Genetic Characterization of Multidrug-Resistant E. coli Isolates from Bloodstream Infections in Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Clinical E. coli Isolates, Identification, and Genomic DNA Extraction
2.2. Characterization of E. coli Genes Associated with Antibiotic Resistance and Virulence
2.3. Phylogenetic Classification
2.4. Genotyping of E. coli Isolates
2.5. Data Analysis
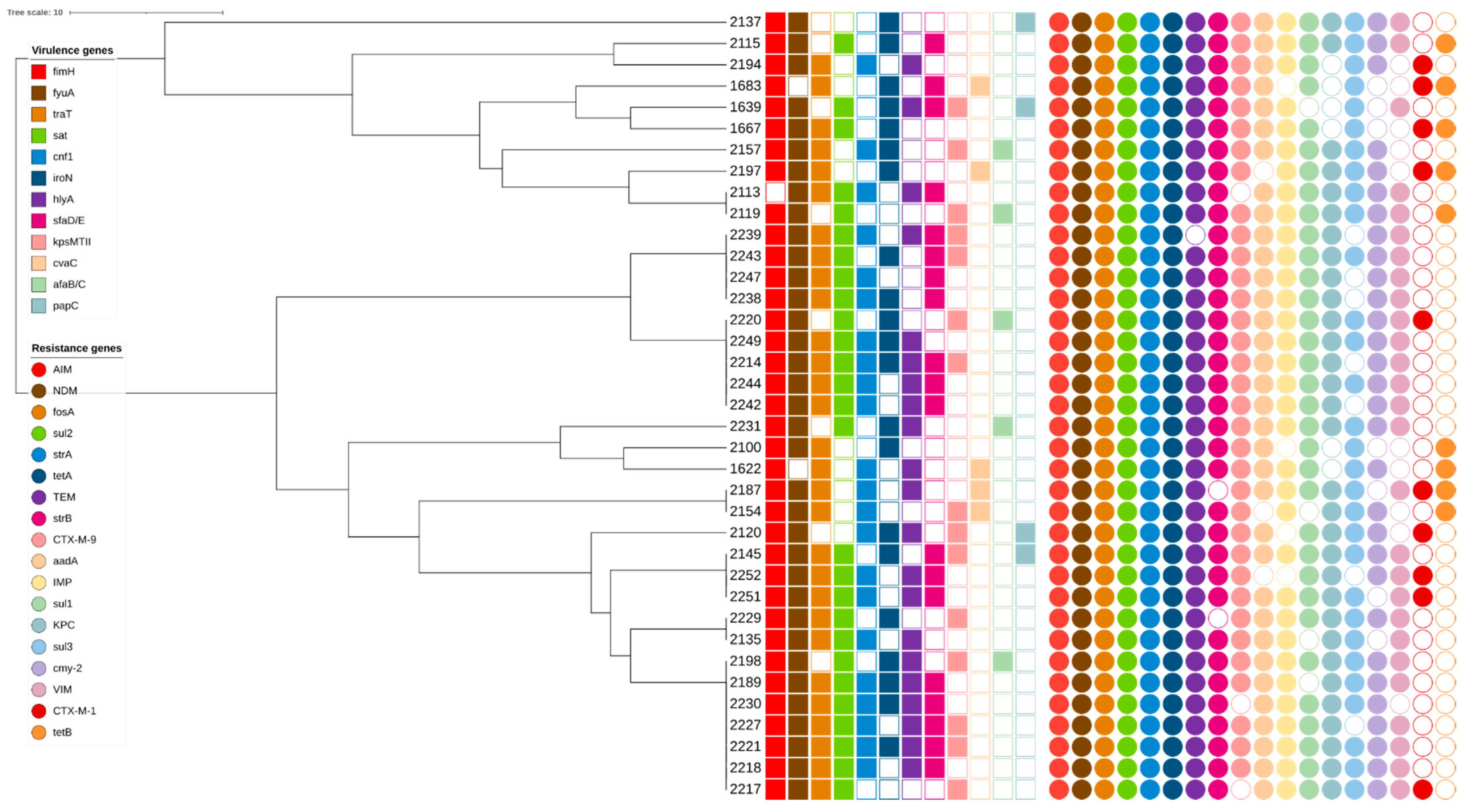
3. Results
3.1. Identification and Characterization of E. coli Antibiotic Resistance Genes
3.2. Identification of Virulence Genes in E. coli
3.3. E. coli Phylogenetic Classification
3.4. rep-PCR Genotyping Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mora-Rillo, M.; Fernández-Romero, N.; Navarro-San Francisco, C.; Díez-Sebastián, J.; Romero-Gómez, M.P.; Arnalich Fernández, F.; Arribas López, J.R.; Mingorance, J. Impact of virulence genes on sepsis severity and survival in Escherichia coli bacteremia. Virulence 2015, 6, 93–100. [Google Scholar] [CrossRef]
- Owrangi, B.; Masters, N.; Kuballa, A.; O’Dea, C.; Vollmerhausen, T.L.; Katouli, M. Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. Eur. J. Clin. Microbiol. 2018, 37, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef]
- Micenková, L.; Beňová, A.; Frankovičová, L.; Bosák, J.; Vrba, M.; Ševčíková, A.; Kmeťová, M.; Šmajs, D. Human Escherichia coli isolates from hemocultures: Septicemia linked to urogenital tract infections is caused by isolates harboring more virulence genes than bacteraemia linked to other conditions. Int. J. Med. Microbiol. 2017, 307, 182–189. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef]
- Blake, D.; Hillman, K.; Fenlon, D.; Low, J. Transfer of antibiotic resistance between commensal and pathogenic members of the Enterobacteriaceae under ileal conditions. J. Appl. Microbiol. 2003, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Dale, A.P.; Woodford, N. Extra-intestinal pathogenic Escherichia coli (ExPEC): Disease, carriage and clones. J. Infect. 2015, 71, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Jauréguy, F.; Carbonnelle, E.; Bonacorsi, S.; Clec’H, C.; Casassus, P.; Bingen, E.; Picard, B.; Nassif, X.; Lortholary, O. Host and bacterial determinants of initial severity and outcome of Escherichia coli sepsis. Clin. Microbiol. Infect. 2007, 13, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B. Incidence of bloodstream infection: A review of population-based studies. Clin. Microbiol. Infect. 2013, 19, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Kirtikliene, T.; Naugzemys, D.; Steponkiene, A.; Bogdevic, R.; Vizuje, G.; Zvingila, D.; Kuisiene, N. Evaluation of the Inter- and Intrahospital Spread of Multidrug Resistant Gram-Negative Bacteria in Lithuanian Hospitals. Microb. Drug Resist. 2019, 25, 326–335. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Xue, W.-C.; Meng, D.-Y. First report of New Delhi metallo-β-lactamase 5 (NDM-5)-producing Escherichia coli from blood cultures of three leukemia patients. Int. J. Infect. Dis. 2016, 42, 45–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, Q.; Du, Y.; Jiang, X.; Tang, J.; Wang, J.; Li, G.; Jiang, Y. Prevalence of plasmid-mediated AmpC β-lactamases in a Chinese university hospital from 2003 to 2005: First report of CMY-2-type AmpC β-lactamase resistance in China. J. Clin. Microbiol. 2008, 46, 1317–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, Y.; Al, Y.B.E.; Hommeril, B.; Genel, N.; Decré, D.; Rottman, M.; Arlet, G. Emergence of plasmid-mediated fosfomycin-resistance genes among Escherichia coli isolates, France. Emerg. Infect. Dis. 2017, 23, 1564–1567. [Google Scholar] [CrossRef] [Green Version]
- Boerlin, P.; Travis, R.; Gyles, C.L.; Reid-Smith, R.; Lim, N.J.H.; Nicholson, V.; McEwen, S.A.; Friendship, R.; Archambault, M. Antimicrobial resistance and virulence genes of Escherichia coli isolates from swine in Ontario. Appl. Environ. Microbiol. 2005, 71, 6753–6761. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Daigle, F.; Harel, J.; Fairbrother, J.M.; Lebel, P. Expression and detection of pap-, sfa-, and afa-encoded fimbrial adhesin systems among uropathogenic Escherichia coli. Can. J. Microbiol. 1994, 40, 286–291. [Google Scholar] [CrossRef]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef]
- Ananias, M.; Yano, T. Serogroups and virulence genotypes of Escherichia coli isolated from patients with sepsis. Braz. J. Med. Biol. Res. 2008, 41, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dombek, P.E.; Johnson, L.K.; Zimmerley, S.T.; Sadowsky, M.J. Use of repetitive DNA sequences and the PCR to differentiate Escherichia coli isolates from human and animal sources. Appl. Environ. Microbiol. 2000, 66, 2572–2577. [Google Scholar] [CrossRef] [Green Version]
- Versalovic, J.; Schneider, M.; De Bruijn, F.J.; Lupski, J.R. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 1994, 5, 25–40. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Domínguez, E.; Zarazaga, M.; Sáenz, Y.; Briñas, L.; Torres, C. Mechanisms of antibiotic resistance in Escherichia coli isolates obtained from healthy children in Spain. Microb. Drug Resist. 2002, 8, 321–327. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Sepp, E.; Andreson, R.; Balode, A.; Bilozor, A.; Brauer, A.; Egorova, S.; Huik, K.; Ivanova, M.; Kaftyreva, L.; Kõljalg, S.; et al. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in Northern and Eastern Europe. Front. Microbiol. 2019, 10, 2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, V.L.; Tomazetto, G.; Cyoia, P.S.; Neves, M.S.; Vidotto, M.C.; Nakazato, G.; Kobayashi, R.K.T. Molecular screening of virulence genes in extraintestinal pathogenic Escherichia coli isolated from human blood culture in Brazil. BioMed Res. Int. 2014, 2014, 465054. [Google Scholar] [CrossRef] [Green Version]
