers and the probe used to quantify the bacterial strains were tested against uninoculated soil and showed no amplification.
2.6. Phenotypic Characterization of the Root System of Sand-Grown Plants
The 20 isogenic plant mutants used in this study and the wild type were grown in sand, as described above, to facilitate the retrieval of the entire root system for phenotypic characterization. The plants were harvested 6 weeks after sowing, the same period as the plants grown in the peat-based substrate that were inoculated with bacteria. The root system was washed in water to separate sand particles from it. It was then put in a transparent tank filled with water, and photographed (Lumix DMC-ZX1, Panasonic, Kadoma, Osaka, Japan). Some roots were cut, and representative root hair zones were photographed using a microscope (Leitz DMRB and MC170 HD, Leica Microsystems GmbH, Wetzlar, Germany). The roots and aboveground parts were dried at 70 °C for 10 d and weighed. For each plant genotype, 3 replicates were used for a total of 63 experimental units.
2.7. Statistical Analyses
All statistical analyses were performed using RStudio version 1.2.5001 (Boston, MA, USA). The ‘agricolae’ package version 1.3-1 [80] and the ‘nparcomp’ package version 3.0 [81,82] were used to perform multiple comparisons using a Benjamini–Hochberg correction. Unless stated otherwise, test results were interpreted at a 5% significance level. Inter-experiment effects were considered.
3. Results
3.1. Rhizosphere Colonization of Mutant Plants by Both Pseudomonas Strains
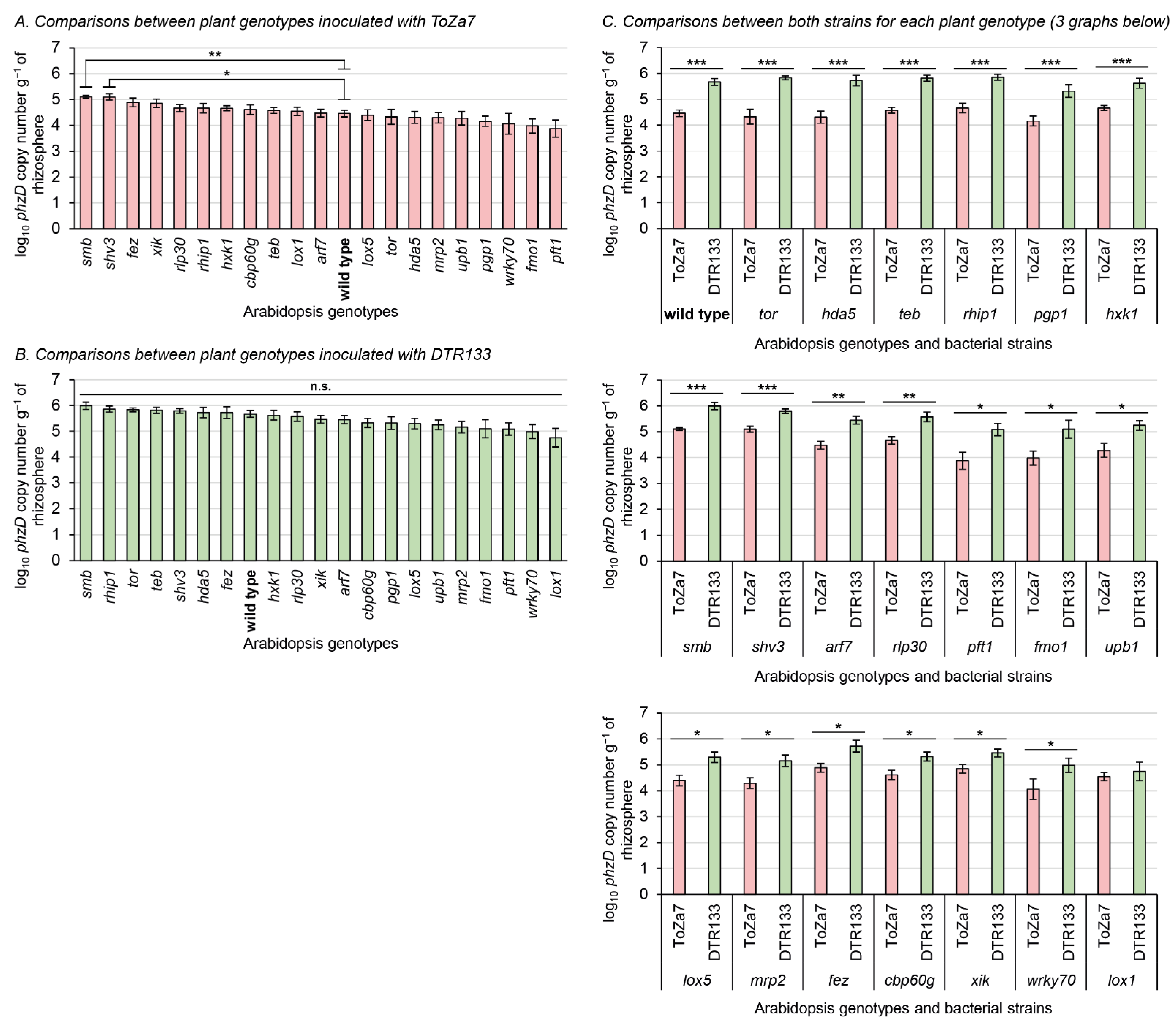
To assess rhizosphere colonization by the two P. chlororaphis subsp. piscium strains, DTR133 and ToZa7, twenty distinct A. thaliana isogenic mutants (and the wild type) affected in root exudation, immunity, and root system architecture were grown in non-sterile potting soil and inoculated. A culture-independent qPCR approach, targeting a conserved DNA motif in the phzD gene, was used to specifically detect and quantify P. chlororaphis subsp. piscium strains DTR133 and ToZa7 in the rhizosphere, three weeks following inoculation (Figure 1). Uninoculated soil was also tested to ensure the specificity of the qPCR bioassay, which led to no amplification. Mutations in plants were all confirmed using PCR with primers specific to the inserted T-DNA.
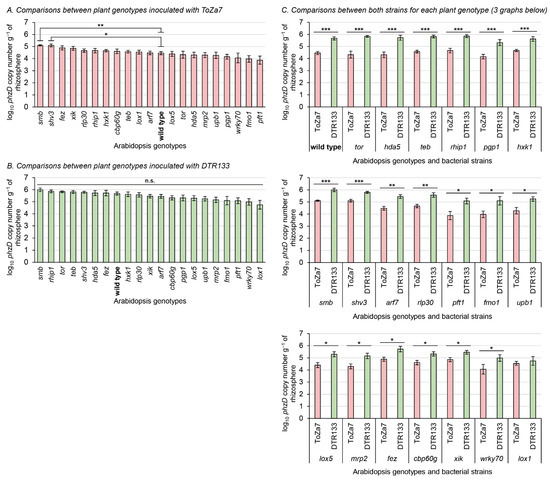
Figure 1.
Rhizosphere colonization three weeks following bacterial inoculation with P. chlororaphis subsp. piscium strains ToZa7 and DTR133 for 21 A. thaliana genotypes. Asterisks refer to significant differences between groups. (A,B): nonparametric multiple test procedure for many-to-one comparisons [81], allowing the comparison of all mutants against the wild type. (C): Wilcoxon–Mann–Whitney tests. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001; n.s.: non-significant. Bars indicate standard errors (n = 10).
ToZa7 colonized the rhizosphere of plants impaired in smb and shv3 on average four-times more than wild-type plants (Figure 1A). When plants were inoculated with DTR133, rhizosphere colonization did not statistically differ between mutants and wild-type plants (Figure 1B). As expected, colonization of wild-type A. thaliana was significantly higher for DTR133 than for ToZa7, on average with a 16-times difference (Figure 1C). The colonization difference between both strains was significant in 19 mutant genotypes (Figure 1C). In those genotypes, DTR133 colonized the rhizosphere 4- to 32-times more than ToZa7. In lox1 mutant plants only, no significant difference in rhizosphere colonization pattern was observed between DTR133 and ToZa7 (Figure 1C). This loss in colonization advantage for DTR133 seems to arise from its reduced colonization capabilities, rather than to an increased colonization by ToZa7 (Figure 1B).
3.2. Aboveground Biomass Accumulation in the Inoculated Mutants Plants
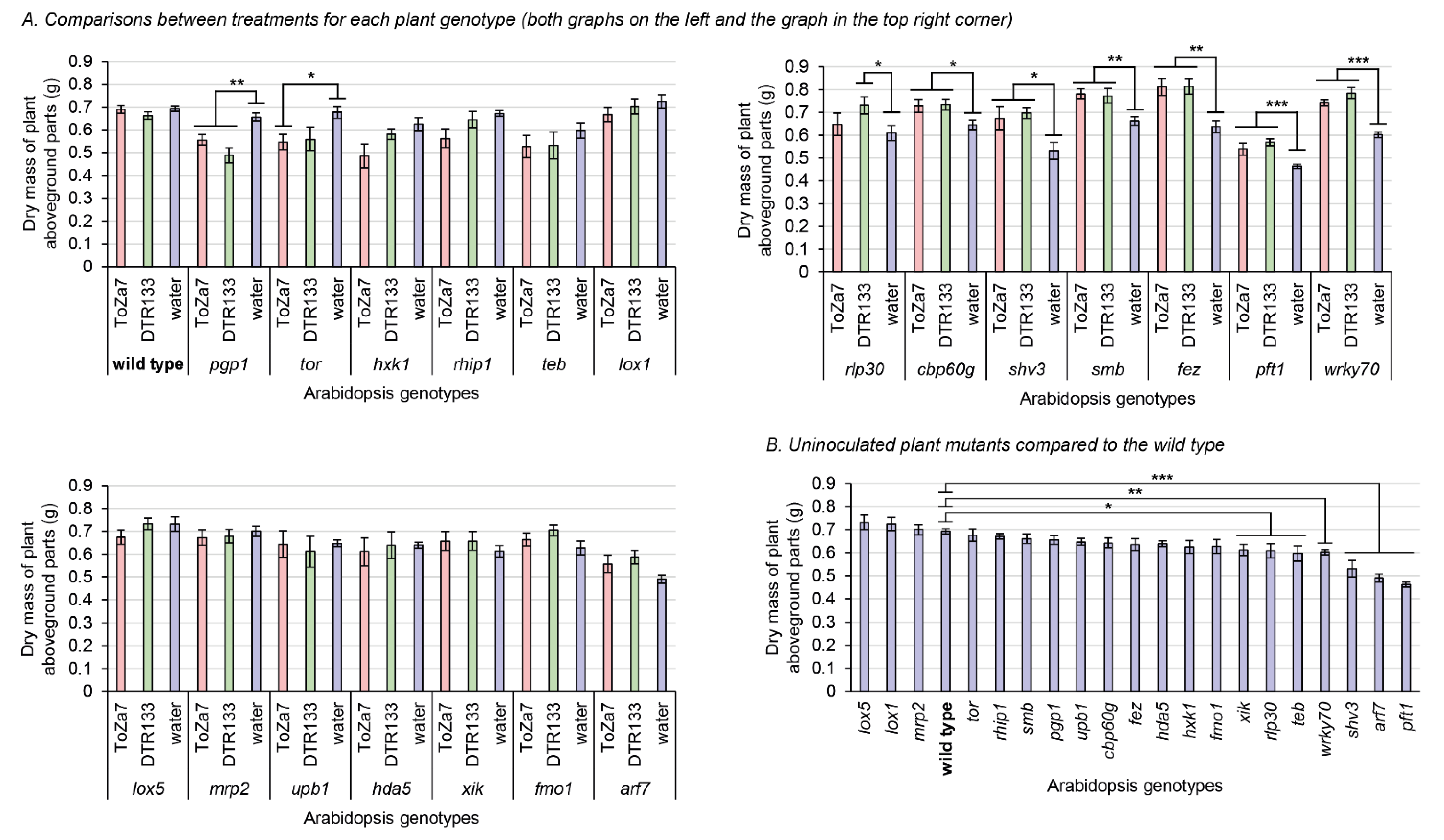
Three weeks following inoculation, plant aboveground parts were harvested, dried, and weighed (Figure 2). Wild-type plants, inoculated with either bacterial strain, did not significantly differ in aboveground biomass, indicating that neither P. chlororaphis subsp. piscium strain DTR133, nor ToZa7 can be considered plant growth promoters on wild-type A. thaliana (Figure 2A). Some mutations, however, had a negative impact on the biomass of uninoculated plants (Figure 2B). Without bacterial inoculation, seven plant genotypes (xik, rlp30, teb, wrky70, shv3, arf7, and pft1) exhibited an 11% to 33% significant mass reduction, when compared to the wild type.
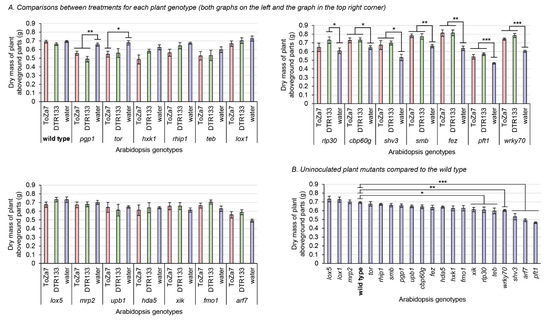
Figure 2.
Dry mass of plant aboveground parts three weeks following inoculation with P. chlororaphis subsp. piscium strains ToZa7 and DTR133 for 21 A. thaliana genotypes. Asterisks refer to significant differences between groups defined by Fisher’s least significant difference test. * p-value < 0.05; ** p-value < 0.01; *** p-value < 0.001. Bars indicate standard errors (n = 10). (A) Comparisons between treatments for each plant genotype (graphs have been divided for easy layout). (B) Uninoculated plant mutants compared to the wild type.
Significant plant biomass differences were found between inoculation treatments for each plant mutant genotype. Seven genotypes displayed increased aboveground biomass when inoculated with DTR133 or ToZa7, compared to water-treated plants (Figure 2A). This increase ranged from 13% to 31% and relates to rlp30, cbp60g, shv3, smb, fez, pft1, and wrky70 plants. Two genotypes instead displayed decreased plant biomass when inoculated with DTR133 or ToZa7: pgp1 and tor. This decrease ranged from 15% to 25%. For most of these mutants displaying different biomasses when inoculated, inoculation had a similar effect in comparison to wild-type plants, whether they were inoculated by DTR133 or ToZa7. However, two plant mutants reacted differently: tor and rlp30. These plants displayed a biomass distinct from wild-type plants when inoculated with one strain, but not with the other. All these results point to a role for these genes in plant–bacteria interactions.
3.3. Phenotypic Characterization of the Mutants Grown in Sterile Sand
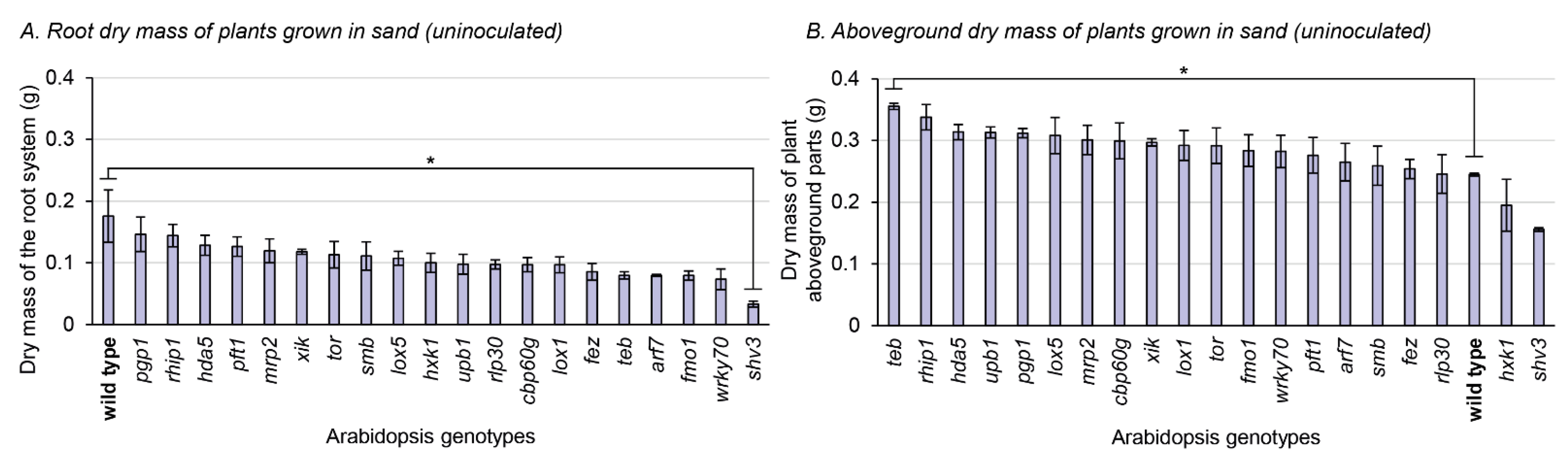
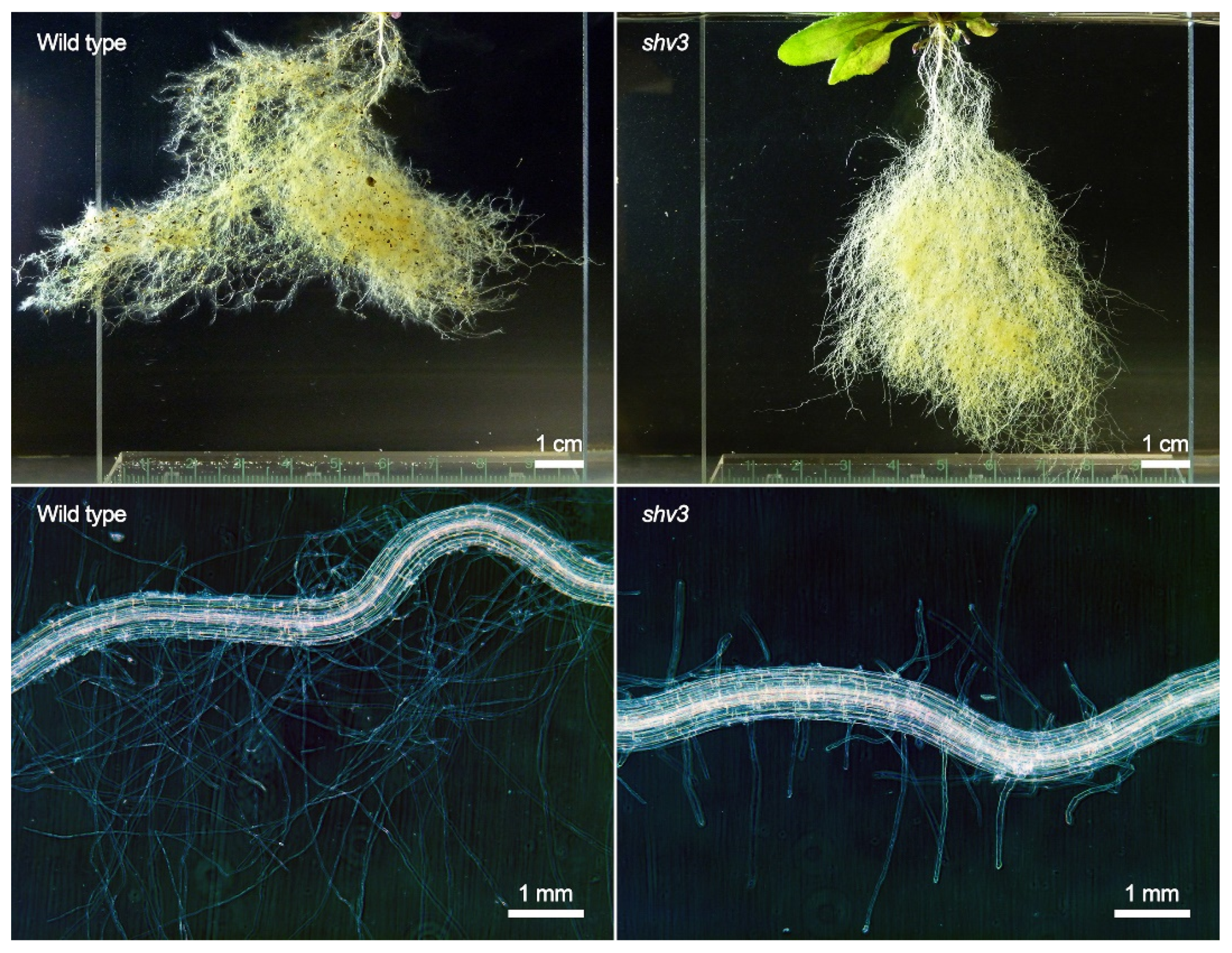
To better assess how mutations could impact on the root system and the rhizosphere, the 21 plant genotypes were grown in sterile sand, without any bacterial inoculation. This allowed for a clear separation between the root system and the substrate, where the mass of the roots and of the aboveground parts can be separately measured (Figure 3), and microscopic observations of the root system can be achieved (Figure 4). Only one mutation significantly impacted the root dry mass compared to the wild type: shv3 (Figure 3A). On average, the dried root system of shv3 plants was about five-times lighter than the wild type. When examined under the microscope, shv3 roots exhibited shorter root hairs than the wild type (Figure 4), which was not the case for the other mutants under study.
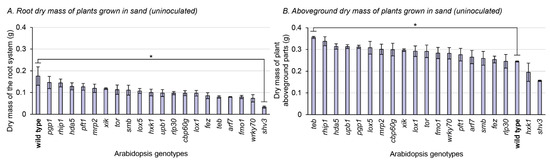
Figure 3.
Dry mass of roots and aboveground parts of 21 A. thaliana genotypes grown in sterile sand (not inoculated). Asterisks refer to significant differences between the wild type and another genotype, defined by Fisher’s least significant difference test. * p-value < 0.05. Bars indicate standard errors (n = 3). (A) Root dry mass of plants grown in sand (uninoculated). (B) Aboveground dry mass of plants grown in sand (uninoculated).
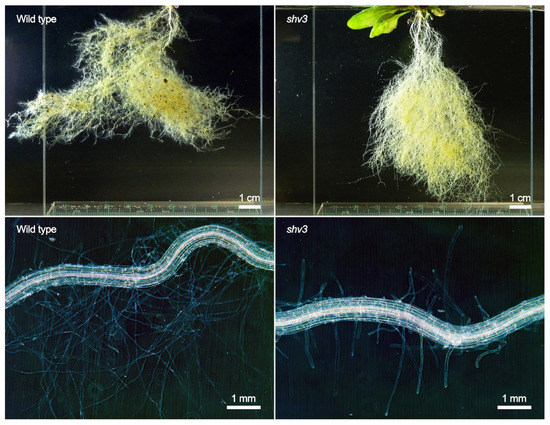
Figure 4.
Root system (top) and root hair zone (bottom) of representative uninoculated A. thaliana wild type (left) and shv3 mutant plants (right) grown in sterile sand.
For the aboveground biomass part of plants grown in sterile sand, only a single genotype displayed a significant difference with the wild type: teb. Plants impaired in teb displayed, on average, 45% higher aboveground biomass parts than the wild type. When instead grown in peat-based substrate without inoculation, plants impaired in teb displayed on average 14% lower biomass than the wild type (Figure 3B).
4. Discussion
This study aimed at evaluating the effect of specific mutations in A. thaliana on rhizosphere colonization, by two phenazine-producing P. chlororaphis subsp. piscium strains of biocontrol interest, DTR133 and ToZa7, known for their distinct colonization abilities on A. thaliana. The rhizosphere colonization of 20 different A. thaliana isogenic mutants (and the wild type) was assessed in non-sterile potting soil, three weeks following bacterial inoculation. In parallel, plant aboveground biomass was measured to further assess the interactions existing between bacterial inoculation treatments and specific A. thaliana mutations. The A. thaliana isogenic mutants where chosen based on their phenotypic profiles, in relation to root architecture, immunity, or exudation.
4.1. Rhizosphere Colonization Is Affected by the Plant Genotype
Plants impaired in smb and shv3 displayed higher rhizosphere colonization by ToZa7 than wild-type plants (Figure 1A). SOMBRERO (SMB) is a NAC-domain (for NAM, ATAF1/2, and CUC2) transcription factor controlling the root cap development [61,69]. When plants are impaired in smb, lateral root cap maturation is delayed, and the root cap extends into the differentiation zone instead of sloughing off the root tip. Root caps produce detached active cells, called border cells, involved in exudation and plant defense [83]. Mutations in smb may affect these cells, especially their exudation profiles or their anti-microbial role, leading to increased bacterial colonization by ToZa7. SHAVEN3 (SHV3) is a glycerophosphoryl diester phosphodiesterase-like protein, involved in cell wall organization and root hair morphogenesis [15,68]. When shv3 is impaired, root hair tip growth is blocked because of ruptures in root hair cells, as illustrated by our microscopic observations (Figure 4). The presence of shorter root hairs may explain the lighter root system observed in shv3 plants, in comparison to the wild type (Figure 3A). Root hairs represent up to 77% of root surface in cultivated crops and are, with the root caps, important sources of root exudates [16,84]. An altered root system architecture may improve plant–bacteria interactions, leading to an increased rhizosphere colonization. Contrary to ToZa7, DTR133 colonized the rhizosphere of mutant plants to the same extent as the wild-type plants (Figure 1B). It may, therefore, display a different colonization strategy than ToZa7, thus, differently interacting with the mutant phenotypes.
As previously shown, there is a significant rhizosphere colonization-level difference between both bacterial strains in A. thaliana wild-type plants [53]. P. chlororaphis subsp. piscium strain DTR133 is a strong rhizosphere colonizer, while ToZa7 displays a lower rhizocompetence. This significant difference in rhizosphere colonization patterns was also observed in 19 out of the 20 A. thaliana mutants under study. Interestingly, no colonization differences between the two P. chlororaphis strains were observed in one A. thaliana mutant: lox1 (Figure 1C). LOX1 is a lipoxygenase, initiating the biosynthesis of plant oxylipins, which are lipid derivatives, such as jasmonic acid, involved in plant physiological processes, including growth and fertility [65]. It may also be involved in stress signalling from roots to shoots [85]. Plants impaired in LOX1 develop more emergent and lateral roots than the wild type. In lox1 mutants, the absence of colonization differences between both P. chlororaphis strains seems to arise from a reduced colonization by DTR133, rather than an increased colonization by ToZa7 (Figure 1B). This differential impact of lox1 mutant plants on rhizosphere colonization by P. chlororaphis strains could be explained by distinct colonization patterns interacting with the altered root system architecture. Noirot-Gros et al. have shown that two P. fluorescens strains, SBW25 and WH6, had distinct patterns of biofilm formation on Aspen roots [86]. Further, Pliego et al. demonstrated that P. alcaligenes AVO73 and P. pseudoalcaligenes AVO110 had distinct avocado root colonization strategies [87]. It would be interesting, in a follow-up study, to assess ToZa7 and DTR133 colonization strategies in the rhizosphere of lox1 mutant plants, compared to the wild type, for example, by using fluorescent labeling and confocal microscopy. Interestingly, LOX1 is involved in jasmonic acid-dependant plant defense pathways. Jasmonic acid and its derivatives are known for mediating beneficial plant–bacteria interactions, especially through induced systemic resistance (ISR) [88]. ToZa7 has been shown to induce the expression of defense-related genes, potentially involved in ISR [56]. To our knowledge, DTR133 has not been shown to induce such a mechanism yet. Jasmonic acid could play a critical role in its interaction with A. thaliana, affecting rhizosphere colonization.
4.2. Bacterial Inoculation on Several Plant Mutants Differently Impacts Plant Biomass
Plants impaired in cbp60g, shv3, smb, fez, pft1, or wrky70, inoculated with ToZa7 or DTR133, displayed increased biomass compared to water-treated plants (Figure 2A). These genes are mostly involved in the plant immunity. ToZa7, the less efficient rhizosphere colonizer, was shown to better colonize the rhizosphere of smb and shv3 plants than the wild type, which could potentially improve its interactions with A. thaliana and increase biomass. This was, however, not the case for DTR133, for which no significant colonization differences between the 20 mutants under study were observed. ToZa7 and DTR133 have previously been shown to harbor genes related to plant growth promotion, involved in the biosynthesis of auxin, pyrroloquinoline quinone, and 2,3-butanediol, that could potentially explain the biomass increase observed in different A. thaliana mutants [89]. However, the mechanisms underlying plant growth promotion in mutants and not in the wild type remain to be elucidated. FEZ, like SMB, is a NAC-domain transcription factor, controlling the root cap development [61,69]. FEZ and SMB regulate each other, controlling stem cells divisions in the root cap. Plants impaired in fez display fewer columella and lateral root cap cell layers. The effect of both mutations on root structure may have affected the Pseudomonas spp. colonization process and their ability to impact the plant by modifying root tip exudation or root colonization sites. WRKY70 is a transcription factor, playing a crucial role in the balance between salicylic acid- and jasmonic acid-dependant defense pathways in A. thaliana [73]. Plants impaired in wrky70 are more susceptible to bacterial, fungal, and oomycete pathogens [90,91]. Further, wrky70 has been shown to be upregulated, following rhizosphere inoculation with PGPR, such as P. fluorescens SS101 [92] and Bacillus cereus AR156 [93], leading to induced systemic resistance against P. syringae pv. tomato. The disruption of wrky70 could impair the A. thaliana immune system and enhance plant–bacteria interactions, leading to an increased biomass. CBP60g (calmodulin-binding protein 60-like g) is a transcription factor involved in MAMP-triggered (microbe-associated molecular patterns) immunity, especially in salicylic acid signalling [60]. It has been proposed to function as a master regulator of the plant immune system [94]. Plants impaired in cbp60g display enhanced growth of the bacterial phytopathogen P. syringae. PFT1 (phytochrome and flowering time, also called MED25) is a subunit of the Mediator complex, a large multiprotein complex involved in transcription regulation [66]. PFT1 plays a role in jasmonate-dependent plant defenses, but is also involved in root hair development [95]. Considering these results, we hypothesise that an altered plant immune response, due to at least some of these mutations, may improve plant–bacteria interactions, leading to an increased plant biomass.
Plants impaired in rlp30, inoculated with DTR133, displayed increased biomass compared to water-treated plants, while inoculation with ToZa7 had no significant effect on biomass (Figure 2A). RLP30 is a receptor-like protein, located at the cell surface, involved in disease resistance [67]. Mutant plants display enhanced susceptibility against P. syringae pv phaseolicola, Sclerotinia sclerotiorum, and Botrytis cinerea, probably because of a defect in the plant MAMP-triggered immune response [96]. This mutation seems to specifically affect the interaction between DTR133 and the plant. DTR133 may harbor an elicitor of the plant immune system that ToZa7 does not produce. It may, thus, not be recognized by the mutant plant anymore, allowing the bacteria to better interact with it in the rhizosphere.
Bacterial inoculation of plants impaired in pgp1 or tor negatively impacted plant biomass. PGP1 (multi-drug resistance P-glycoprotein) is an ATP-binding cassette transporter involved in auxin transport [21]. Plants impaired in pgp1 display more lateral roots, as well as an altered root exudation profile. TOR (target of rapamycin) is a conserved eukaryotic kinase regulating cell growth, according to nutrient availability [71]. Plants harboring a T-DNA upstream TOR (accession SALK_007846) overexpress TOR mRNA in roots, leading to increased root growth. Deprost et al. suggested that this is probably caused by a strong promoter, carried by the inserted T-DNA [71]. Bacterial inoculation might lead to an imbalance between root growth and shoot growth, in favor of the root system, which could be detrimental to the accumulation of biomass in the aboveground plant parts.
