Abstract
Postbiotic feed additives may aid foodborne pathogen reduction during poultry rearing. The study objective was to evaluate a postbiotic additive in parallel to an industry control diet and the subsequent associated burden of Salmonella enterica on a single, commercial broiler farm in Honduras. Twelve houses were matched and assigned the standard diet (CON) or standard diet plus postbiotic (SCFP). New litter was placed in each house and retained across flock cycles with sampling prior to each chick placement and three consecutive rearing cycles. At ~33–34 days, 25 ceca were collected on-farm from each house, treatment, and cycle. Salmonella prevalence in litter for CON (30.6%) and SCFP (27.8%) were equivalent; however, Salmonella load within positive samples was lower (p = 0.04) for SCFP (3.81 log10 MPN/swab) compared to CON (5.53 log10 MPN/swab). Cecal prevalence of Salmonella was lower (p = 0.0006) in broilers fed SCFP (3.4%) compared to CON (12.2%). Salmonella load within positive ceca were numerically reduced (p = 0.121) by 1.45 log10 MPN/g for SCFP (2.41 log10 MPN/g) over CON (3.86 log10 MPN/g). Estimated burden was lower (p = 0.003) for SCFP flocks (3.80 log10 MPN) compared to CON (7.31 log10 MPN). These data demonstrate the preharvest intervention potential of postbiotics to reduce Salmonella enterica in broiler chickens.
Keywords:
Salmonella; postbiotic; poultry; broiler; ceca; preharvest; feed additive; food safety; intervention; Honduras 1. Introduction
Countries with robust public health surveillance, reporting, and industry regulatory oversight routinely monitor for agents associated with foodborne illness, especially serovars of Salmonella enterica. In the United States, foodborne disease associated with 31 known pathogens results in an estimated 9.4 million illnesses annually and, of those, non-typhoidal Salmonella are estimated to cause approximately 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths [1]. In a report from the Interagency Food Safety Analytics Collaboration, more than 75% of illnesses reported from 811 outbreaks between 1998 and 2017 were linked to Salmonella and were attributable to seven food categories of which chicken products (14.0%) and eggs (7.9%) together constituted the primary source of over 20% of Salmonella outbreaks [2]. In contrast, many developing countries lack robust surveillance programs and public health data on the domestic incidence and attribution of foodborne salmonellosis. Nonetheless, the association of poultry products with Salmonella and its corresponding public health threat are globally recognized and, therefore, necessitate Salmonella control in an increasingly globalized import and export market [3,4,5].
As developing countries seek to increase poultry production capacity to meet increasing domestic consumption trends, additional opportunities to increase export volumes are often met with more stringent regulatory and food safety-based compliance specifications. For instance, while Honduran raw poultry products have yet to be exported to the United States due to endemic Newcastle disease concerns, raw beef products are approved for export. Establishments producing these products are regulated by SENASA, the federal national service of health and food safety. Export approval is based on demonstrated equivalence of SENASA-specified regulations and food safety process controls, including microbiological testing, to criteria established by the United States Department of Agriculture’s Food Safety and Inspection Service, who conducts the Foreign Supplier Audits and Reports [6]. SENASA has recently established Salmonella performance standards for livestock and poultry. As in the United States, Honduran poultry establishments have inspectors who collect chicken carcass rinse samples post-chill, utilizing the reference microbiological methods specified in the FSIS’s Microbiology Laboratory Guidebook 4.11 at the Laboratorio Nacional de Residuos (LANAR) [7]. To remain in compliance, weekly samplings may result in no more than five qualitatively positive Salmonella detections within a moving 51-week window [6]. To meet these criteria, robust process controls and post-harvest microbial interventions are commonly deployed. As may be evidenced from the establishment of compliance reports in the United States, and a largely unchanging public health burden of salmonellosis attributed to poultry products, postharvest microbial interventions are not fully efficacious despite many years of technology use, development, and regulatory oversight [8,9].
In recent years, qualitative performance standards have come under scrutiny in favor of a more quantitative-based approach to pathogen testing and associated product safety risk [10]. The FSIS’s recent Roadmap to Reducing Salmonella includes potential assessment or evaluation of semiquantitative- or quantitative-based methods to inform Salmonella risk assessments [11]. Recent risk modeling studies for Salmonella in ground turkey have strengthened the argument that contamination load, and not prevalence alone, may be a more impactful metric to utilize [12,13]. Ultimately, this potential shift towards a load-based approach could impact countries seeking export to the United States and other countries. As such, a continual effort to identify technologies capable of further reducing Salmonella risk are also increasingly focused on live production. Interventions and products reducing the prevalence and loads of Salmonella in live animals during rearing and at harvest are generally assumed to enhance the efficacy of postharvest interventions utilized in downstream processing plants.
In primary production, chick source, biosecurity, farm inputs, and management practices, including sanitation practices, are fundamental to managing Salmonella and general pathogen risk [14,15,16]. In poultry, vaccination with live, attenuated strains of Salmonella are commonplace and continue to evolve with variable efficacy [17,18]. While vaccination may confer early protection against wild type Salmonella colonization, durability of protection may decrease over time, requiring booster administration [18,19,20]. Feed additive solutions that can be continually coadministered throughout the life of the animal while promoting gut health, performance, and protection against pathogen colonization are therefore attractive. Such technologies promote health, performance, immunity, and pathogen control through a variety of mechanisms generally impacting the gastrointestinal tract and immune system [21,22,23,24,25]. The most commonly used products are often classified as prebiotics, probiotics, synbiotics, or postbiotics, based upon composition and functionality, though these definitions continue to evolve [26,27,28].
