Localization of the Swainsonine-Producing Chaetothyriales Symbiont in the Seed and Shoot Apical Meristem in Its Host Ipomoea carnea
Abstract
:1. Introduction
2. Materials and Method
2.1. Seeds and Plants
2.2. Stereofluorescence Microscopy and Laser Scanning Confocal Microscopy
2.3. Scanning Electron Microscopy
2.4. Fluorescent In Situ Hybridization (FISH)
3. Results
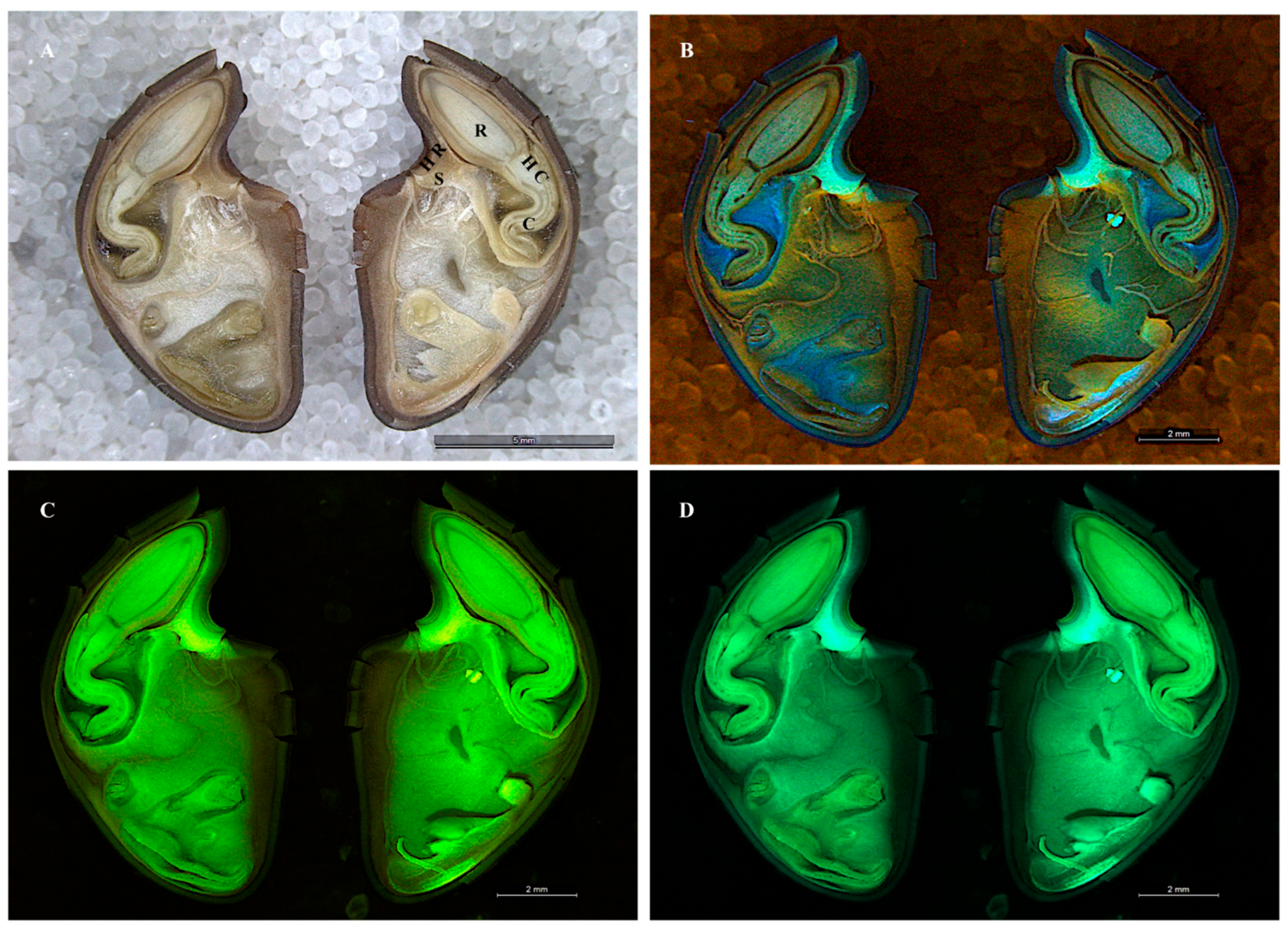
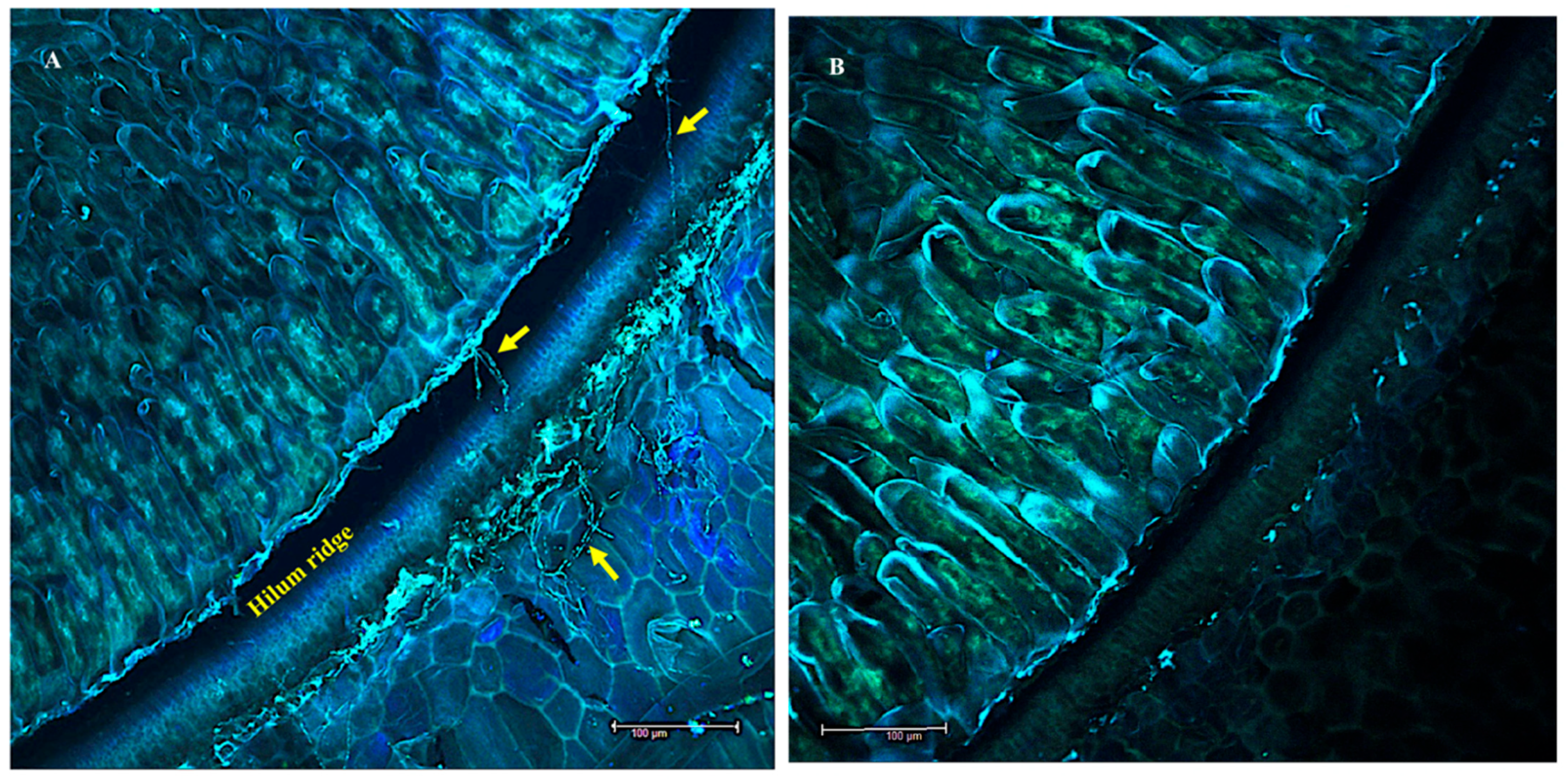
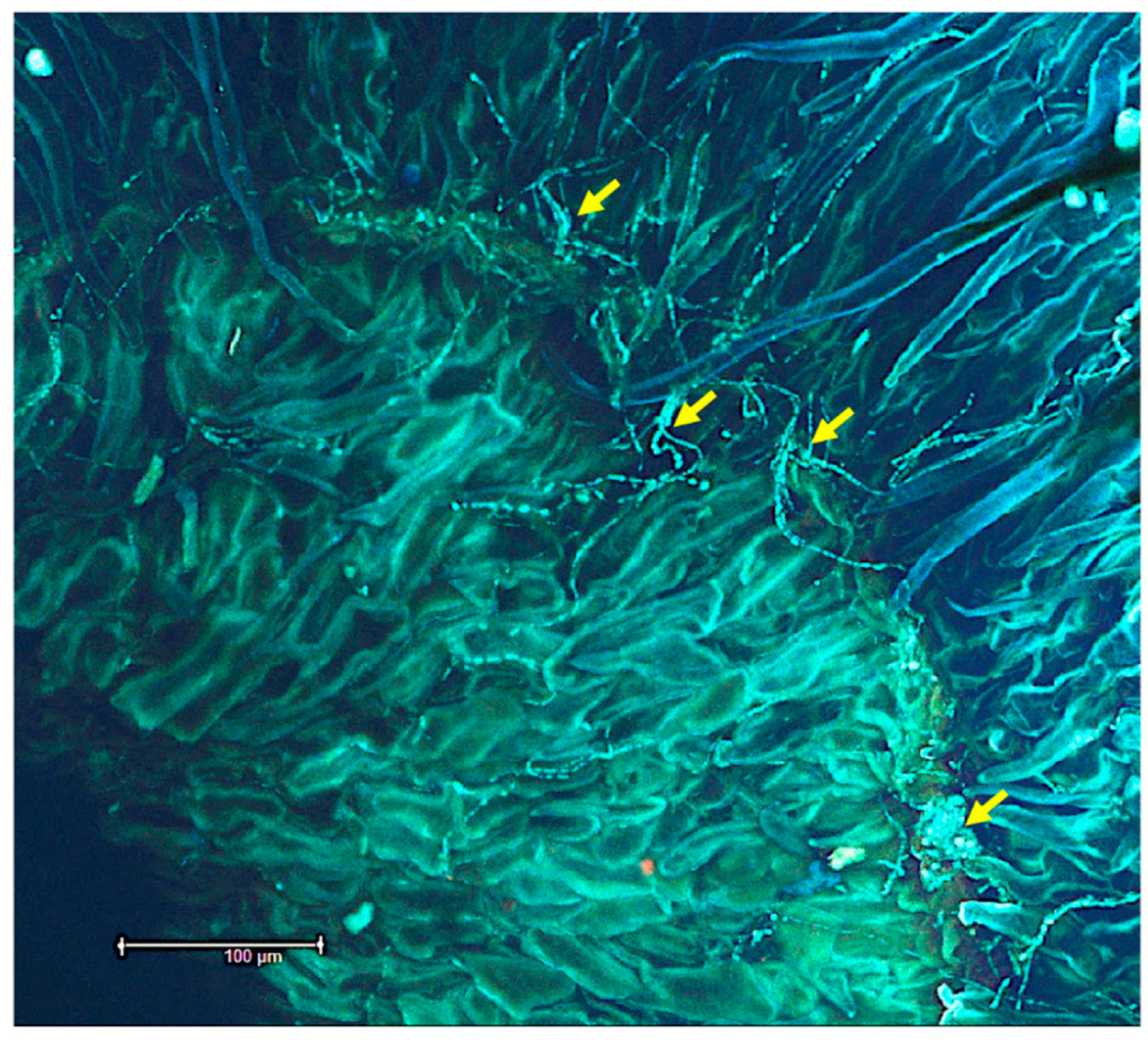
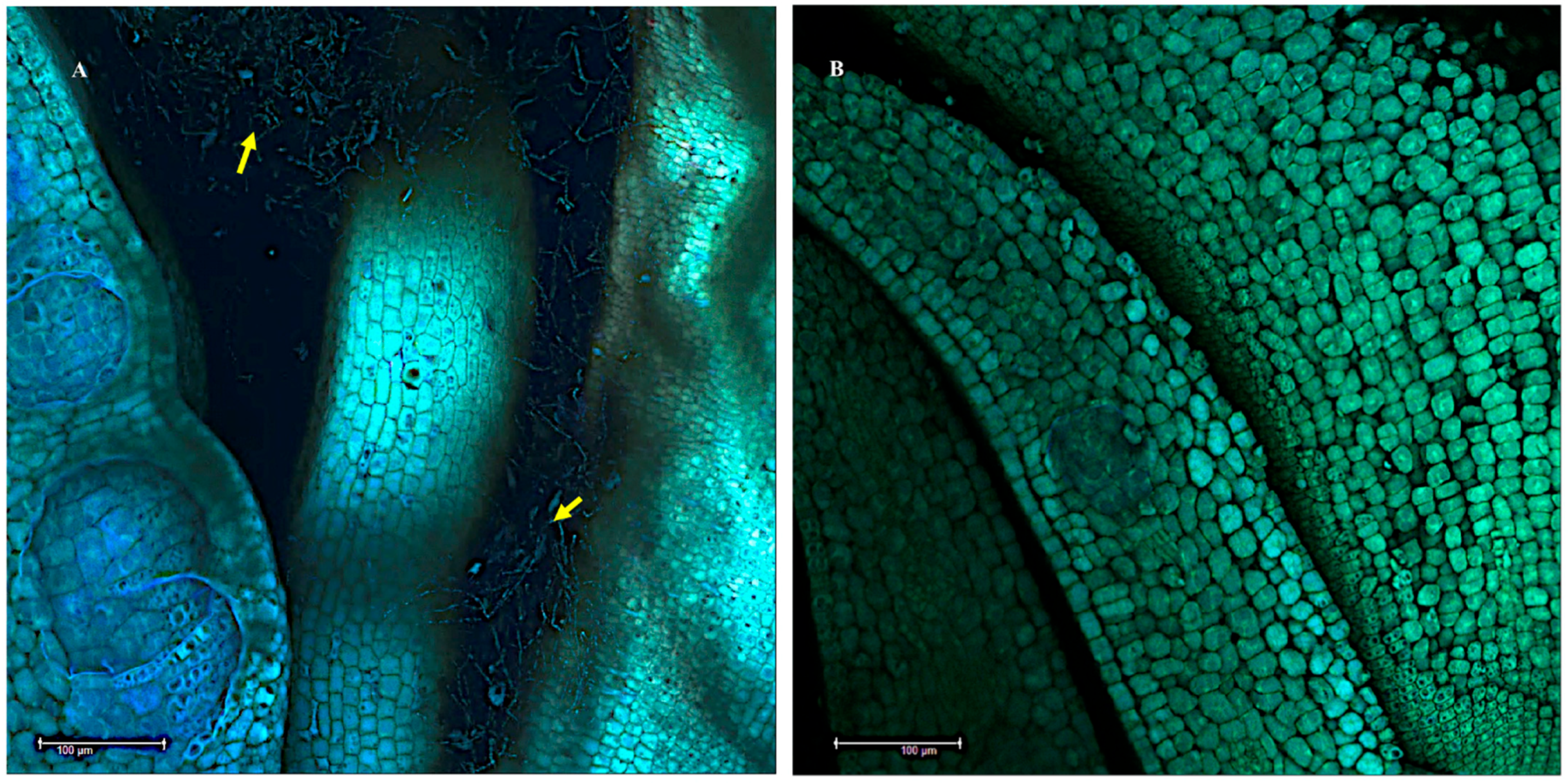
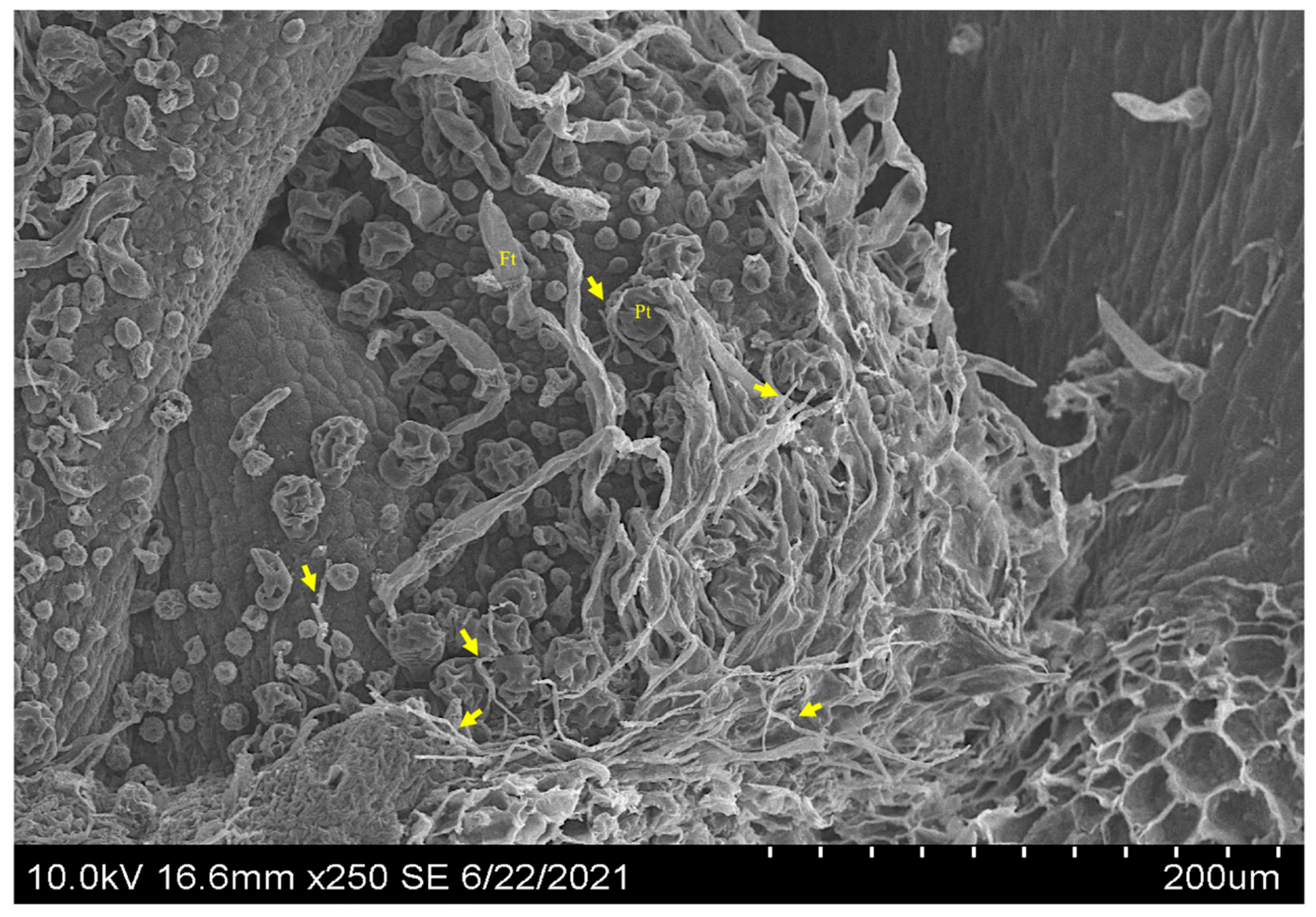
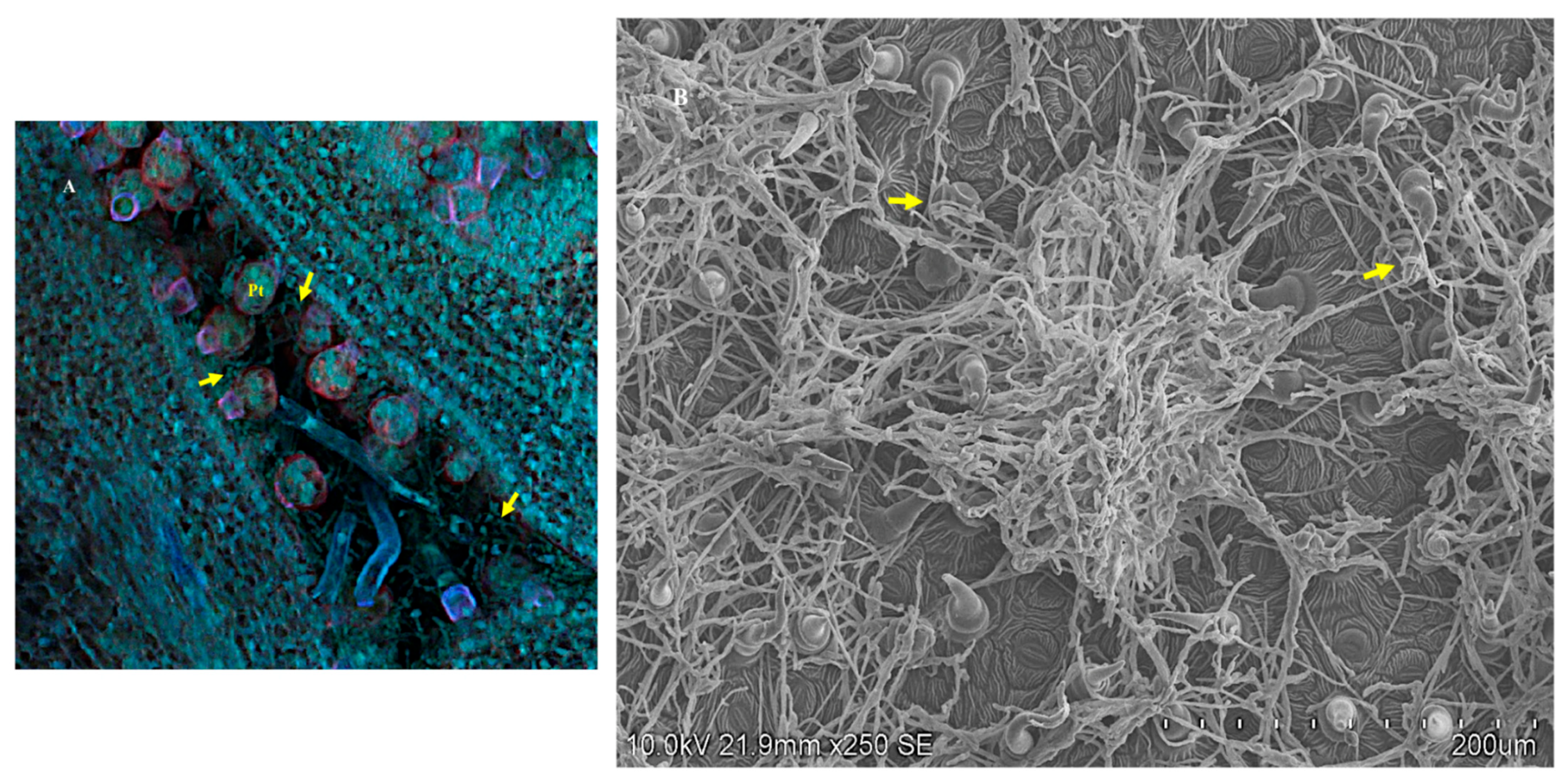
3.1. Seeds
3.2. The Shoot Apical Meristem SAM
3.3. Leaves and Other Plant Parts
3.4. Fluorescent In Situ Hybridization (FISH)
3.5. Swainsonine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; Volume 26–27, pp. 398–401. [Google Scholar]
- Ewald, P.W. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 1987, 503, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Siller, S.; Nowak, M.A. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution 1996, 50, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Panaccione, D.G.; Beaulieu, W.T.; Cook, D. Bioactive alkaloids in vertically transmitted fungal endophytes. Funct. Ecol. 2014, 28, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Colegate, S.M.; Dorling, P.R.; Huxtable, C.R. A spectroscopic investigation of swainsonine: An α-mannosidase inhibitor isolated from Swainsona canescens. Aust. J. Chem. 1979, 32, 2257–2264. [Google Scholar] [CrossRef]
- Molyneux, R.; James, L. Loco intoxication: Indolizidine alkaloids of spotted locoweed (Astragalus lentiginosus). Science 1982, 216, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, R.J.; Mckenzie, R.A.; O’Sullivan, B.M.; Elbein, A.D. Identification of the glycosidase inhibitors swainsonine and calystegine B2 in Weir vine (Ipomoea sp. Q6 [aff. calobra]) and correlation with toxicity. J. Nat. Prod. 1995, 58, 878–886. [Google Scholar]
- De Balogh, K.K.; Dimande, A.P.; van der Lugt, J.J.; Molyneux, R.J.; Naudé, T.W.; Welman, W.G. A lysosomal storage disease induced by Ipomoea carnea in goats in Mozambique. J. Vet. Diagn. Investig. 1999, 11, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Dantas, A.F.M.; Riet-Correa, F.; Gardner, D.R.; Medeiros, R.M.T.; Barros, S.S.; Anjos, B.L.; Lucena, R.B. Swainsonine-induced lysosomal storage disease in goats caused by the ingestion of Turbina cordata in Northeastern Brazil. Toxicon 2007, 49, 111–116. [Google Scholar] [CrossRef]
- Cook, D.; Gardner, D.R.; Pfister, J.A. Swainsonine-containing plants and their relationship to endophytic fungi. J. Agric. Food Chem. 2014, 62, 7326–7334. [Google Scholar] [CrossRef]
- Dorling, P.R.; Huxtable, C.R.; Colgate, S.M. Inhibition of lysomal mannosidase by swainsonine and indolizidine alkaloid isolated from Swainsona canescens. Biochem. J. 1980, 191, 649–651. [Google Scholar] [CrossRef]
- Tulsiani, D.R.P.; Broquist, H.P.; James, L.F.; Touster, O. The similar effects of swainsonine and locoweed on tissue glycosidases and oligosaccharides of the pig indicate that the alkaloid is the principal toxin responsible for the induction of locoism. Arch. Biochem. Biophys. 1984, 232, 76–85. [Google Scholar] [CrossRef]
- Panter, K.E.; James, L.F.; Stegelmeier, B.L.; Ralphs, M.H.; Pfister, J.A. Locoweeds: Effects on reproduction in livestock. J. Nat. Toxins 1999, 8, 53–62. [Google Scholar] [PubMed]