4.3. Changes in Root Exudates Composition Do Not Alter Rhizosphere Colonization
Badri et al. showed that pgp1 and mrp2 mutant plants display altered root exudates compositions [21], which could have impacted rhizosphere colonization levels of DTR133 and ToZa7 or their colonization trends. Results obtained in this study did not support this hypothesis: both strains inoculated in the rhizosphere of these mutants showed no significant difference in colonization compared to the wild type. However, inoculated pgp1 plants displayed reduced biomass compared to water-treated plants. Because rhizosphere colonization was not significantly impacted by this mutation, this decrease probably arose from changes in plant–bacteria interactions.
4.4. The teb Mutation Interplays with the Plant Substrate to Affect Aboveground Biomass Accumulation
Uninoculated plants, impaired in teb, demonstrated a higher aboveground biomass accumulation than the wild type when grown in sand (Figure 3B), while they showed reduced biomass accumulation compared to the wild type when grown in the peat-based substrate (Figure 2B). TEBICHI is a DNA polymerase, involved in the plant DNA damage responses [97]. Plants lacking this protein display shorter roots and a smaller aerial system [70]. The differential impact of substrates on biomass accumulation in this mutant remains unexplained.
The various A. thaliana mutants used in this study have been generated by different research teams, who phenotypically characterized them according to their own topics of interest. The information available for these plants is consequently heterogeneous. Further phenotypic characterization of these mutants could strengthen our understanding of the underlying plant–bacteria interaction mechanisms, especially regarding plant growth promotion and biocontrol.
In conclusion, this study aimed at evaluating the impact of specific mutations in A. thaliana on rhizosphere colonization by two distinct phenazine-producing P. chlororaphis subsp. piscium strains of biocontrol interest, and their interacting impacts on plant biomass. Mutations in two plant genes, involved in the root system architecture, smb and shv3, have been shown to positively impact ToZa7 rhizosphere colonization and to allow for biomass increase, when bacterial inoculation was performed. The inactivation of another gene, related to the root system architecture, lox1, induced a change in colonization patterns between ToZa7 and DTR133. Mutations in several genes, mostly involved in the plant immune system, were associated with biomass increase only, in relation to bacterial inoculation: fez, wrky70, cbp60g, pft1, and rlp30. The results indicate an interplay between plant genotype, plant growth and bacterial rhizosphere colonization. Further investigation will be required to decipher the mechanisms underlying the impact of these genes on rhizospheric bacteria colonization, to improve Pseudomonas spp. rhizocompetence, and ultimately, their biocontrol abilities in the field. Some of the candidate genes studied here could become interesting targets for assisted plant breeding programs.
Author Contributions
Conceptualization, A.Z. and M.F.; methodology, A.Z. and M.F.; formal analysis, A.Z.; investigation, A.Z., H.S. and A.N.; writing—original draft preparation, A.Z.; writing—review and editing, A.Z. and M.F.; supervision, M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the New Brunswick Innovation Foundation (NBIF) grants to M. Filion.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Arabidopsis Biological Resource Center for providing all the seeds used in this study. We also thank Adrien Biessy for proofreading the manuscript and contributing to the rhizosphere harvests, as well as Loïck Ducros, who also helped in rhizosphere sampling.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Primer sequences used to screen mutants for homozygosity. Sequences were retrieved from the T-DNA Express website (http://signal.salk.edu/cgi-bin/tdnaexpress, accessed on 13 March 2018) through the iSect tool, except for the pgp1, the wrky70 and the cbp60g primers, which were designed using Geneious Prime v.2019.2.1. LBb1.3 was used as left border primer to detect the inserted T-DNA. Ta: Annealing temperature.
Table A1.
Primer sequences used to screen mutants for homozygosity. Sequences were retrieved from the T-DNA Express website (http://signal.salk.edu/cgi-bin/tdnaexpress, accessed on 13 March 2018) through the iSect tool, except for the pgp1, the wrky70 and the cbp60g primers, which were designed using Geneious Prime v.2019.2.1. LBb1.3 was used as left border primer to detect the inserted T-DNA. Ta: Annealing temperature.
| Accession Number | Affected Gene | Forward Primer | Reverse Primer | Ta (°C) |
|---|---|---|---|---|
| CS66051 | pgp1 | GTTGAAGAACCCGGCGATAC | AGCAACAACTACGGGGAAGA | 53 |
| CS66052 | mrp2 | TGGATGGGTTACATCTATGCG | TGCAATCACTATTCATAGCCAATC | 49 |
| SALK_059431C | lox1-1 | AGCTCCTTGAACCTCACTTCC | GAGACGCTATTTGGAATTCCC | 50 |
| SALK_044826C | lox5-1 | ATCACATGACAGGCCCAATAG | TGATCGATTCGATTCGAAATC | 47 |
| CS69137 | rhip1-1 | CTTCTTCTGCAGAAATGGTGG | CTTTTTCACGAAAATTGGCTG | 47 |
| SALK_093312C | hda5 | ATCTGGGAGAAGCTTCAGCTC | CTGCATTCAGGAAAAGCAAAG | 49 |
| CS69135 | hxk1-3 | TTGTTTTTGATTCCAAATCGG | TCATCAAATGAGGAGGAATCG | 46 |
| CS24607 | arf7-1 | CAGCTAGATCGTTCGAAATGG | AGCACATCACCATTTAGGTGC | 50 |
| SALK_024208C | shv3-2 | GAAGGTTGTCACGAAGACTCG | TCCAAAACAGAAAAATGCTGG | 48 |
| SALK_143526C | smb-3 | GTCGTCATCATCATCTGCATC | GTGTATAACGCGCACACACAC | 50 |
| SALK_007846C | tor | CGTTAACACTTGGACCCTGTC | ACCCCTTTTTGGTTCAACAAC | 51 |
| SALK_018851C | teb-5 | ATTCATTGCTCGGCATCTATG | CGATTCATTGGATTGTTTTGG | 47 |
| SALK_026163C | fmo1-1 | CTTTTCGGTTGGACTTGGAAC | CTGCTTTGGACGTATCCTACG | 51 |
| SALK_025663C | fez-2 | TAGACATTGTGTCGGTGCTTG | GTATAGGAACAGCTCGAGGCC | 52 |
| SALK_025198C | wrky70-1 | TGATCTTCGGAATCCATGAAG | CAAACCACACCAAGAGGAAAG | 49 |
| CS868100 | upb1-1 | CTGAACTTCGAATTTGGATGC | ACACCCTTGTGGACACTTGTC | 49 |
| SALK_129555C | pft1-2 | TGGAACTGGTCCAACAGAAAC | TGCATTGGCTTTCTTCCATAC | 50 |
| SALK_067972C | xik-2 | GGGTAGCAAGATACTCCTCGG | GCAAGAGCAACTCAATTCTGG | 51 |
| SALK_023199C | cbp60g-1 | TCAATGAAGATTCGGAACAGC | ACTTCCGACTCCTAGTCCAGC | 50 |
| CS65465 | rlp30-2 | TCCCGACAAATGAATTCTCAC | TGTCGACGAGAAGCTTAGCTC | 49 |
| LBb1.3—Primer targeting the inserted T-DNA | ATTTTGCCGATTTCGGAAC | Reverse primer of each genotype | 48 | |
References
- Hiltner, L. Über Neuere Erfahrungen Und Probleme Auf Dem Gebiete Der Bodenbakteriologie Unter Besonderer Berücksichtigung Der Gründüngung Und Brache. Arb. Dtsch. Landwirtsch. Ges. 1904, 98, 59–78. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.; Bloemberg, G.V. Life in the Rhizosphere. In Pseudomonas Vol. 1—Genomics, Life Style and Molecular Architecture; Ramos, J.-L., Ed.; Springer: New York, NY, USA, 2004; pp. 403–430. ISBN 1-4613-4788-2. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.M.; Whipps, J.M. Substrate Flow in the Rhizosphere. Plant Soil 1990, 129, 1–10. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. The Release of Root Exudates as Affected by the Plant Physiological Status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 23–72. [Google Scholar]