Original XPC™ (Diamond V, Cedar Rapids, IA, USA) is a postbiotic product consisting of functional metabolites produced as a proprietary Saccharomyces cerevisiae fermentation product (SCFP). As a newer category of products, postbiotics are generally considered preparations consisting of the bioactive compounds produced in controlled fermentation processes by specific microorganisms that ultimately confer a health benefit to the target host [29,30]. SCFP has demonstrated improvements in a variety of gut health, immunity, and production performance measures in commercial poultry, including lower corticosterone levels, heterophil/lymphocyte ratios, and physical asymmetry during stress events, reduced intestinal lesions and improved immune function during coccidia challenge, improved feed efficiency, growth, meat yield, and egg production, as well as foodborne pathogen reduction, including Salmonella [31,32,33,34,35,36,37,38,39]. In vitro gut fermentation models have associated SCFP with modulation of the microflora composition in a manner to synergistically reduce Salmonella Typhimurium and Campylobacter jejuni concentrations [40,41,42]. The purpose of this study was to evaluate dietary inclusion of SCFP, under real-world production conditions, on Salmonella enterica colonization, load, and overall burden in the litter and ceca of commercial broilers on a Honduran farm.
2. Materials and Methods
2.1. Experimental Design
A single commercial broiler farm was selected by the collaborating producer for inclusion into the study. The farm consisted of 12 open-sided houses with an average placement population of ~9400 birds per house. Houses were matched by feeder and drinker system type and insulation parameters to assign six houses each to two dietary treatments consisting of the standard industry diet (CON) or the standard industry diet supplemented with 1.25 kg/MT of postbiotic (SCFP). Prior to placement of the first flock, each house was fully cleaned and sanitized according to the company’s standard operating procedures, and new wood shavings or rice hull litter sourced and placed into each house with litter sampling prior to chick placement for the first rearing cycle. Litter was reutilized without amendment for subsequent rearing cycles except for top dressing in the brooding area at chick placements, wherein the same litter type was utilized with the exception of one house after the second rearing cycle. For each cycle, day of hatch Cobb 500 slow or Ross 308 slow broiler chicks sourced from the company’s suppliers were placed into each house and exposed to dietary treatments from Day 0 to the targeted market age of approximately 33–34 days. Sampling and laboratory personnel were blinded to treatment. Three consecutive flock cycles were evaluated in the study with houses remaining in the respective assigned treatment cohort.
2.2. Litter Sampling
Environmental sampling of the litter was conducted on four occasions to include sampling of the new litter prior to placement of the first flock cycle and at harvest of each cycle prior to placement of the subsequent flock. On each occasion and within each house, four litter swabs were collected. Briefly, for each house, new 1/2” × 9” nap paint rollers (Bates Choice Pro, Lafayette, LA, USA) were prehydrated with 200 mL of Buffered Peptone Water (BPW) (Symmag Suministros Industriales, San Pedro Sula, Honduras) and the roller device handle (Rubbermaid, Atlanta, GA, USA) utilized to furrow into the litter and roll the length of each house in a U-pattern in four replicates. Immediately upon sampling, each roller was placed into an appropriately labeled sterile sample bag (Twirl’Em®, LabPlas Inc., Montreal, QC, Canada) and placed on ice in a cooler for transport to the laboratory for analysis.
2.3. Ceca Sampling
For each flock and house within cycle at the target market age, 25 birds were randomly caught and removed from the house for sampling. Birds were humanely euthanized by cervical dislocation, dipped in a 400 ppm quaternary ammonium solution (Edwards Councilor, Virginia Beach, VA, USA), and prepared for necropsy. One cecal pouch was aseptically removed from each bird and placed into an appropriately labeled sterile sample bag and immediately placed on ice in a cooler for transport to the laboratory for analysis.
2.4. Salmonella Analysis
All samples were received into the laboratory and processed within 12–24 h of collection. Nap roller litter samples were prepared for analysis by the addition of 200 mL of BPW with subsequent hand agitation and rinsing for approximately 1–2 min, creating the primary sample. Each ceca sample was weighed, lacerated to expose contents, and 40 mL of BPW added to the tissue and contents with agitation for 1 min (Vortex Genie 2, USA Scientific, Ocala, FL, USA) to create a primary sample slurry. For both matrices, culture-based qualitative and quantitative Salmonella analyses were conducted in parallel. For detection of Salmonella, a 1 mL aliquot from each primary sample was removed and combined with 9 mL of BPW and incubated at 35° ± 2 °C for 18–24 h. After incubation, 0.5 ± 0.05 mL of the BPW enrichments were transferred into 10 mL Tetrathionate broth (TT) and 0.1 ± 0.02 mL into 10 mL Rappaport–Vassiliadis (RV) broth with incubation at 42°± 0.5 °C for 22–24 h. Tetrathionate and RV broths were then sampled with a 10 µL sterile loop and streak-plated for isolation of Salmonella on xylose lysine desoxycholate (XLD) and Rambach agar plates with incubation at 35° ± 2 °C for 18–24 h. Morphologically typical colonies were then confirmed as Salmonella enterica by colony-based Salmonella PCR (Biocontrol GDS, MilliporeSigma, Burlington, VT, USA) and an immunochromatographic lateral flow (Singlepath® Salmonella, MilliporeSigma, Burlington, VT, USA). In parallel, three 1 mL aliquots of primary slurry were placed into the first three wells of a 96-well plasma tube plate and serially diluted in BPW to achieve a seven-dilution miniaturized most probable number assay (mMPN) according to Pavic et al. [43]. The sample mMPN positive well patterns were input into an MPN calculator to estimate MPN/mL of the primary sample which was then back-calculated to obtain MPN/swab and weight-adjusted MPN/g of ceca.
2.5. Statistical Analysis
Paired analytical outcomes reported by the laboratory for each sample were input into a spreadsheet by study variables including sample type, flock cycle, treatment code, and house. Data were analyzed in SAS Version 9.4 (SAS Institute, Cary, NC, USA) and R Version 4.1.0 (R Core Team, Vienna, Austria) with house as the experimental unit. The binomial response variable for the outcome of Salmonella prevalence (% positive) was determined for each individual sample when confirmation of a typical isolate was obtained from either the detection or mMPN assay or both. Litter swab Salmonella prevalence and load were modeled using a generalized linear mixed model and linear mixed model that were fit using the packages “lme4” [44] and “lmerTest” [45]. For prevalence, a binomial mixed-effects logistic regression was fit with litter type, treatment, their interaction, and a covariate for baseline prevalence at preplacement as fixed effects, and random effects for rearing cycle and pen. Rearing cycle was fit as a random effect because of the need to include additional covariates; as a result, the regression coefficients represent the average of the cycle-specific estimates. The interaction term between litter type and treatment was removed if p > 0.05. For litter swab Salmonella load, a linear mixed-effects model was fit with the same fixed and random effects, except the baseline prevalence covariate was replaced with a covariate for the Salmonella load measured at preplacement. Least-square/marginal means were estimated using the package “emmeans” [46]. Cecal prevalence data were modeled in PROC GLIMMIX using events/trials at the house level with fixed effects of treatment, cycle, and the interaction. Raw MPN/mL estimates were back-calculated by dilution volume to obtain MPN/swab and ceca adjusted by sample weight to obtain MPN/g prior to log10 transformation. Estimates of total Salmonella burden were calculated utilizing the cecal prevalence and load values obtained for each individual flock based on total birds at harvest. Cecal quantitative estimates were modeled in PROC MIXED with fixed effects of treatment, cycle, and the interaction. LS means estimates with pairwise differences for fixed effect of treatment were considered significant at p < 0.05. An observational and statistical outlier was removed from the SCFP cohort’s ceca samples in cycle 3 because of a sampling protocol deviation wherein the subsample of birds was removed and held overnight in a coop prior to sampling, which did not occur for any other house or cycle in the study. This was removed because this sampling deviation, in combination with feed and water withdrawal, may increase Salmonella in the sampled birds and thus be confounding to the estimate for the specific experimental unit [47,48,49].
3. Results
Salmonella litter prevalence across cycles of the trial were not different (p = 0.09) between CON (30.6%; adjusted 30.7%) or SCFP (27.8%; adjusted 7.5%), despite a lower observed and estimated prevalence for SCFP (Figure 1A). Preplacement litter swabs indicated variability of Salmonella presence in the fresh litter of some houses, and means were higher for those within the SCFP cohort (33.3%) as compared to CON (25.0%) (Table 1).
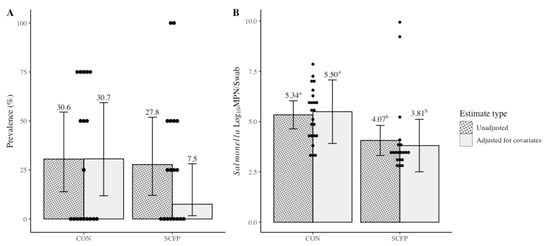
Figure 1.
Treatment level Salmonella enterica litter prevalence (A) and load (B) LS means estimates with and without adjustment for covariates. Error bars reflect 95% confidence intervals with significant differences within estimate types denoted by superscripts a,b at p < 0.05. Dots represent individual observed values.

Table 1.
Observed prevalence and load of Salmonella enterica in the litter of broiler houses freshly sourced and after each of three consecutive rearing cycles of birds fed either CON or SCFP diets.
Cycle to cycle, observed litter prevalence was quite variable, with SCFP litter prevalence of 20.8%, 20.8%, and 41.7% for cycles 1, 2, and 3 and CON prevalence of 12.5%, 50.0%, and 33.3%, respectively (Table 1).
The difference in preplacement prevalence and litter types resulted in markedly different observed and adjusted mean estimates after controlling for their unbalanced distribution across treatment groups. Preplacement, mean Salmonella load in the culture-positive fresh litter samples was also observed to be higher in the houses assigned to the SCFP cohort (6.17 log10 MPN/swab) when compared to houses in the CON cohort (3.35 log10 MPN/swab), indicating that incoming litter or house contamination with Salmonella was not equivalent between cohorts (Table 1). Mean litter load across cycles in culture-positive houses after adjustment for litter type and preplacement load was different (p = 0.04) between CON (5.34 log10 MPN/swab; adjusted 5.50 log10 MPN/swab) and SCFP (4.07 log10 MPN/swab; adjusted 3.81 log10 MPN/swab), with Salmonella load in SCFP houses being lower by 1.69 log10 MPN/swab (95% CI: 0.107 to 3.26; Figure 1B). After each successive rearing cycle, observed mean litter loads in the SCFP cohort houses were 3.01, 3.54, and 4.85 log10 MPN/swab compared to CON houses at 5.77, 5.47, and 4.98 log10 MPN/swab, respectively (Table 1).
Prevalence of Salmonella across all cycles within the ceca of birds from the SCFP cohort were significantly lower (3.4%; 95% CI: 1.8–6.4%) as compared to CON (12.2%; 95% CI: 9.2–15.9%), reflecting a 72.1% reduction (Figure 2A).
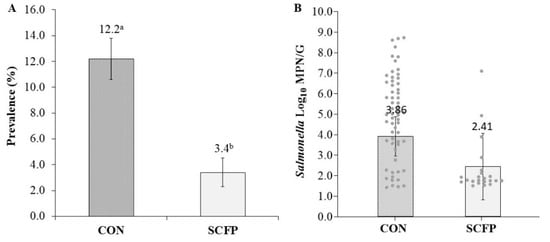
Figure 2.
Treatment-level Salmonella enterica cecal prevalence (A) and load (B) LS means estimates. Error bars reflect prevalence estimate standard error and load estimate 95% confidence interval with significant differences denoted by superscripts a,b at p > 0.05.
Within flock cycles, prevalence of Salmonella remained lower (p < 0.05) in SCFP cohort houses at 1.3%, 12.0%, and 2.4% as compared to CON houses at 7.3%, 22.0%, and 10.7% for cycles 1, 2, and 3, respectively (Table 2 and Table 3). Culture-positive Salmonella load for SCFP (2.41 log10 MPN/g; 95% CI: 0.81–4.01) was numerically (1.45 log10 MPN/g) lower (p = 0.121) than CON (3.86 log10 MPN/g; 95% CI: 2.91–4.81) (Figure 2B). Within flock cycles, SCFP cecal loads were 1.67, 1.95, and 3.62 log10 MPN/g as compared to CON loads at 2.98, 4.72, and 3.88 log10 MPN/g, reflecting no difference in cycle 1 (p = 0.53), a reduction (p = 0.016) of 2.77 for cycle 2, and no difference (p = 0.853) in cycle 3 (Table 3).

Table 2.
Observed prevalence and load of Salmonella enterica in the ceca of broilers at market age fed either CON or SCFP diets by flock-rearing cycle and estimated treatment level within-cycle total burden.

Table 3.
LS means estimates for prevalence and load of Salmonella enterica in the ceca of broilers at market age fed either CON or SCFP diets by flock-rearing cycle and estimated treatment level within-cycle total burden.
Estimates of total Salmonella burden going to harvest were 7.31 log10 MPN (95% CI: 5.80–8.82) and 3.80 log10 MPN (95% CI: 2.23–5.36) for CON and SCFP cohorts, respectively, a more than 1000-fold reduction (p = 0.0026) in cecal Salmonella total load associated with SCFP inclusion into the dietary ration (Figure 3 and Figure 4).
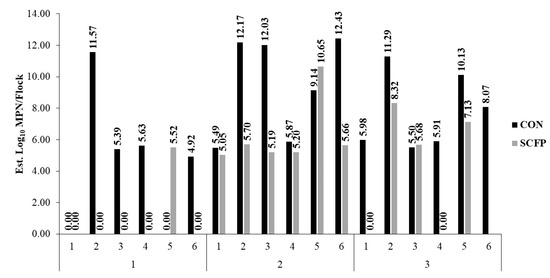
Figure 3.
Estimated Salmonella enterica burden by flock within rearing cycle and treatment cohort. Values (MPN/flock) reflect livability-adjusted flock estimates obtained utilizing observed prevalence and load outcomes.
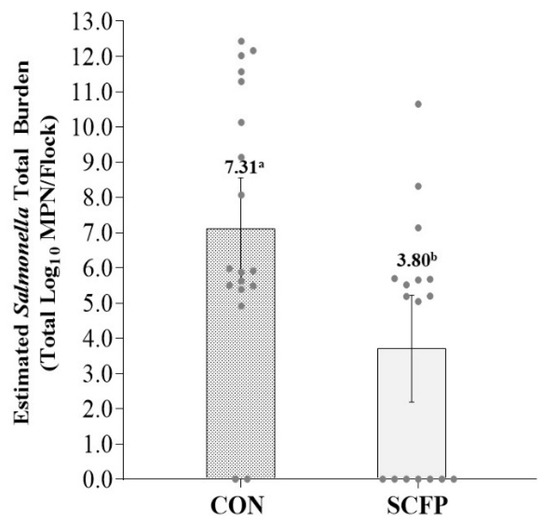
Figure 4.
LS means estimated total Salmonella enterica burden by treatment. Error bars reflect 95% confidence interval with significant differences denoted by superscripts a,b at p < 0.05.
4. Discussion
Salmonella enterica are foodborne pathogens of significant public health risk globally in broiler meat and other poultry products. Feed-additive technologies that promote animal health and production while additionally conferring a preharvest food safety benefit may ultimately aid producers in their efforts to reduce pathogen risk when utilized in conjunction with a comprehensive food safety management plan. In this study conducted on a single Honduran commercial broiler farm, data support that broiler flocks fed SCFP had significantly reduced cecal prevalence and reduced loads of Salmonella when compared to same-farm flocks fed a typical industry diet without SCFP (CON). Additionally, these data present Salmonella prevalence information from within a representative commercial broiler operation in Honduras. To our knowledge, no such recent reports are available in the scientific literature.
In broiler and other poultry production, litter management practices, quality, and microbial composition are significant factors associated with pathogen status in flocks, particularly in regions where reuse may be a common practice. Litter microbiome analyses have demonstrated associations between the physicochemical characteristics of the litter, the abundance of specific taxa, the associated ability to isolate foodborne pathogens such as Salmonella and Campylobacter, and the litter influence on gut colonization [50,51,52]. Specifically, for Salmonella, Machado and Hagerman [53] reported decreasing odds for the probability of detecting Salmonella in litter prior to harvest with successive litter reuses up to six rearing cycles, after which, however, the odds began to increase. Conversely, in another study, Salmonella detections in recycled litter continued to decrease with up to 14 reuse cycles [54]. Other factors, such as house construction, pad composition, soil type, and litter type may also influence the ability of Salmonella to persist in litter [55]. The microbial ecology of litter is dynamic and complex with environmental conditions and management practices, potentially creating conditions favoring the persistence of Salmonella and other pathogens. These risks, however, likely vary greatly between regions, practices, and operations.
What may be more consistent is the cyclical influence between the litter and the host’s gut, thereby influenced by environmental and dietary treatments [56]. In this study, we observed that including SCFP in the diets of broilers did appear to affect a subtle shift in litter Salmonella status over the course of three consecutive reuses. Despite an observed higher mean prevalence and load in the fresh litter for the SCFP cohort of houses pre-placement, the model-adjusted across-cycle estimate indicated an overall lower Salmonella MPN/swab load for the SCFP cohort. The commercial farm utilized two different litter types in the houses’ preplacement of the first rearing cycle: specifically, rice hull or wood shavings. While comparing litter types was not an objective of the study, the effect of litter type was investigated utilizing general linear mixed models. For these models, the SCFP cohort demonstrated significantly less Salmonella loads in the litter regardless of type. Notably, litter type was not equally balanced between treatments. Sampling conducted pre-placement revealed two houses within each treatment testing positive, for which both litter types were represented. Considering all other houses in the trial were negative with both fresh litter types at preplacement, this observation may suggest house contamination (e.g., ineffective sanitation or cross-contamination from outside source into the specific barns) rather than a contaminated litter source. This observation highlights a key challenge and example of the limitations in conducting real-world research in commercial operations and may warrant future research needs to investigate Salmonella survival and proliferation as influenced by litter type on a commercial operation scale.
Regardless of origin, Salmonella in the rearing environment leads to host exposure and the chance for colonization of the gut. The epidemiological triad between the agent, environment, and host is a dynamic that contributes to the persistence of a pathogen such as Salmonella in the production and controlled rearing of broilers. Though not quantifiable in the present study, the observation of reduced litter load could contribute to a lower re-infection pressure and, therefore, decreased Salmonella cecal prevalence and loads observed within the SCFP cohort as compared to the CON cohort flocks reared on the respective litters over the course of the trial. In controlled research, Salmonella serotype status in contaminated litter has been associated with isolation in the crop and ceca, even within a short 12 h window of feed withdrawal and contaminated litter exposure [57]. Feed withdrawal prior to harvest has been demonstrated to significantly increase Salmonella detection in the crop and could lead to carcass contamination during harvesting [58]. Indeed, Berghaus and colleagues reported that Salmonella loads in environmental boot swabs and litter samples had the strongest association with loads of Salmonella in pre- and post-chill carcass rinses [59]. The risk of transfer from litter to organ colonization and subsequently carcass contamination during harvest is multifactorial and complex; nonetheless, it is likely to be influenced by the quantitative loads of Salmonella exposure. As such, the reduced Salmonella litter loads observed in the SCFP cohort of this study may be key in reducing individual exposure in subsequent flock placements and thereby contribute to overall long-term management. Future research could explore litter microbiome composition and Salmonella survival as specifically influenced by dietary treatments of birds.
While litter and other farm inputs (such as feed, water, and personnel) expose naïve individuals or populations to Salmonella, the ability of Salmonella to colonize and propagate within and between individuals in the population is largely influenced at the individual level by the host’s gut microbiome and immune system. Resistance to colonization by Salmonella may be partially attributed to short-chain volatile fatty acid (VFA) and other metabolite products from members of the microbiome, as well as maintenance of gut barrier function and immunity [60]. SCFPs have been shown to increased VFA concentrations for acetate and butyrate and promote favorable shifts in abundance of beneficial taxa and inhibition of Salmonella propagation during challenge [40,41,42,61]. In vivo research has identified immunomodulatory benefits of feeding SCFP to broilers, including those challenged with Eimeria tenella, though no Salmonella-specific mechanisms have been investigated to date [36,37]. These reports suggest that SCFP may impart an anti-Salmonella effect through multiple pathways and modes of action, though more research is warranted to determine anti-Salmonella-specific mechanisms.
Controlled research evaluating the effects of SCFP to reduce Salmonella Enteritidis challenges has demonstrated quantitative reductions in cecal contents [62,63]; however, reductions in prevalence (% positive) for direct challenge studies are commonly not observed due to extremely high challenge doses administered via oral gavage. Real-world industry conditions have exposure routes and doses which vary greatly [59]. When combined with stress, disease challenge, and other factors of commercial production not easily replicated in controlled research settings, the efficacy of SCFP and similar products may become more evident. This study demonstrated that SCFP reduced Salmonella prevalence compared to control flocks, and prevalence within SCFP flocks were consistently lower within and between flock-rearing cycles. The consistent reduction in the number of individuals positive for Salmonella was also coupled with those individuals and the populations, on average, having significantly lower cecal Salmonella loads. Notably, the combined reductions observed cycle over cycle in both cecal Salmonella prevalence and loads in populations of the SCFP cohort indicate that there would be fewer individuals in a flock shedding Salmonella at lower loads back into the litter during rearing and thus likely influencing the subsequent flock placement. While our trial only observed three consecutive cycles, the data do appear to directionally support this concept of Salmonella transmission, and future work may consider following an increased number of cycles. If this is the case, products such as SCFP may impart a compounding benefit over time, particularly in regions where litter reuse and litter amendment interventions are commonly utilized.
Ultimately, efforts to reduce Salmonella risk rely on multiple interventions strategically utilized throughout the processing continuum. At harvest and in processing, several physical and chemical interventions, often validated for multi-log reductions of indicator organisms or pathogens such as Salmonella, are commonly employed to reduce microbial contamination. These include, but are not limited to, hot water, steam, chlorine, organic acids, and others [64,65]. The efficacy of these interventions may, therefore, be impacted by the incoming microbial burden that must be mitigated. Utilizing the Salmonella estimates obtained for each flock on trial in combination with placement numbers adjusted for mortality, we estimated the total burden of Salmonella to processing. While sufficient evidence is lacking to directly correlate cecal prevalence and loads to finished product contamination risk, litter loads have been associated with carcass contamination, and thus these relationships at the flock-level may be useful, in the very least, as estimates of incoming Salmonella burden to be managed. Flock-level burden estimates varied greatly across cycles and within treatment cohorts. Notably, fewer overall flocks within the SCFP cohort (n = 4) had a load burden exceeding 6.00 log10 MPN as compared to the CON cohort which had eight flocks, of which six exceeded 10.00 log10 MPN (Figure 3). From a practical point of view, higher cumulative burden at a processing plant could constrain efficacy of postharvest processing controls and the food safety management system. Products such as SCFPs may be beneficial towards reducing the overall load burden at these facilities, and future research may warrant sampling of postharvest matrices.
Salmonella prevalence and serovar distribution in food animal production are recognized as being both geographically diverse and influenced by seasonality [66,67,68,69]. Therefore, limitations to this study may include the geographical isolation of a single farm in Honduras studied over an approximate 4-month window. However, a critical benefit in this study was the replication of dietary treatments within the single farm in parallel, which is often not feasible for many farm operations. Serotyping or molecular typing of isolates may have yielded additional insights into this study. However, serotyping in commercial farms is not always approved by stakeholders, thus another general limitation of the study. Lastly, as previously noted, global diversity in soil type, pad construction, litter types, house types, and farm management practices exist, all of which may influence Salmonella ecology uniquely and thus warrant continued evaluation of SCFP postbiotic.
Together, these data demonstrate the association and influence that dietary inclusion of SCFP imparts on multi-flock qualitative and quantitative Salmonella reductions. These reductions, therefore, may lead to an overall lower Salmonella burden on farms and are likely to challenge a processing facility. SCFP postbiotic demonstrates potential to serve as an effective preharvest intervention, thus contributing to a multi-hurdle farm-to-fork Salmonella food safety program.
Author Contributions
Conceptualization, W.E.C., A.G. and T.J.J.; methodology, W.E.C., A.G. and M.G.; data curation, M.G. and W.E.C.; formal analysis, W.E.C., M.G., S.A.N. and D.P.; writing—original draft preparation, W.E.C.; writing—review and editing, W.E.C., S.A.N., A.G., M.G. and T.J.J.; supervision, D.P. and A.G.; project administration, W.E.C., A.G. and T.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding but was made possible by the collaborating commercial producer and the intrinsic costs shared within the routine commercial operation.
Institutional Review Board Statement
An animal use protocol was not required for this study as live animals were utilized only for a small portion of the study, and these animals were owned and were being commercially reared primarily for food production by the collaborating producer. The test article already has regulatory acceptance for use in animal food. The live animal portion of the study only included on-farm sampling and necropsy of animals, and these activities were supervised by veterinary personnel according to AVMA-approved practices for humane on-farm euthanasia from the commercially raised flocks at time of harvest and transfer to the commercial poultry processing plant.
Informed Consent Statement
Not applicable.
Data Availability Statement
Observed microbiological data is reported within the article. Restrictions apply to the availability of additional information due to 3rd party proprietary information associated with collaborating producer.
Acknowledgments
The authors would like to thank the Honduran poultry company and its personnel who engaged in this collaboration and its shared costs and allowed the opportunity to conduct this insightful real-world commercial trial amidst the challenges of the COVID-19 pandemic.
Conflicts of Interest
Authors affiliated with Diamond V. provided the feed additive, SCFP, as well as analytical financial support for the study. Manuel Gutierrez served as an independent, treatment blinded contractor for analytical sample collection, processing, and reporting.
References
- Scallen, E.; Hoekstra, R.M.; Tauxe, R.V.; Widdowson, M.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Interagency Food Safety Analytics Collaboration. Foodborne Illness Source Attribution Estimates for 2017 for Salmonella, Escherichia coli O157, Listeria monocytogenes, and Campylobacter Using Multi-Year Outbreak Surveillance Data, United States. Available online: https://www.cdc.gov/foodsafety/ifsac/pdf/P19-2017-report-TriAgency-508-archived.pdf (accessed on 21 September 2021).
- Popa, G.L.; Papa, M.L. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Wong, D.; Patrick, M.E.; Binsztein, N.; Cieslik, A.; Chalermchaikit, T.; Aidara-Kane, A.; Ellis, A.; Angulott, F.J.; Wegener, H.C. Web-based surveillance and global Salmonella distribution, 200–2002. Emerg. Infect. Dis. 2006, 12, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Parisi, A.; Stanaway, J.D.; Sarkar, K.; Crump, J.A. The global burden of non-typhoidal Salmonella invasive disease: A systemic analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service. Final Report of a Remote Audit Conducted for Honduras: Evaluating the Food Safety Systems Governing Raw Beef and Raw Poultry Products Exported to the United States of America. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-09/honduras-foreign-audit-report.pdf (accessed on 21 September 2021).
- United States Department of Agriculture, Food Safety and Inspection Service. Microbiology Laboratory Guidebook 4.11: Isolation and Identification of Salmonella from Meat, Poultry, Pasteurized Egg, and Siluriformes (Fish) Products and Carcass and Environmental Sponges. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2021-08/MLG-4.11.pdf (accessed on 21 September 2021).
- United States Centers for Disease Control and Prevention, Foodborne Diseases Active Surveillance Network (FoodNet). Available online: https://www.cdc.gov/foodnet/index.html (accessed on 21 September 2021).
- United States Department of Agriculture, Food Safety and Inspection Service. Salmonella Verification Testing Program Monthly Posting. Available online: https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/microbiological-testing-program-rte-meat-and-0 (accessed on 21 September 2021).
- McEntire, J.; Acheson, D.; Siemens, A.; Eilert, S.; Robach, M. The public health value of reducing Salmonella levels in raw meat and poultry. Food Prot. Trends 2014, 34, 386–392. [Google Scholar]
- United States Department of Agriculture, Food Safety and Inspection Service. Roadmap to Reducing Salmonella: Driving Change through Science-Based Policy. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/2020-12/FSISRoadmaptoReducingSalmonella.pdf (accessed on 21 September 2021).