- Braun, K.; Romero, J.; Liddell, C.; Creamer, R. Production of swainsonine by fungal endophytes of locoweed. Mycol. Res. 2003, 107, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, Q.; Wang, J.; Wang, J.; Wang, Y.; Song, Y.; Geng, G.; Li, Q. Swainsonine-producing fungal endophytes from major locoweed species in China. Toxicon 2010, 56, 330–338. [Google Scholar] [CrossRef]
- Baucom, D.L.; Romero, M.; Belfon, R.; Creamer, R. Two new species of Undifilum, fungal endophytes of Astragalus (locoweeds) in the United States. Botany 2012, 90, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grum, D.S.; Cook, D.; Baucom, D.; Mott, I.W.; Gardner, D.R.; Creamer, R.; Allen, J.G. Production of the alkaloid swainsonine by a fungal endophyte in the host Swainsona canescens. J. Nat. Prod. 2013, 76, 1984–1988. [Google Scholar] [CrossRef]
- Cook, D.; Beaulieu, W.T.; Mott, I.W.; Riet-Correa, F.; Gardner, D.R.; Grum, D.; Pfister, J.A.; Clay, K.; Marcolongo-Pereira, C. Production of the alkaloid swainsonine by a fungal endosymbiont of the Ascomycete order Chaetothyriales in the host Ipomoea carnea. J. Agric. Food Chem. 2013, 61, 3797–3803. [Google Scholar] [CrossRef]
- Oldrup, E.; McLain-Romero, J.; Padilla, A.; Moya, A.; Gardner, D.; Creamer, R. Localization of endophytic Undifilum fungi in locoweed seed and influence of environmental parameters on a locoweed in vitro culture system. Botany 2010, 88, 512–521. [Google Scholar] [CrossRef] [Green Version]
- Reyna, R.; Cooke, P.; Grum, D.; Cook, D.; Creamer, R. Detection and localization of the endophyte Undifilum oxytropis in locoweed tissues. Botany 2012, 90, 1229–1236. [Google Scholar] [CrossRef]
- Noor, A.I.; Nava, A.; Cooke, P.; Cook, D.; Creamer, R. Evidence for nonpathogenic relationships of Alternaria section Undifilum endophytes within three host locoweed plant species. Botany 2018, 96, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Noor, A.I.; Nava, A.; Neyaz, M.; Cooke, P.; Creamer, R.; Cook, D. Ectopic growth of the fungal symbiont (Chaetothyriales) on Ipomoea carnea. Botany 2021, 99, 619–627. [Google Scholar] [CrossRef]
- Gardner, D.R.; Molyneux, R.J.; Ralphs, M.H. Analysis of swainsonine: Extraction methods, detection, and measurement in populations of locoweeds (Oxytropis spp.). J. Agric. Food Chem. 2001, 49, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.R.; Cook, D. A comparison of alternative sample preparation procedures for the analysis of swainsonine using LC-MS/MS. Phytochem. Anal. 2011, 22, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.C.F.; Riet-Correa, R.M.T.; Medeiros, E.F.; Lima, S.S.; Barros, E.J.; Gimeno, R.J.; Molyneux, R.; Gardner, D.R. Intoxication by Ipomoea sericophylla and Ipomoea riedelii in goats in the state of Paraíba, Northeastern Brazil. Toxicon 2006, 47, 371–379. [Google Scholar] [CrossRef]
- Nation, J.L. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol. 1983, 58, 347–351. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Saha, S.; Kostina, O.; Muravnik, L.; Mitra, A. Replacing critical point drying with a low-cost chemical drying provides comparable surface image quality of glandular trichomes from leaves of Millingtonia hortensis L. f. in scanning electron micrograph. Appl. Microsc. 2020, 50, 15. [Google Scholar] [CrossRef]
- Tirichine, L.; Andrey, P.; Biot, E.; Maurin, Y.; Gaudin, V. 3D fluorescent in situ hybridization using Arabidopsis leaf cryosections and isolated nuclei. Plant Methods 2009, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Noor, A.I.; Neyaz, M.; Cook, D.; Creamer, R. Molecular characterization of a fungal ketide synthase gene among swainsonine-producing Alternaria species in the USA. Curr. Microbiol. 2020, 77, 2554–2563. [Google Scholar] [CrossRef]
- Steiner, U.; Leibner, S.; Schardl, C.L.; Leuchtmann, A.; Leistner, E. Periglandula a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 2011, 103, 1133–1145. [Google Scholar] [CrossRef]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef] [Green Version]
- Steiner, U.; Hellwig, S.; Ahimsa-Muller, M.A.; Grundmann, N.; Li, S.M.; Drewke, C.; Leistner, E. The key role of peltate glandular trichomes in symbiota comprising Clavicipitaceous fungi of the genus Periglandula and their host plants. Toxins 2015, 7, 1355–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leistner, E.; Steiner, U. The genus Periglandula and its symbiotum with morning glory plants (Convolvulaceae). In Physiology and Genetics; Springer: Cham, Switzerland, 2018; pp. 131–147. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neyaz, M.; Gardner, D.R.; Creamer, R.; Cook, D. Localization of the Swainsonine-Producing Chaetothyriales Symbiont in the Seed and Shoot Apical Meristem in Its Host Ipomoea carnea. Microorganisms 2022, 10, 545. https://doi.org/10.3390/microorganisms10030545
Neyaz M, Gardner DR, Creamer R, Cook D. Localization of the Swainsonine-Producing Chaetothyriales Symbiont in the Seed and Shoot Apical Meristem in Its Host Ipomoea carnea. Microorganisms. 2022; 10(3):545. https://doi.org/10.3390/microorganisms10030545
Chicago/Turabian StyleNeyaz, Marwa, Dale R. Gardner, Rebecca Creamer, and Daniel Cook. 2022. "Localization of the Swainsonine-Producing Chaetothyriales Symbiont in the Seed and Shoot Apical Meristem in Its Host Ipomoea carnea" Microorganisms 10, no. 3: 545. https://doi.org/10.3390/microorganisms10030545
APA StyleNeyaz, M., Gardner, D. R., Creamer, R., & Cook, D. (2022). Localization of the Swainsonine-Producing Chaetothyriales Symbiont in the Seed and Shoot Apical Meristem in Its Host Ipomoea carnea. Microorganisms, 10(3), 545. https://doi.org/10.3390/microorganisms10030545





