Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Cultivation
2.2. Protein Extraction and Enzymatic Activity Analysis
2.3. Protein Secretion and Signal Peptide
2.4. Statistical Analysis
3. Results
3.1. Sugar Utilization
3.2. Enzymatic Activities in the Cytosol
3.3. Enzymatic Activities in the Culture Medium
4. Discussion
4.1. Utilization of Plant Sugars
4.2. Cytoplasmic Activity of Glycolytic Enzymes
4.3. Extracellular Activities of Glycolytic Enzymes
4.4. Secretion
4.5. A New Holobiontic Model
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.-H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Butehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a New Root-Colonizing Fungus. Mycologia 1998, 90, 896. [Google Scholar] [CrossRef]
- Varma, A.; Verma, S.; Sahay, N. Piriformospora Indica, Ble Plant-Growth-Promoting Root Endophyte. Appl. Environ. Microbiol. 1999, 65, 22–26. [Google Scholar] [CrossRef]
- Banhara, A.; Ding, Y.; Kühner, R.; Zuccaro, A.; Parniske, M. Colonization of root cells and plant growth promotion by Piriformospora indica occurs independently of plant common symbiosis genes. Front. Plant Sci. 2015, 6, 667. [Google Scholar] [CrossRef]
- Fakhro, A.; Andrade-Linares, D.R.; Von Bargen, S.; Bandte, M.; Büttner, C.; Grosch, R.; Schwarz, D.; Franken, P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza 2010, 20, 191–200. [Google Scholar] [CrossRef]
- Gill, S.S.; Gill, R.; Trivedi, D.K.; Anjum, N.A.; Sharma, K.K.; Ansari, M.W.; Ansari, A.A.; Johri, A.K.; Prasad, R.; Pereira, E.; et al. Piriformospora indica: Potential and Significance in Plant Stress Tolerance. Front. Microbiol. 2016, 7, 332. [Google Scholar] [CrossRef]
- Varma, A.; Verma, S.; Sahay, N.; Tehorn, B.B.; Franken, P.; Varma, A.; Sahay, N.; Bütehorn, B.; Franken, P.V.S.S. Piriformospora Indica, a Cultivable Plant-Growth-Promoting Root Endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef]
- Riess, K.; Oberwinkler, F.; Bauer, R.; Garnica, S. Communities of Endophytic Sebacinales Associated with Roots of Herbaceous Plants in Agricultural and Grassland Ecosystems Are Dominated by Serendipita herbamans sp. nov. PLoS ONE 2014, 9, e94676. [Google Scholar] [CrossRef][Green Version]
- Blechert, O.; Kost, G.; Hassel, A.; Rexer, K.-H.; Varma, A. First Remarks on the Symbiotic Interaction Between Piriformospora indica and Terrestrial Orchids. In Mycorrhiza; Springer: Singapore, 1999; pp. 683–688. [Google Scholar]
- Vohník, M.; Pánek, M.; Fehrer, J.; Selosse, M.-A. Experimental evidence of ericoid mycorrhizal potential within Serendipitaceae (Sebacinales). Mycorrhiza 2016, 26, 831–846. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant–fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef]
- Gianinazzi-Pearson, V. Plant Cell Responses to Arbuscular Mycorrhizal Fungi: Getting to the Roots of the Symbiosis. Plant Cell 1996, 8, 1871–1883. [Google Scholar] [CrossRef][Green Version]
- Parniske, M. Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 2004, 7, 414–421. [Google Scholar] [CrossRef]
- Harrison, M.J. Signaling in the Arbuscular Mycorrhizal Symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef]
- Zuccaro, A.; Lahrmann, U.; Güldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M.; et al. Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont Piriformospora indica. PLoS Pathog. 2011, 7, e1002290. [Google Scholar] [CrossRef]
- Rani, M.; Raj, S.; Dayaman, V.; Kumar, M.; Dua, M.; Johri, A.K. Functional Characterization of a Hexose Transporter from Root Endophyte Piriformospora indica. Front. Microbiol. 2016, 7, 1083. [Google Scholar] [CrossRef]
- Kaschuk, G.; Hungria, M.; Leffelaar, P.A.; Giller, K.E.; Kuyper, T.W. Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol. 2010, 12, 60–69. [Google Scholar] [CrossRef]
- Paul, M.J.; Pellny, T.K. Carbon metabolite feedback regulation of leaf photosynthesis and development. J. Exp. Bot. 2003, 54, 539–547. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink regulation of photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Ramon, M.; Rolland, F.; Sheen, J. Sugar Sensing and Signaling. Arab. Book 2008, 6, e0117. [Google Scholar] [CrossRef]
- Jang, J.C.; Sheen, J. Sugar Sensing in Higher Plants. Trends Plant Sci. 1997, 15, 773–785. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. d-Mannose: Properties, Production, and Applications: An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef]
- Engel, J.; Schmalhorst, P.; Routier, F.H. Biosynthesis of the Fungal Cell Wall Polysaccharide Galactomannan Requires Intraluminal GDP-mannose. J. Biol. Chem. 2012, 287, 44418–44424. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Sasaki, M.; Vertès, A.A.; Inui, M.; Yukawa, H. Identification and Functional Analysis of the Gene Cluster for l-Arabinose Utilization in Corynebacterium glutamicum. Appl. Environ. Microbiol. 2009, 75, 3419–3429. [Google Scholar] [CrossRef]
- Xiao, H.; Gu, Y.; Ning, Y.; Yang, Y.; Mitchell, W.J.; Jiang, W.; Yang, S. Confirmation and Elimination of Xylose Metabolism Bottlenecks in Glucose Phosphoenolpyruvate-Dependent Phosphotransferase System-Deficient Clostridium acetobutylicum for Simultaneous Utilization of Glucose, