Plasmid-Mediated Quinolone Resistance (PMQR) in Two Clinical Strains of Salmonella enterica Serovar Corvallis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates and Antimicrobial Susceptibility
2.2. Whole Genome Sequencing, Bioinformatics Analysis and Phylogenetic Relationships
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Archambault, M.; Petrov, P.; Hendriksen, R.S.; Asseva, G.; Bangtrakulnonth, A.; Hasman, H.; Aarestrup, F.M. Molecular characterization and occurrence of extended-spectrum beta-lactamase resistance genes among Salmonella enterica serovar Corvallis from Thailand, Bulgaria, and Denmark. Microb. Drug Resist. 2006, 12, 192–198. [Google Scholar] [CrossRef]
- Asseva, G.; Petrov, P.; Ivanov, I.; Kantardjiev, T. Surveillance of human salmonellosis in Bulgaria, 1999–2004: Trends, shifts and resistance to antimicrobial agents. Eurosurveillance 2006, 11, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Ben Aissa, R.; Al-Gallas, N. Molecular typing of Salmonella enterica serovars Enteritidis, Corvallis, Anatum and Typhimurium from food and human stool samples in Tunisia, 2001–2004. Epidemiol. Infect. 2008, 136, 468–475. [Google Scholar] [CrossRef]
- Yamatogi, R.S.; Oliveira, H.C.; Camargo, C.H.; Fernandes, S.A.; Hernandes, R.T.; Pinto, J.P.; Rall, V.L.; Araujo, J.P., Jr. Clonal relatedness and resistance patterns of Salmonella Corvallis from poultry carcasses in a Brazilian slaughterhouse. J. Infect. Dev. Ctries. 2015, 9, 1161–1165. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Hendriksen, R.S.; Aarestrup, F.M. Plasmid-mediated quinolone resistance determinant qnrS1 detected in Salmonella enterica serovar Corvallis strains isolated in Denmark and Thailand. J. Antimicrob. Chemother. 2007, 60, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Schmoger, S.; Jahn, S.; Helmuth, R.; Guerra, B. NDM-1 carbapenemase-producing Salmonella enterica subsp. enterica serovar Corvallis isolated from a wild bird in Germany. J. Antimicrob. Chemother. 2013, 68, 2954–2956. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine; 6th Revision 2018; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Fabrega, A.; du Merle, L.; Le Bouguenec, C.; Jimenez de Anta, M.T.; Vila, J. Repression of invasion genes and decreased invasion in a high-level fluoroquinolone-resistant Salmonella Typhimurium mutant. PLoS ONE 2009, 4, e8029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef]