- Miajlovic, H.; Mac Aogáin, M.; Collins, C.J.; Rogers, T.; Smith, S. Characterization of Escherichia coli bloodstream isolates associated with mortality. J. Med. Microbiol. 2016, 65, 71–79. [Google Scholar] [CrossRef]
- Bozcal, E.; Eldem, V.; Aydemir, S.; Skurnik, M. The relationship between phylogenetic classification, virulence and antibiotic resistance of extraintestinal pathogenic Escherichia coli in İzmir province, Turkey. PeerJ 2018, 6, e5470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daga, A.P.; Koga, V.L.; Soncini, J.G.M.; De Matos, C.M.; Perugini, M.R.E.; Pelisson, M.; Kobayashi, R.; Vespero, E.C. Escherichia coli bloodstream infections in patients at a university hospital: Virulence factors and clinical characteristics. Front. Cell. Infect. Microbiol. 2019, 9, 191. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonnen, A.F.-P.; Henneke, P. Role of pore-forming toxins in neonatal sepsis. Clin. Dev. Immunol. 2013, 2013, 608456. [Google Scholar] [CrossRef]
- Giedraitienė, A.; Vitkauskienė, A.; Pavilonis, A.; Patamsytė, V.; Genel, N.; Decre, D.; Arlet, G. Prevalence of O25b-ST131 clone among Escherichia coli strains producing CTX-M-15, CTX-M-14 and CTX-M-92 β-lactamases. Infect. Dis. 2017, 49, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Prakapaite, R.; Saab, F.; Planciuniene, R.; Petraitis, V.; Walsh, T.J.; Petraitiene, R.; Semoskaite, R.; Baneviciene, R.; Kalėdienė, L.; Kavaliauskas, P. Molecular characterization of uropathogenic Escherichia coli reveals emergence of drug resistant O15, O22 and O25 serogroups. Medicina 2019, 55, 733. [Google Scholar] [CrossRef] [Green Version]
- Usein, C.-R.; Papagheorghe, R.; Oprea, M.; Condei, M.; Strãuţ, M. Molecular characterization of bacteremic Escherichia coli isolates in Romania. Folia Microbiol. 2015, 61, 221–226. [Google Scholar] [CrossRef]
- Fratamico, P.M.; DebRoy, C.; Liu, Y.; Needleman, D.S.; Baranzoni, G.M.; Feng, P. Advances in molecular serotyping and subtyping of Escherichia coli. Front. Microbiol. 2016, 7, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurieva, T.; Dautzenberg, M.J.D.; Gniadkowski, M.; Derde, L.P.G.; Bonten, M.J.M.; Bootsma, M.C.J. The transmissibility of antibiotic-resistant Enterobacteriaceae in intensive care units. Clin. Infect. Dis. 2017, 66, 489–493. [Google Scholar] [CrossRef] [Green Version]
| Gene | Reference to Gene Primers | |
|---|---|---|
| Antibiotic Resistance Genes | Resistance Mechanism | |
| blaNDM | β-lactams resistance genes | [11] |
| blaKPC | ||
| Multiplex I TEM | [12] | |
| Multiplex SHV | ||
| Multiplex II CTX-M group 1 | ||
| Multiplex II CTX-M group 2 | ||
| Multiplex II CTX-M group 9 | ||