- Sampedro, F.; Wells, S.J.; Bender, J.B.; Hedberg, C.W. Developing a risk management framework to improve public health outcomes by enumerating Salmonella in ground turkey. Epidemiol. Infect. 2019, 147, E69. [Google Scholar] [CrossRef] [Green Version]
- Oscar, T.P. Salmonella prevalence alone is not a good indicator of poultry food safety. Risk Anal. 2021, 41, 110–130. [Google Scholar] [CrossRef]
- Volkova, V.V.; Wills, R.W.; Hubbard, S.A.; Magee, D.L.; Byrd, J.A.; Bailey, R.H. risk factors associated with detection of Salmonella in broiler litter at the time of new flock placement. Zoonoses Public Health 2011, 58, 158–168. [Google Scholar] [CrossRef]
- Pulido-Landinez, M. Food safety—Salmonella update in broilers. Anim. Feed Sci. Technol. 2019, 250, 53–58. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Mathis, D.L.; Bramwell, R.K.; Macklin, K.S.; Wilson, J.L.; Wineland, M.J.; Maurer, J.J.; Lee, M.D. Multilevel analysis of environmental Salmonella prevalences and management practices on 49 broiler breeder farms in four south-eastern states, USA. Zoonoses Public Health 2012, 59, 365–374. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef] [PubMed]
- Dorea, F.C.; Cole, D.J.; Hofacre, C.L.; Zamperini, K.; Mathis, D.L.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesse, M.; Weber, R.; Glunder, G. Antibody titers in turkeys increase after multiple booster vaccinations with an attenuated Salmonella live vaccine. BMC Res. Notes 2018, 11, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousaalkar, K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines 2020, 8, 696. [Google Scholar] [CrossRef]
- Tellez, G.; Pixley, C.; Wolfenden, R.E.; Layton, S.L.; Hargis, B.M. Probiotics/direct fed microbials for Salmonella control in poultry. Food Res. Int. 2012, 45, 628–633. [Google Scholar] [CrossRef]
- Johny, A.K.; Venkitanarayanan, K. Chatper 17—Preharvest Food Safety—Potential Use of Plant-Derived Compounds in Layer Chickens in Producing Safe Eggs; Ricke, S.C., Gast, R.K., Eds.; Elsevier: London, UK, 2016; pp. 347–372. [Google Scholar]
- Adhikari, P.; Lee, C.H.; Cosby, D.E.; Cox, N.A.; Kim, W.K. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2019, 98, 1235–1242. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A Review of prebiotics against Salmonella in poultry: Current and future potential for microbiome research applications. Front. Vet. Sci. 2018, 5, 191. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.I.; Sadekuzzaman, M.; Sang-Do, H. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott KStanton, C.; Swanson, K.S.; Cani, P.D.; Verbeke, K.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toala, J.E.; Garcia-Varela, G.; Garcia, H.S.; Mata-Haro, V.; Gonsalez-Cordova, A.F.; Vallejo-Cordoba, B.; Hernandez-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; M.E., S.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; McIntyre, D.R.; Pavlidis, H.O.; Archer, G.S. Reducing stress susceptibility of broiler chickens by supplementing a yeast fermentation product in the feed or drinking water. Animals 2018, 8, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lensing, M.; van der Klis, J.D.; Yoon, I.; Moore, D.T. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poult. Sci. 2012, 91, 1590–1597. [Google Scholar] [CrossRef]
- Labib, Z.M.; Elsamadony, H.A.; El Gabaly, L.S.; Zoghbi, A.F. Immunopathological studies on ducks experimentally infected with duck virus enteritis and Salmonella enteritidis with special references to the effect of XPC prebiotic. Zagazig Vet. J. 2014, 42, 41–62. [Google Scholar] [CrossRef] [Green Version]
- Firman, J.D.; Moore, D.; McIntyre, D. Effects of dietary inclusion of a Saccharomyces cerevisiae fermentation product on performance and gut characteristics of male turkeys to market weight. Int. J. Poult. Sci. 2013, 12, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Price, P.T.; Byrd, J.A.; Alvarado, C.Z.; Pavlidis, H.O.; McIntyre, D.R.; Archer, G.S. Utilizing original XPCTM in feed to reduce stress susceptibility of broilers. Poult. Sci. 2018, 97, 855–859. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Moore, D.; Gao, Y.P.; Yan, H.J.; Qi, G.H. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 2009, 88, 2141–2151. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Wu, S.G.; Yu, S.H.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G.H. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Park, S.; Roto, S.; Pavlidis, H.; McIntyre, D.; Striplin, K.; Brammer, L.; Ricke, S. Effects of feeding original XPC to broilers with a live coccidiosis vaccine under industrial conditions: Part 2. Cecal microbiota analysis. Poult. Sci. 2017, 96, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Roto, S.; Park, S.; Lee, S.; Kaldhone, P.; Pavlidis, H.; Frankenbach, S.; McIntyre, D.; Striplin, K.; Brammer, L.; Ricke, S. Effects of feeding Original XPCTM to broilers with a live coccidiosis-vaccine under industry conditions: Part 1. Growth performance and Salmonella inhibition. Poult. Sci. 2017, 96, 1831–1837. [Google Scholar] [CrossRef]
- Feye, K.M.; Rubinelli, P.M.; Chaney, W.E.; Pavlidis, H.O.; Kogut, M.H.; Ricke, S.C. The preliminary development of an in vitro poultry cecal culture model to evaluate the effects of original XPCTM for the reduction of Campylobacter jejuni and its potential effects on the microbiota. Front. Microbiol. 2020, 10, 3062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong Park, S.; Ae Kim, S.; In Lee, S.; Rubinelli, P.; Roto, S.; Pavlidis, H.; McIntyre, D.; Ricke, S. Original XPCTM Effect on Salmonella typhimurium and cecal microbiota from three different ages of broiler chickens when incubated in an anaerobic in vitro culture system. Front. Microbiol. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Rubinelli, P.; Roto, S.; Ae Kim, S.; Hong Park, S.; Pavlidis, H.; McIntyre, D.; Ricke, S. Reduction of Salmonella typhimurium by fermentation metabolites of diamond V original XPC in an in vitro anaerobic mixed chicken cecal culture. Front. Vet. Sci. 2016, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Pavic, A.; Goves, P.J.; Bailey, G.; Cox, J.M. A validated miniaturized MPN method, based on ISO 6579:2002, for the enumeration of Salmonella from poultry matrices. J. Appl. Microbiol. 2010, 109, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.7.0. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 9 December 2021).