- Jacoby, G.A. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 2005, 41 (Suppl. 2), S120–S126. [Google Scholar] [CrossRef]
- Ruiz, J. Mechanisms of resistance to quinolones: Target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 2003, 51, 1109–1117. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Strahilevitz, J.; Hooper, D.C. Plasmid-mediated quinolone resistance. Microbiol. Spectr. 2014, 2, 475–503. [Google Scholar] [CrossRef]
- Robicsek, A.; Jacoby, G.A.; Hooper, D.C. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 2006, 6, 629–640. [Google Scholar] [CrossRef]
- CLSI. Performance standards for antimicrobial susceptibility testing. In CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne PA, USA, 2019. [Google Scholar]
- Vielva, L.; de Toro, M.; Lanza, V.F.; de la Cruz, F. PLACNETw: A web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics 2017, 33, 3796–3798. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Moller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Ponten, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef]
- Zankari, E.; Allesoe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Akiyama, T.; Khan, A.A. Isolation and characterization of small qnrS1-carrying plasmids from imported seafood isolates of Salmonella enterica that are highly similar to plasmids of clinical isolates. FEMS Immunol. Med. Microbiol. 2012, 64, 429–432. [Google Scholar] [CrossRef]
- Chen, K.; Yang, C.; Dong, N.; Xie, M.; Ye, L.; Chan, E.W.C.; Chen, S. Evolution of ciprofloxacin resistance-encoding genetic elements in Salmonella. mSystems 2020, 5, e01234-20. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Fortini, D.; Veldman, K.; Mevius, D.; Carattoli, A. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J. Antimicrob. Chemother. 2009, 63, 274–281. [Google Scholar] [CrossRef]
- Murray, A.; Mather, H.; Coia, J.E.; Brown, D.J. Plasmid-mediated quinolone resistance in nalidixic-acid-susceptible strains of Salmonella enterica isolated in Scotland. J. Antimicrob. Chemother. 2008, 62, 1153–1155. [Google Scholar] [CrossRef]
- Sjolund-Karlsson, M.; Howie, R.; Rickert, R.; Krueger, A.; Tran, T.T.; Zhao, S.; Ball, T.; Haro, J.; Pecic, G.; Joyce, K.; et al. Plasmid-mediated quinolone resistance among non-Typhi Salmonella enterica isolates, USA. Emerg. Infect. Dis. 2010, 16, 1789–1791. [Google Scholar] [CrossRef]
- Veldman, K.; Cavaco, L.M.; Mevius, D.; Battisti, A.; Franco, A.; Botteldoorn, N.; Bruneau, M.; Perrin-Guyomard, A.; Cerny, T.; De Frutos Escobar, C.; et al. International collaborative study on the occurrence of plasmid-mediated quinolone resistance in Salmonella enterica and Escherichia coli isolated from animals, humans, food and the environment in 13 European countries. J. Antimicrob. Chemother. 2011, 66, 1278–1286. [Google Scholar] [CrossRef]
- Antunes, P.; Mourao, J.; Machado, J.; Peixe, L. First description of qnrS1-IncN plasmid in a ST11 Salmonella Enteritidis clinical isolate from Portugal. Diagn. Microbiol. Infect. Dis. 2011, 69, 463–465. [Google Scholar] [CrossRef]
- Cui, M.; Zhang, P.; Li, J.; Sun, C.; Song, L.; Zhang, C.; Zhao, Q.; Wu, C. Prevalence and characterization of fluoroquinolone resistant Salmonella isolated from an integrated broiler chicken supply chain. Front. Microbiol. 2019, 10, 1865. [Google Scholar] [CrossRef]
- Dolejska, M.; Villa, L.; Hasman, H.; Hansen, L.; Carattoli, A. Characterization of IncN plasmids carrying blaCTX-M-1 and qnr genes in Escherichia coli and Salmonella from animals, the environment and humans. J. Antimicrob. Chemother. 2013, 68, 333–339. [Google Scholar] [CrossRef]
- Guerra, B.; Helmuth, R.; Thomas, K.; Beutlich, J.; Jahn, S.; Schroeter, A. Plasmid-mediated quinolone resistance determinants in Salmonella spp. isolates from reptiles in Germany. J. Antimicrob. Chemother. 2010, 65, 2043–2045. [Google Scholar] [CrossRef]
- Kehrenberg, C.; Friederichs, S.; de Jong, A.; Michael, G.B.; Schwarz, S. Identification of the plasmid-borne quinolone resistance gene qnrS in Salmonella enterica serovar Infantis. J. Antimicrob. Chemother. 2006, 58, 18–22. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Wootton, L.; Day, M.R.; Threlfall, E.J. Plasmid-mediated quinolone resistance determinant qnrS1 found in Salmonella enterica strains isolated in the UK. J. Antimicrob. Chemother. 2007, 59, 1071–1075. [Google Scholar] [CrossRef][Green Version]
- Kehrenberg, C.; Hopkins, K.L.; Threlfall, E.J.; Schwarz, S. Complete nucleotide sequence of a small qnrS1-carrying plasmid from Salmonella enterica subsp. enterica Typhimurium DT193. J. Antimicrob. Chemother. 2007, 60, 903–905. [Google Scholar] [CrossRef]