| Multiplex III CTX-M group 8/25 | ||
| blaCMY-2 | [13] | |
| blaIMP | [14] | |
| blaVIM | ||
| blaSPM | ||
| blaAIM | ||
| blaGIM | ||
| blaSIM | ||
| blaDIM | ||
| fosA | Fosfomycin resistance gene (glutathione S-transferase) | [15] |
| aadA | Aminoglycoside resistance genes | [16] |
| strA | ||
| strB | ||
| aac(3)IV | ||
| tetA | Tetracycline resistance genes | [16] |
| tetB | ||
| tetC | ||
| sul1 | Sulphonamide resistance genes | [16] |
| sul2 | ||
| sul3 | ||
| mcr-1 | Colistin resistance gene | [17] |
| Virulence genes | Virulence mechanism | |
| Siderophores | ||
| iroN | salmocherin | [18] |
| fyuA | yersiniabactin | |
| Adhesins | ||
| sfaD/E | S fimbriae | [19] |
| papC | P fimbriae | |
| afaB/C | Dr binding adhesin | |
| fimH | type 1 fimbriae | [18] |
| Toxins | ||
| hlyA | α-hemolysin | [20] |
| cnf1 | necrotizing cytotoxic factor type 1 | |
| sat | autotransporter toxin | [21] |
| cvaC | colicin V | [18] |
| ibeA | Ibe A cell invasin | |
| Serum resistance | ||
| kpsMTII | K1 and K5 capsules | [18] |
| traT | complement resistance protein | |
| Resistance Genes Group | Resistance Gene | Total Number of Isolates (n = 256), n (%) | Number of Isolates in 2014 (n = 91), n (%) | Number of Isolates in 2018 (n = 165), n (%) | % of Increase (+%)/or Decrease (−%) | p Value |
|---|---|---|---|---|---|---|
| β-lactams resistance genes | NDM | 252 (98.4%) | 88 (96.7%) | 164 (99.4%) | +2.7% | >0.05 |
| KPC | 186 (72.7%) | 37 (40.7%) | 149 (90.3%) | +49.6% | 0.00002 | |
| TEM | 253 (98.8%) | 90 (98.9%) | 163 (98.8%) | +0.1% | >0.05 | |
| AIM | 249 (97.3%) | 89 (97.8%) | 160 (96.7%) | −1.1% | >0.05 | |
| CTX-M-1 | 87 (34%) | 45 (49.5%) | 42 (25.5%) | −24% | 0.009 | |
| CTX-M-2 | 35 (13.7%) | 11 (12.1%) | 24 (14.5%) | +2.4% | >0.05 | |
| CTX-M-9 | 232 (90.6%) | 90 (98.9%) | 142 (86.1%) | −12.8% | >0.05 | |
| IMP | 227 (88.7%) | 77 (84.6%) | 150 (90.9%) | +6.3% | >0.05 | |
| VIM | 134 (52.3%) | 36 (39.5%) | 98 (59.4%) | +19.9% | 0.050 | |
| CMY-2 | 183 (71.5%) | 54 (59.3%) | 129 (78.2%) | +18.9% | >0.05 | |
| Sulphonamide resistance genes | sul1 | 198 (77.3%) | 63 (69.2%) | 135 (81.8%) | +12.6% | >0.05 |
| sul2 | 254 (99.2%) | 90 (98.9%) | 164 (99.4%) | +0.5% | >0.05 | |
| sul3 | 237 (92.6%) | 91 (100%) | 146 (88.5%) | −11.5% | >0.05 | |
| Aminoglycoside resistance genes | strA | 252 (97.7%) | 90 (98.9%) | 162 (98.2%) | −0.7% | >0.05 |
| strB | 254 (99.2%) | 91 (100%) | 163 (98.8%) | −1.2% | >0.05 | |
| aac3 (IV) | 45 (17.6%) | 9 (9.9%) | 36 (21.8%) | +11.9% | 0.037 | |
| aadA | 237 (92.6%) | 82 (90.1%) | 155 (93.8%) | +3.7% | >0.05 | |
| Tetracycline resistance genes | tetA | 256 (100%) | 91 (100%) | 165 (100%) | 0 | >0.05 |
| tetB | 53 (20.7%) | 23 (25.3%) | 30 (18.2%) | −7.1% | >0.05 | |
| tetC | 38 (14.8%) | 23 (25.3%) | 15 (9.1%) | −16.2% | 0.0009 | |
| Fosfomycin resistance gene | fosA | 249 (97.3%) | 88 (96.7%) | 161 (97.6%) | +0.9% | >0.05 |
| Colistin resistance gene | mrc-1 | 13 (5.1%) | 8 (8.8%) | 5 (3.03%) | −5.77% | 0.043 |
| Virulence Factor | Genes | Number of Isolates in Healthcare Institutions (n = 256), n (%) | Number of Isolates in 2014 (n = 91), n (%) | Number of Isolates in 2018 (n = 165), n (%) | % of Increase (+%)/or Decrease (−%) | p Value |
|---|---|---|---|---|---|---|
| Toxins | hlyA | 104 (40.6%) | 21 (23.1%) | 83 (50.3%) | +27.2% | 0.004 |
| cnf1 | 86 (33.6%) | 16 (17.6%) | 70 (42.2%) | +24.6% | 0.003 | |
| cvaC | 46 (18%) | 23 (25.3%) | 23 (13.9%) | −11.4% | 0.018 | |
| ibeA | 17 (6.6%) | 10 (11%) | 7 (4.2%) | −6.8% | 0.027 | |
| sat | 138 (53.9%) | 40 (44%) | 98 (59.4%) | +15.4 | >0.050 | |
| Adhesins | staD/E | 122 (47.7%) | 31 (34.1%) | 91 (55.2%) | +21.1 | 0.05 |
| fimH | 252 (98.4%) | 89 (97.8%) | 163 (98.8%) | +1% | >0.05 | |
| papC | 47 (18.4%) | 28 (30.8%) | 19 (11.5%) | −19.3% | 0.0002 | |
| afaB/C | 34 (13.3%) | 19 (20.9%) | 15 (9.1%) | −11.8% | 0.006 | |
| Siderophores | fyuA | 235 (91.8%) | 81 (89%) | 154 (93.3%) | +4.3% | >0.05 |
| iroN | 161 (62.9%) | 54 (59.3%) | 107 (64.8%) | +5.5% | >0.05 | |
| Serum resistance | traT | 208 (81.3%) | 69 (75.8%) | 139 (84.2%) | +8.4% | >0.05 |
| kpsMTII | 126 (49.2%) | 47 (51.6%) | 79 (47.9%) | −3.7% | >0.05 |
| Phylogenetic Group A | Phylogenetic Group B1 | Phylogenetic Group B2 | Phylogenetic Group F | |
|---|---|---|---|---|
| Total number of isolates (n = 256), n/% | 203/79.3% | 2/0.8% | 40/15.6% | 11/4.3% |
| Number of isolates in 2014 (n = 91), n/% | 77/84.6% | 2/2.2% | 10/11% | 2/2.2% |
| Number of isolates in 2018 (n = 165), n/% | 126/76.4% | - | 30/18.2% | 9/5.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirtikliene, T.; Mierauskaitė, A.; Razmienė, I.; Kuisiene, N. Genetic Characterization of Multidrug-Resistant E. coli Isolates from Bloodstream Infections in Lithuania. Microorganisms 2022, 10, 449. https://doi.org/10.3390/microorganisms10020449
Kirtikliene T, Mierauskaitė A, Razmienė I, Kuisiene N. Genetic Characterization of Multidrug-Resistant E. coli Isolates from Bloodstream Infections in Lithuania. Microorganisms. 2022; 10(2):449. https://doi.org/10.3390/microorganisms10020449
Chicago/Turabian StyleKirtikliene, Tatjana, Aistė Mierauskaitė, Ilona Razmienė, and Nomeda Kuisiene. 2022. "Genetic Characterization of Multidrug-Resistant E. coli Isolates from Bloodstream Infections in Lithuania" Microorganisms 10, no. 2: 449. https://doi.org/10.3390/microorganisms10020449
APA StyleKirtikliene, T., Mierauskaitė, A., Razmienė, I., & Kuisiene, N. (2022). Genetic Characterization of Multidrug-Resistant E. coli Isolates from Bloodstream Infections in Lithuania. Microorganisms, 10(2), 449. https://doi.org/10.3390/microorganisms10020449