- Moran, E.T.; Bilgili, S.F. Influence of feeding and fasting broilers prior to marketing on cecal access of orally administered Salmonella. J. Food Prot. 1990, 53, 205–207. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Sarlin, L.L.; Caldwell, D.J.; Yezak, C.R., Jr.; Hume, M.E.; Corrier, D.E.; Deloach, J.R.; Hargis, B.M. Effect of feed withdrawal on the incidence of Salmonella in the crops and ceca of market age broiler chickens. Poult. Sci. 1997, 76, 654–656. [Google Scholar] [CrossRef]
- Corrier, D.E.; Byrd, J.A.; Hargis, B.M.; Hume, M.E.; Bailey, R.H.; Stanker, L.H. Survival of Salmonella in the crop contents of market-age broilers during feed withdrawal. Avian Dis. 1999, 43, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Valeris-Chacin, R.; Pieters, M.; Hwang, H.; Johnson, T.J.; Singer, R.S. Association of Broiler Litter Microbiome Composition and Campylobacter Isolation. Front. Vet. Sci. 2021, 8, 4927. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.G.; Zwirzitz, B.; Oladeinde, A.; Cook, K.L.; Plymel, C.; Zock, G.; Lakin, S.; Aggrey, S.E.; Ritz, C.; Looft, T.; et al. Reused poultry litter microbiome with competitive exclusion potential against Salmonella Heidelberg. J. Environ. Qual. 2019, 49, 869–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Machado Junior, P.C.; Chung, C.; Hagerman, A. Modeling Salmonella spread in broiler production: Identifying determinants and control strategies. Front. Vet. Sci. 2020, 7, 564. [Google Scholar] [CrossRef]
- Roll, V.F.B.; Dai Pra, M.A.; Roll, A.P. Research on Salmonella in broiler litter reused for up to 14 consecutive flocks. Poult. Sci. 2011, 2257–2262. [Google Scholar] [CrossRef]
- Volkova, V.V.; Bailey, R.H.; Wills, R.W. Salmonella in broiler litter and properties of soil at farm location. PLoS ONE 2009, 4, e6403. [Google Scholar] [CrossRef]
- Cressman, M.D.; Zhongtang, Y.; Nelson, M.C.; Moeller, S.J.; Lilburn, M.S.; Zerby, H.N. Interreltations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010, 76, 6572–6582. [Google Scholar] [CrossRef] [Green Version]
- Buhr, R.J.; Bourassa, D.V.; Hinton, A., Jr.; Fairchild, B.D.; Ritz, C.W. Impact of litter Salmonella status during feed withdrawal on Salmonella recovery from the broiler crop and ceca. Poult. Sci. 2017, 96, 4361–4369. [Google Scholar] [CrossRef]
- Corrier, D.E.; Byrd, J.A.; Hargis, B.M.; Hume, M.E.; Bailey, R.H.; Stanker, L.H. Presence of Salmonella in the crop and ceca of broiler chickens before and after preslaughter feed withdrawal. Poult. Sci. 1999, 78, 45–49. [Google Scholar] [CrossRef]
- Berghaus, R.D.; Thayer, S.G.; Law, B.F.; Mild, R.M.; Hofacre, C.L.; Singer, R.S. Enumeration of Salmonella and Campylobacter spp. in environmental farm samples and processing plant carcass rinses from commercial broiler chicken flocks. Appl. Environ. Microbiol. 2013, 79, 4106–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.W.L.; Tsolis, R.M.; Baumler, A.J. Salmonella versus the microbiome. Microbiol. Molec. Biol. Rev. 2020, 85, e00027-19. [Google Scholar] [CrossRef]
- Feye, K.M.; Dittoe, D.K.; Rubinelli, P.M.; Olson, E.G.; Ricke, S.C. Yeast fermentate-mediated reduction of Salmonella reading and typhimurium in an in vitro turkey cecal culture model. Front. Microbiol. 2021, 12, 645301. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.; Chaney, W.E. Effect of feeding a postbiotic derived from Saccharomyces cerevisiae fermentation as a preharvest food safety hurdle for reducing Salmonella Enteritidis in the ceca of layer pullets. J. Food Protection 2021, 84, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis reduction in layer ceca with a Bacillus probiotic. Vet. World 2020, 13, 184–187. [Google Scholar] [CrossRef]
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating best practices for Campylobacter and Salmonella reduction in poultry processing plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef]
- Loretz, M.; Stephan, R.; Zweifel, C. Antimicrobial activity of decontamination treatments for poultry carcasses: A literature survey. Food Control 2010, 21, 791–804. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.S.; Conte-Junior, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A Meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [Green Version]
- Hendriksen, R.S.; Vieira, A.R.; Karlsmose, S.; Lo Fo Wong, D.M.A.; Jensen, A.B.; Wgener, H.C.; Aarestrup, F.M. Global monitoring of Salmonella serovar distribution from the World Health Organization Global Foodborne Infection Network Country Data Bank: Results of quality assured laboratories from 2001 to 2007. Foodborne Pathog. Dis. 2011, 8, 887–900. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Pangloli, P.; Richards, H.A.; Mount, J.R.; Draughon, F.A. Prevalence of Salmonella in diverse environmental farm samples. J. Food Prot. 2006, 69, 2576–2580. [Google Scholar] [CrossRef]
- Velasquez, C.G.; Macklin, K.S.; Kumar, S.; Bailey, M.; Ebner, P.E.; Oliver, H.F.; Martin-Gonzalez, F.S.; Singh, M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018, 97, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).