- Wu, J.J.; Ko, W.C.; Chiou, C.S.; Chen, H.M.; Wang, L.R.; Yan, J.J. Emergence of Qnr determinants in human Salmonella isolates in Taiwan. J. Antimicrob. Chemother. 2008, 62, 1269–1272. [Google Scholar] [CrossRef][Green Version]
- Selzer, G.; Som, T.; Itoh, T.; Tomizawa, J. The origin of replication of plasmid p15A and comparative studies on the nucleotide sequences around the origin of related plasmids. Cell 1983, 32, 119–129. [Google Scholar] [CrossRef]
- Baucheron, S.; Chaslus-Dancla, E.; Cloeckaert, A.; Chiu, C.H.; Butaye, P. High-level resistance to fluoroquinolones linked to mutations in gyrA, parC, and parE in Salmonella enterica serovar Schwarzengrund isolates from humans in Taiwan. Antimicrob. Agents Chemother. 2005, 49, 862–863. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, J.H.; Kwak, H.S.; Woo, G.J. Characterization of the quinolone resistance mechanism in foodborne Salmonella isolates with high nalidixic acid resistance. Int. J. Food Microbiol. 2011, 146, 52–56. [Google Scholar] [CrossRef]
- Weill, F.X.; Bertrand, S.; Guesnier, F.; Baucheron, S.; Cloeckaert, A.; Grimont, P.A. Ciprofloxacin-resistant Salmonella Kentucky in travelers. Emerg. Infect. Dis. 2006, 12, 1611–1612. [Google Scholar] [CrossRef]
- Lindstedt, B.A.; Aas, L.; Kapperud, G. Geographically dependent distribution of gyrA gene mutations at codons 83 and 87 in Salmonella Hadar, and a novel codon 81 Gly to His mutation in Salmonella Enteritidis. APMIS 2004, 112, 165–171. [Google Scholar] [CrossRef]
- Hong, Y.P.; Wang, Y.W.; Huang, I.H.; Liao, Y.C.; Kuo, H.C.; Liu, Y.Y.; Tu, Y.H.; Chen, B.H.; Liao, Y.S.; Chiou, C.S. Genetic relationships among multidrug-resistant Salmonella enterica serovar Typhimurium strains from humans and animals. Antimicrob. Agents Chemother. 2018, 62, e00213-18. [Google Scholar] [CrossRef]
- Hooton, S.P.; Timms, A.R.; Cummings, N.J.; Moreton, J.; Wilson, R.; Connerton, I.F. The complete plasmid sequences of Salmonella enterica serovar Typhimurium U288. Plasmid 2014, 76, 32–39. [Google Scholar] [CrossRef]
- Oliva, M.; Monno, R.; D’Addabbo, P.; Pesole, G.; Dionisi, A.M.; Scrascia, M.; Chiara, M.; Horner, D.S.; Manzari, C.; Luzzi, I.; et al. A novel group of IncQ1 plasmids conferring multidrug resistance. Plasmid 2017, 89, 22–26. [Google Scholar] [CrossRef]
- Scholz, P.; Haring, V.; Wittmann-Liebold, B.; Ashman, K.; Bagdasarian, M.; Scherzinger, E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 1989, 75, 271–288. [Google Scholar] [CrossRef]