- Badri, D.V.; Vivanco, J.M. Regulation and Function of Root Exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic Root Exudate Chemistry and Microbial Substrate Preferences Drive Patterns in Rhizosphere Microbial Community Assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.-R.; Borriss, R.; von Wirén, N. Root Exudation of Sugars, Amino Acids, and Organic Acids by Maize as Affected by Nitrogen, Phosphorus, Potassium, and Iron Deficiency. J. Plant. Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Uren, N.C. Types, Amounts, and Possible Functions of Compounds Released into the Rhizosphere by Soil-Grown Plants. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–21. [Google Scholar]
- Jaeger, C.H.; Lindow, S.E.; Miller, W.; Clark, E.; Firestone, M.K. Mapping of Sugar and Amino Acid Availability in Soil around Roots with Bacterial Sensors of Sucrose and Tryptophan. Appl. Environ. Microbiol. 1999, 65, 2685–2690. [Google Scholar] [CrossRef] [Green Version]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Reverdy, A.; She, Q.; Sun, B.; Chai, Y. The Role of Rhizodeposits in Shaping Rhizomicrobiome. Environ. Microbiol. Rep. 2019, 12, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Cavell, A.C.; Dolan, L.; Roberts, K.; Grierson, C.S. Genetic Interactions during Root Hair Morphogenesis in Arabidopsis. Plant Cell 2000, 12, 1961–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertin, C.; Yang, X.; Weston, L.A. The Role of Root Exudates and Allelochemicals in the Rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, P.R.; Miller, A.J.; Dennis, P.G. Do Root Exudates Exert More Influence on Rhizosphere Bacterial Community Structure than Other Rhizodeposits. In Molecular Microbial Ecology of the Rhizosphere: Volume 1 & 2; de Bruijn, F.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Volume 1, pp. 229–242. [Google Scholar]
- Zboralski, A.; Biessy, A.; Filion, M. Rhizosphere Colonization by Plant-Beneficial Pseudomonas spp.: Thriving in a Heterogeneous and Challenging Environment. In Advances in PGPR Research; Singh, H.B., Ed.; CAB International: Oxfordshire, UK, 2017; pp. 197–217. [Google Scholar]
- Weston, L.A.; Ryan, P.R.; Watt, M. Mechanisms for Cellular Transport and Release of Allelochemicals from Plant Roots into the Rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef]
- Badri, D.V.; Loyola-Vargas, V.M.; Broeckling, C.D.; De-la-Pena, C.; Jasinski, M.; Santelia, D.; Martinoia, E.; Sumner, L.W.; Banta, L.M.; Stermitz, F.; et al. Altered Profile of Secondary Metabolites in the Root Exudates of Arabidopsis ATP-Binding Cassette Transporter Mutants. Plant Physiol. 2008, 146, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Cox, D.E.; Dyer, S.; Weir, R.; Cheseto, X.; Sturrock, M.; Coyne, D.; Torto, B.; Maule, A.G.; Dalzell, J.J. ABC Transporter Genes ABC-C6 and ABC-G33 Alter Plant-Microbe-Parasite Interactions in the Rhizosphere. Sci. Rep. 2019, 9, 19899. [Google Scholar] [CrossRef]
- Badri, D.V.; Loyola-Vargas, V.M.; Du, J.; Stermitz, F.R.; Broeckling, C.D.; Iglesias-Andreu, L.; Vivanco, J.M. Transcriptome Analysis of Arabidopsis Roots Treated with Signaling Compounds: A Focus on Signal Transduction, Metabolic Regulation and Secretion. New Phytol. 2008, 179, 209–223. [Google Scholar] [CrossRef]
- Doornbos, R.F.; Geraats, B.P.J.; Kuramae, E.E.; Van Loon, L.C.; Bakker, P.A.H.M. Effects of Jasmonic Acid, Ethylene, and Salicylic Acid Signaling on the Rhizosphere Bacterial Community of Arabidopsis Thaliana. Mol. Plant Microbe Interact. 2011, 24, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G.; et al. Salicylic Acid Modulates Colonization of the Root Microbiome by Specific Bacterial Taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Kidd, B.N.; Vivanco, J.M.; Schenk, P.M. Linking Jasmonic Acid Signaling, Root Exudates, and Rhizosphere Microbiomes. Mol. Plant Microbe Interact. 2015, 28, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Mulders, I.H.M.; Dekkers, L.C.; Lugtenberg, B.J.J. Root Colonization by Phenazine-1-Carboxamide-Producing Bacterium Pseudomonas Chlororaphis PCL1391 Is Essential for Biocontrol of Tomato Foot and Root Rot. Mol. Plant Microbe Interact. 2000, 13, 1340–1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloemberg, G.V.; Lugtenberg, B.J. Molecular Basis of Plant Growth Promotion and Biocontrol by Rhizobacteria. Curr. Opin. Plant. Biol. 2001, 4, 343–350. [Google Scholar] [CrossRef]
- Haas, D.; Défago, G. Biological Control of Soil-Borne Pathogens by Fluorescent Pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.M. Biological Control of Soilborne Plant Pathogens in the Rhizosphere with Bacteria. Annu. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Weller, D.M. Pseudomonas Biocontrol Agents of Soilborne Pathogens: Looking Back over 30 Years. Phytopathology 2007, 97, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Blanco, J. Pseudomonas Strains That Exert Biocontrol of Plant Pathogens. In Pseudomonas; Ramos, J.-L., Goldberg, J.B., Filloux, A., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 121–172. ISBN 94-017-9554-1. [Google Scholar]
- Palleroni, N.J. Genus I Pseudomonas. In Bergey’s Manual of Systematic Bacteriology; Krieg, N.R., Holt, J.C., Eds.; The Williams and Wilkins Co.: Baltimore, MD, USA, 1984; pp. 141–199. [Google Scholar]
- Stanier, R.Y.; Palleroni, N.J.; Doudoroff, M. The Aerobic Pseudomonads a Taxonomic Study. J. Gen. Microbiol. 1966, 43, 159–271. [Google Scholar] [CrossRef] [Green Version]
- Chin-A-Woeng, T.F.C.; Bloemberg, G.V.; Lugtenberg, B.J.J. Phenazines and Their Role in Biocontrol by Pseudomonas Bacteria. New Phytol. 2003, 157, 503–523. [Google Scholar] [CrossRef] [Green Version]
- Mavrodi, D.V.; Parejko, J.A.; Mavrodi, O.V.; Kwak, Y.-S.; Weller, D.M.; Blankenfeldt, W.; Thomashow, L.S. Recent Insights into the Diversity, Frequency and Ecological Roles of Phenazines in Fluorescent Pseudomonas spp. Environ. Microbiol. 2013, 15, 675–686. [Google Scholar] [CrossRef]
- Biessy, A.; Filion, M. Phenazines in Plant-Beneficial Pseudomonas spp.: Biosynthesis, Regulation, Function and Genomics. Environ. Microbiol. 2018, 20, 3905–3917. [Google Scholar] [CrossRef] [Green Version]
- Thomashow, L.S.; Weller, D.M. Role of a Phenazine Antibiotic from Pseudomonas Fluorescens in Biological Control of Gaeumannomyces Graminis Var. Tritici. J. Bacteriol. 1988, 170, 3499–3508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrodi, D.V.; Blankenfeldt, W.; Thomashow, L.S. Phenazine Compounds in Fluorescent Pseudomonas spp. Biosynthesis and Regulation. Annu. Rev. Phytopathol. 2006, 44, 417–445. [Google Scholar] [CrossRef] [PubMed]
- Selin, C.; Habibian, R.; Poritsanos, N.; Athukorala, S.N.P.; Fernando, D.; De Kievit, T.R. Phenazines Are Not Essential for Pseudomonas Chlororaphis PA23 Biocontrol of Sclerotinia Sclerotiorum, but Do Play a Role in Biofilm Formation. FEMS Microbiol. Ecol. 2010, 71, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeTourneau, M.K.; Marshall, M.J.; Cliff, J.B.; Bonsall, R.F.; Dohnalkova, A.C.; Mavrodi, D.V.; Devi, S.I.; Mavrodi, O.V.; Harsh, J.B.; Weller, D.M.; et al. Phenazine-1-Carboxylic Acid and Soil Moisture Influence Biofilm Development and Turnover of Rhizobacterial Biomass on Wheat Root Surfaces. Environ. Microbiol. 2018, 20, 2178–2194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wilks, J.C.; Danhorn, T.; Ramos, I.; Croal, L.; Newman, D.K. Phenazine-1-Carboxylic Acid Promotes Bacterial Biofilm Development via Ferrous Iron Acquisition. J. Bacteriol. 2011, 193, 3606–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeTourneau, M.; Marshall, M.J.; Grant, M.R.; Freeze, P.M.; Strawn, D.; Lai, B.; Dohnalkova, A.; Harsh, J.B.; Weller, D.M.; Thomashow, L.S. Phenazine-1-Carboxylic Acid-Producing Bacteria Enhance the Reactivity of Iron Minerals in Dryland and Irrigated Wheat Rhizospheres. Environ. Sci. Technol. 2019, 53, 14273–14284. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Cook, R.J.; Thomashow, L.S.; Weller, D.M.; Pierson, L.S. Contribution of Phenazine Antibiotic Biosynthesis to the Ecological Competence of Fluorescent Pseudomonads in Soil Habitats. Appl. Environ. Microbiol. 1992, 58, 2616–2624. [Google Scholar] [CrossRef] [Green Version]
- Mirleau, P.; Philippot, L.; Corberand, T.; Lemanceau, P. Involvement of Nitrate Reductase and Pyoverdine in Competitiveness of Pseudomonas Fluorescens Strain C7R12 in Soil. Appl. Environ. Microbiol. 2001, 67, 2627–2635. [Google Scholar] [CrossRef] [Green Version]
- Latour, X.; Delorme, S.; Mirleau, P.; Lemanceau, P. Identification of Traits Implicated in the Rhizosphere Competence of Fluorescent Pseudomonads: Description of a Strategy Based on Population and Model Strain Studies. Agronomie 2003, 23, 397–405. [Google Scholar] [CrossRef]
- Zboralski, A.; Filion, M. Genetic Factors Involved in Rhizosphere Colonization by Phytobeneficial Pseudomonas spp. Comput. Struct. Biotechnol. J. 2020, 18, 3539–3554. [Google Scholar] [CrossRef]
- Mazzola, M. Mechanisms of Natural Soil Suppressiveness to Soilborne Diseases. Antonie Van Leeuwenhoek 2002, 81, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.; Funnell, D.L.; Raaijmakers, J.M. Wheat Cultivar-Specific Selection of 2,4-Diacetylphloroglucinol-Producing Fluorescent Pseudomonas Species from Resident Soil Populations. Microb. Ecol. 2004, 48, 338–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Fuente, L.; Landa, B.B.; Weller, D.M. Host Crop Affects Rhizosphere Colonization and Competitiveness of 2, 4-Diacetylphloroglucinol-Producing Pseudomonas Fluorescens. Phytopathology 2006, 96, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.P.; Handelsman, J.; Goodman, R.M. Genetic Basis in Plants for Interactions with Disease-Suppressive Bacteria. Proc. Natl. Acad. Sci. USA 1999, 96, 4786–4790. [Google Scholar] [CrossRef] [Green Version]
- Persello-Cartieaux, F.; David, P.; Sarrobert, C.; Thibaud, M.-C.; Achouak, W.; Robaglia, C.; Nussaume, L. Utilization of Mutants to Analyze the Interaction between Arabidopsis Thaliana and Its Naturally Root-Associated Pseudomonas. Planta 2001, 212, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zboralski, A.; Biessy, A.; Savoie, M.-C.; Novinscak, A.; Filion, M. Metabolic and Genomic Traits of Phytobeneficial Phenazine-Producing Pseudomonas spp. Are Linked to Rhizosphere Colonization in Arabidopsis Thaliana and Solanum Tuberosum. Appl. Environ. Microbiol. 2020, 86, e02443-19. [Google Scholar] [CrossRef]
- Biessy, A.; Novinscak, A.; St-Onge, R.; Léger, G.; Zboralski, A.; Filion, M. Inhibition of Three Potato Pathogens by Phenazine-Producing Pseudomonas spp. Is Associated with Multiple Biocontrol-Related Traits. mSphere 2021, 6, e00427-21. [Google Scholar] [CrossRef]
- Ghirardi, S.; Dessaint, F.; Mazurier, S.; Corberand, T.; Raaijmakers, J.M.; Meyer, J.-M.; Dessaux, Y.; Lemanceau, P. Identification of Traits Shared by Rhizosphere-Competent Strains of Fluorescent Pseudomonads. Microb. Ecol. 2012, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Kamou, N.N.; Cazorla, F.; Kandylas, G.; Lagopodi, A.L. Induction of Defense-Related Genes in Tomato Plants after Treatments with the Biocontrol Agents Pseudomonas Chlororaphis ToZa7 and Clonostachys Rosea IK726. Arch. Microbiol. 2019, 202, 257–267. [Google Scholar] [CrossRef]
- Kamou, N.N.; Karasali, H.; Menexes, G.; Kasiotis, K.M.; Bon, M.C.; Papadakis, E.N.; Tzelepis, G.D.; Lotos, L.; Lagopodi, A.L. Isolation Screening and Characterisation of Local Beneficial Rhizobacteria Based upon Their Ability to Suppress the Growth of Fusarium Oxysporum f. Sp. Radicis-Lycopersici and Tomato Foot and Root Rot. Biocontrol Sci. Technol. 2015, 25, 928–949. [Google Scholar] [CrossRef]
- Landa, B.B.; Cachinero-Díaz, J.M.; Lemanceau, P.; Jiménez-Díaz, R.M.; Alabouvette, C. Effect of Fusaric Acid and Phytoanticipins on Growth of Rhizobacteria and Fusarium Oxysporum. Can. J. Microbiol. 2002, 48, 971–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okushima, Y. Functional Genomic Analysis of the AUXIN RESPONSE FACTOR Gene Family Members in Arabidopsis Thaliana: Unique and Overlapping Functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Tsuda, K.; Sato, M.; Cohen, J.D.; Katagiri, F.; Glazebrook, J. Arabidopsis CaM Binding Protein CBP60g Contributes to MAMP-Induced SA Accumulation and Is Involved in Disease Resistance against Pseudomonas Syringae. PLoS Pathog. 2009, 5, e1000301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemsen, V.; Bauch, M.; Bennett, T.; Campilho, A.; Wolkenfelt, H.; Xu, J.; Haseloff, J.; Scheres, B. The NAC Domain Transcription Factors FEZ and SOMBRERO Control the Orientation of Cell Division Plane in Arabidopsis Root Stem Cells. Dev. Cell. 2008, 15, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, M. Salicylic Acid-Independent ENHANCED DISEASE SUSCEPTIBILITY1 Signaling in Arabidopsis Immunity and Cell Death Is Regulated by the Monooxygenase FMO1 and the Nudix Hydrolase NUDT7. Plant Cell 2006, 18, 1038–1051. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.M. Genome-Wide Insertional Mutagenesis of Arabidopsis Thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-P.; Tunc-Ozdemir, M.; Chang, Y.; Jones, A.M. Cooperative Control between AtRGS1 and AtHXK1 in a WD40-Repeat Protein Pathway in Arabidopsis Thaliana. Front. Plant Sci. 2015, 6, 851. [Google Scholar] [CrossRef] [Green Version]
- Vellosillo, T.; Martinez, M.; Lopez, M.A.; Vicente, J.; Cascon, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins Produced by the 9-Lipoxygenase Pathway in Arabidopsis Regulate Lateral Root Development and Defense Responses through a Specific Signaling Cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef] [Green Version]