- Loftie-Eaton, W.; Rawlings, D.E. Diversity, biology and evolution of IncQ-family plasmids. Plasmid 2012, 67, 15–34. [Google Scholar] [CrossRef]
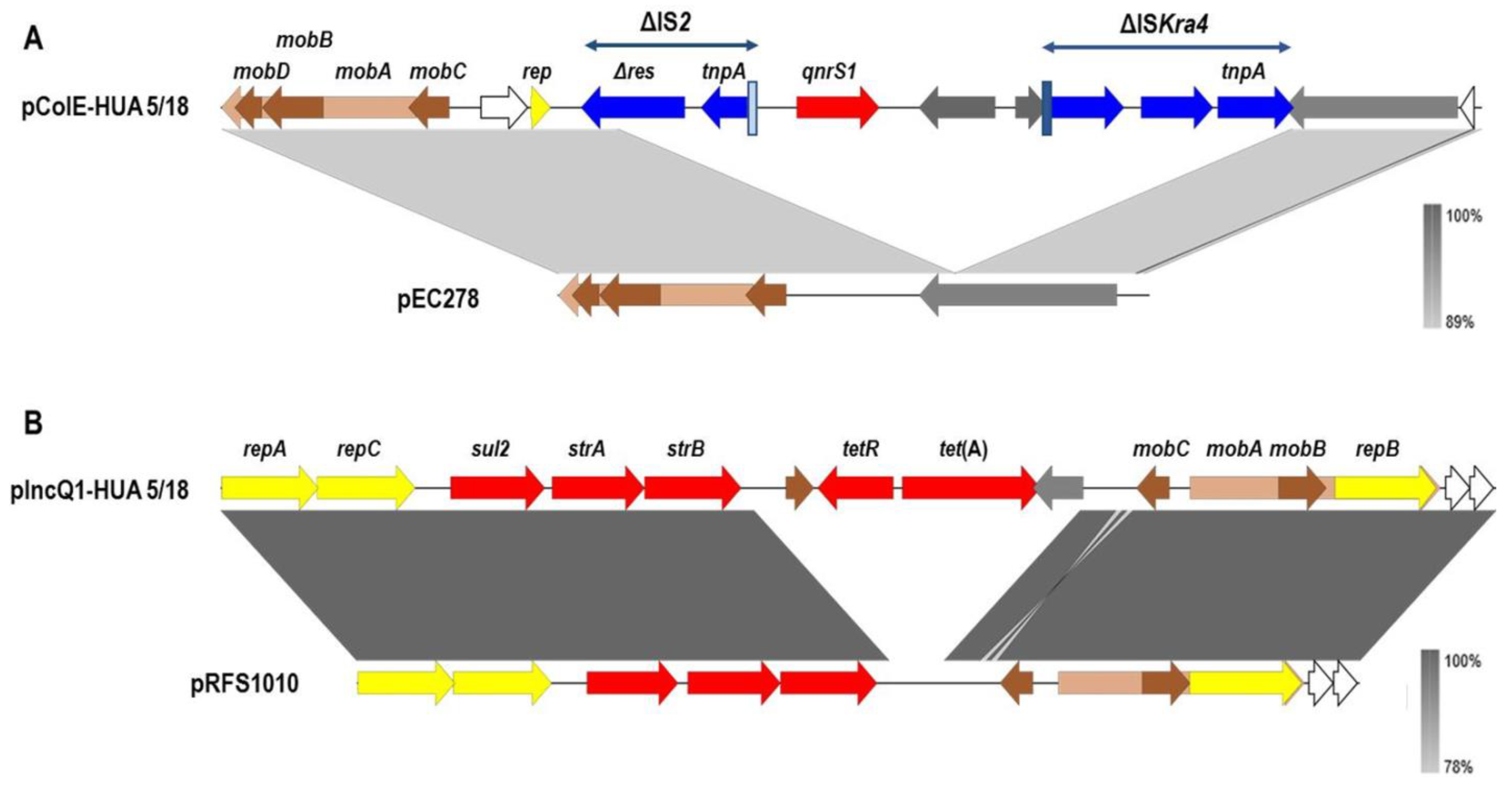
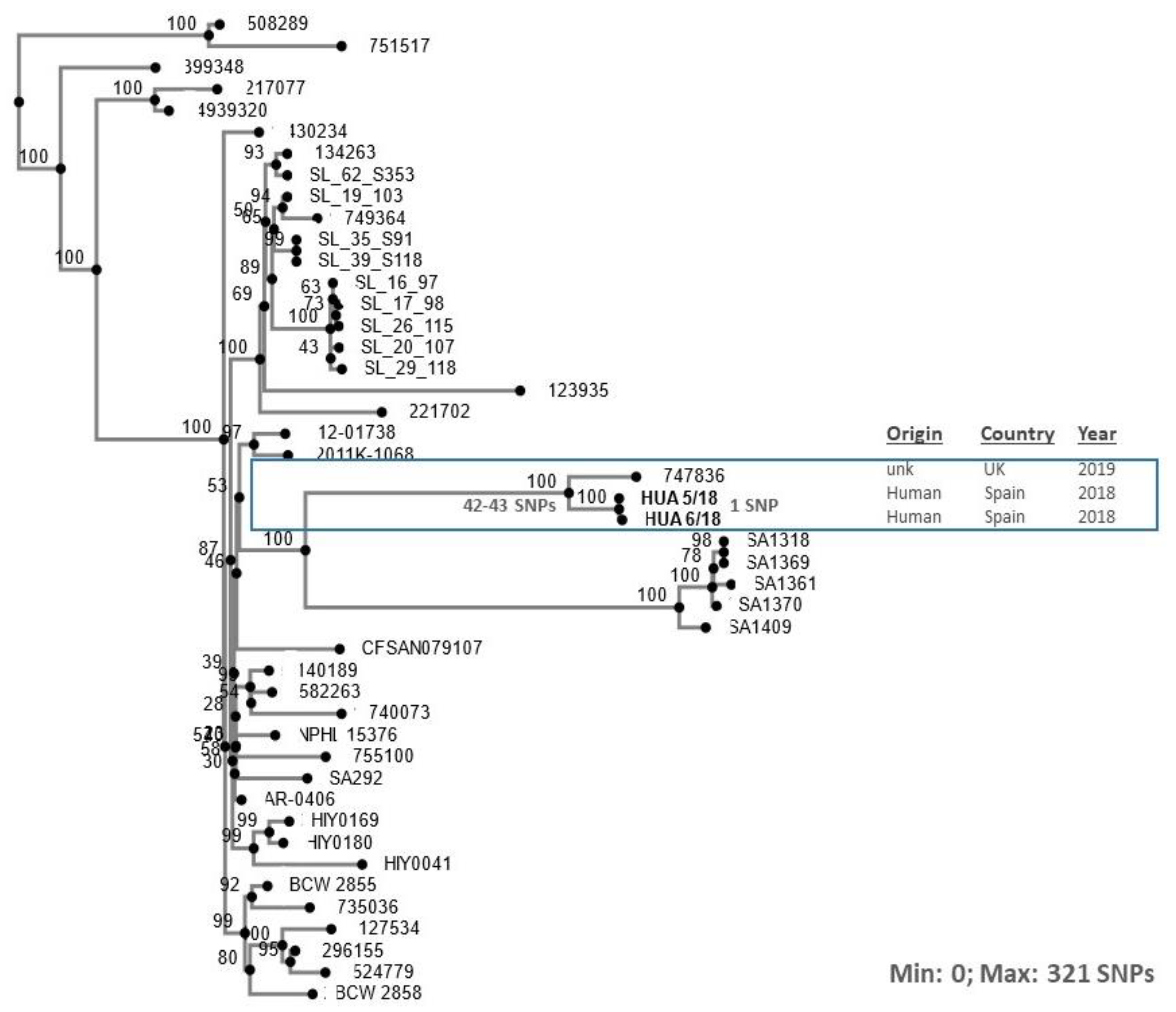
| Isolate a | Kmer | Contigs | N50 | Longest Contig (bp) | Total bp in Contigs | Contigs >1 kb | Library | Coverage |
|---|---|---|---|---|---|---|---|---|
| HUA 5/18 | 85 | 78 | 795,230 | 2,081,448 | 4,887,704 | 22 | 508 ± 126 | 25× |
| HUA 6/18 | 87 | 131 | 407,429 | 1,548,594 | 4,886,051 | 46 | 506 ± 129 | 41× |
| Isolate a | Patient Sex b/Age | NAL-IZD c (mm) | NAL-MIC c (µg/mL) | CIP-MIC c (µg/mL) | PEF-IZD c (mm) | ParC | Resistance Phenotype d Plasmid-Located Resistance Genes | Plasmid Inc. (Size in bp) e |
|---|---|---|---|---|---|---|---|---|
| HUA 5/18 | F/53 | 20 | 3 | 0.5 | 12 | Thr57Ser | CIP, PEF, STR, SUL, TET strA, strB, sul2, tet(A) qnrS1 | IncQ1 (11,044)* ColE (10,036)* nid (5,570; 5,284)*,* ColpVC (2,179) |
| HUA 6/18 | F/6 | 20 | 4 | 0.75 | 12 | Thr57Ser | STR, SUL, TET, CIP, PEF strA, strB, sul2, tet(A) qnrS1 | IncQ1 (11,044)* ColE (10,036)* nid (5,570; 5,284)*,* ColpVC (2,179) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, X.; Fernández, J.; Hernáez, S.; Rodicio, R.; Rodicio, M.R. Plasmid-Mediated Quinolone Resistance (PMQR) in Two Clinical Strains of Salmonella enterica Serovar Corvallis. Microorganisms 2022, 10, 579. https://doi.org/10.3390/microorganisms10030579
Vázquez X, Fernández J, Hernáez S, Rodicio R, Rodicio MR. Plasmid-Mediated Quinolone Resistance (PMQR) in Two Clinical Strains of Salmonella enterica Serovar Corvallis. Microorganisms. 2022; 10(3):579. https://doi.org/10.3390/microorganisms10030579
Chicago/Turabian StyleVázquez, Xenia, Javier Fernández, Silvia Hernáez, Rosaura Rodicio, and Maria Rosario Rodicio. 2022. "Plasmid-Mediated Quinolone Resistance (PMQR) in Two Clinical Strains of Salmonella enterica Serovar Corvallis" Microorganisms 10, no. 3: 579. https://doi.org/10.3390/microorganisms10030579
APA StyleVázquez, X., Fernández, J., Hernáez, S., Rodicio, R., & Rodicio, M. R. (2022). Plasmid-Mediated Quinolone Resistance (PMQR) in Two Clinical Strains of Salmonella enterica Serovar Corvallis. Microorganisms, 10(3), 579. https://doi.org/10.3390/microorganisms10030579