- Kidd, B.N.; Edgar, C.I.; Kumar, K.K.; Aitken, E.A.; Schenk, P.M.; Manners, J.M.; Kazan, K. The Mediator Complex Subunit PFT1 Is a Key Regulator of Jasmonate-Dependent Defense in Arabidopsis. Plant Cell 2009, 21, 2237–2252. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J.W.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.-M.; Woods-Tor, A.; Zipfel, C.; et al. A Genome-Wide Functional Investigation into the Roles of Receptor-like Proteins in Arabidopsis. Plant Physiol. 2008, 147, 503–517. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Ishii, T.; Matsunaga, T.; Tominaga, R.; Kuromori, T.; Wada, T.; Shinozaki, K.; Hirayama, T. The Glycerophosphoryl Diester Phosphodiesterase-like Proteins SHV3 and Its Homologs Play Important Roles in Cell Wall Organization. Plant Cell Physiol. 2008, 49, 1522–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, T.; van den Toorn, A.; Sanchez-Perez, G.F.; Campilho, A.; Willemsen, V.; Snel, B.; Scheres, B. SOMBRERO, BEARSKIN1, and BEARSKIN2 Regulate Root Cap Maturation in Arabidopsis. Plant Cell 2010, 22, 640–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, S. Arabidopsis TEBICHI, with Helicase and DNA Polymerase Domains, Is Required for Regulated Cell Division and Differentiation in Meristems. Plant Cell 2006, 18, 879–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolaï, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR Kinase Links Plant Growth, Yield, Stress Resistance and MRNA Translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional Regulation of ROS Controls Transition from Proliferation to Differentiation in the Root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Brader, G.; Kariola, T.; Tapio Palva, E. WRKY70 Modulates the Selection of Signaling Pathways in Plant Defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef]
- Ojangu, E.-L.; Järve, K.; Paves, H.; Truve, E. Arabidopsis Thaliana Myosin XIK Is Involved in Root Hair as Well as Trichome Morphogenesis on Stems and Leaves. Protoplasma 2007, 230, 193–202. [Google Scholar] [CrossRef]
- Peremyslov, V.V.; Prokhnevsky, A.I.; Avisar, D.; Dolja, V.V. Two Class XI Myosins Function in Organelle Trafficking and Root Hair Development in Arabidopsis. Plant Physiol. 2008, 146, 1109–1116. [Google Scholar] [CrossRef] [Green Version]
- Springer, N.M. Isolation of Plant DNA for PCR and Genotyping Using Organic Extraction and CTAB. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5515. [Google Scholar] [CrossRef]
- O’Malley, R.C.; Barragan, C.C.; Ecker, J.R. A User’s Guide to the Arabidopsis T-DNA Insertion Mutant Collections. In Plant Functional Genomics: Methods and Protocols; Alonso, J.M., Stepanova, A.N., Eds.; Humana Press: New York, NY, USA, 2015; pp. 323–342. [Google Scholar]
- King, E.O.; Ward, M.K.; Raney, D.E. Two Simple Media for the Demonstration of Pyocyanin and Fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Manefield, M.; Whiteley, A.S.; Bailey, M.J. DNA and RNA Extraction from Soil. In Molecular Microbial Ecology Manual; Kowalchuk, G.A., de Bruijn, F.J., Head, I.M., Akkermans, A.D.L., van Elsas, J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 149–158. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-8; 2017; Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 27 March 2020).
- Gao, X.; Alvo, M.; Chen, J.; Li, G. Nonparametric Multiple Comparison Procedures for Unbalanced One-Way Factorial Designs. J. Stat. Plan. Inference 2008, 138, 2574–2591. [Google Scholar] [CrossRef]
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. Nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Hawes, M.; Allen, C.; Turgeon, B.G.; Curlango-Rivera, G.; Minh Tran, T.; Huskey, D.A.; Xiong, Z. Root Border Cells and Their Role in Plant Defense. Annu. Rev. Phytopathol. 2016, 54, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Sengupta, S. Plant-Microbe Cross-Talk in the Rhizosphere: Insight and Biotechnological Potential. Open Microbiol. J. 2015, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Remans, T.; Opdenakker, K.; Jozefczak, M.; Gielen, H.; Guisez, Y.; Vangronsveld, J.; Cuypers, A. A Mutant of the Arabidopsis Thaliana LIPOXYGENASE1 Gene Shows Altered Signalling and Oxidative Stress Related Responses after Cadmium Exposure. Plant Physiol. Biochem. 2013, 63, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Noirot-Gros, M.-F.; Shinde, S.; Larsen, P.E.; Zerbs, S.; Korajczyk, P.J.; Kemner, K.M.; Noirot, P.H. Dynamics of Aspen Roots Colonization by Pseudomonads Reveals Strain-Specific and Mycorrhizal-Specific Patterns of Biofilm Formation. Front. Microbiol. 2018, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Pliego, C.; de Weert, S.; Lamers, G.; de Vicente, A.; Bloemberg, G.; Cazorla, F.M.; Ramos, C. Two Similar Enhanced Root-Colonizing Pseudomonas Strains Differ Largely in Their Colonization Strategies of Avocado Roots and Rosellinia Necatrix Hyphae. Environ. Microbiol. 2008, 10, 3295–3304. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Jasmonic Acid Signalling and the Plant Holobiont. Curr. Opin. Microbiol. 2017, 37, 42–47. [Google Scholar] [CrossRef]
- Biessy, A.; Novinscak, A.; Blom, J.; Léger, G.; Thomashow, L.S.; Cazorla, F.M.; Jošić, D.; Filion, M. Diversity of Phytobeneficial Traits Revealed by Whole-genome Analysis of Worldwide-isolated Phenazine-producing Pseudomonas spp. Environ. Microbiol. 2019, 21, 437–455. [Google Scholar] [CrossRef] [Green Version]
- AbuQamar, S.; Chen, X.; Dhawan, R.; Bluhm, B.; Salmeron, J.; Lam, S.; Dietrich, R.A.; Mengiste, T. Expression Profiling and Mutant Analysis Reveals Complex Regulatory Networks Involved in Arabidopsis Response to Botrytis Infection. Plant J. 2006, 48, 28–44. [Google Scholar] [CrossRef]
- Knoth, C.; Ringler, J.; Dangl, J.L.; Eulgem, T. Arabidopsis WRKY70 Is Required for Full RPP4 -Mediated Disease Resistance and Basal Defense against Hyaloperonospora Parasitica. Mol. Plant Microbe Interact. 2007, 20, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Mortel, J.E.; de Vos, R.C.H.; Dekkers, E.; Pineda, A.; Guillod, L.; Bouwmeester, K.; van Loon, J.J.A.; Dicke, M.; Raaijmakers, J.M. Metabolic and Transcriptomic Changes Induced in Arabidopsis by the Rhizobacterium Pseudomonas Fluorescens SS101. Plant Physiol. 2012, 160, 2173–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.-H.; Huang, Z.-Y.; Xie, P.; Gu, C.; Li, K.; Wang, D.-C.; Yu, Y.-Y.; Fan, Z.-H.; Wang, C.-J.; Wang, Y.-P.; et al. Transcription Factors WRKY70 and WRKY11 Served as Regulators in Rhizobacterium Bacillus Cereus AR156-Induced Systemic Resistance to Pseudomonas Syringae Pv. Tomato DC3000 in Arabidopsis. J. Exp. Bot. 2016, 67, 157–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Zhang, Y.; Li, Y.; Zhang, Q.; Ding, Y.; Zhang, Y. ChIP-Seq Reveals Broad Roles of SARD1 and CBP60g in Regulating Plant Immunity. Nat. Commun. 2015, 6, 10159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaravelpandian, K.; Chandrika, N.N.P.; Tsai, Y.-H.; Schmidt, W. PFT1-Controlled ROS Balance Is Critical for Multiple Stages of Root Hair Development in Arabidopsis. Plant Signal. Behav. 2013, 8, e24066. [Google Scholar] [CrossRef]
- Zhang, W.; Fraiture, M.; Kolb, D.; Löffelhardt, B.; Desaki, Y.; Boutrot, F.F.G.; Tör, M.; Zipfel, C.; Gust, A.A.; Brunner, F. Arabidopsis RECEPTOR-LIKE PROTEIN30 and Receptor-like Kinase SUPPRESSOR OF BIR1-1/EVERSHED Mediate Innate Immunity to Necrotrophic Fungi. Plant Cell 2013, 25, 4227–4241. [Google Scholar] [CrossRef] [Green Version]
- Nisa, M.; Bergis, C.; Pedroza-Garcia, J.; Drouin-Wahbi, J.; Mazubert, C.; Bergounioux, C.; Benhamed, M.; Raynaud, C. The Plant DNA Polymerase Theta Is Essential for the Repair of Replication-associated DNA Damage. Plant J. 2021, 106, 1197–1207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).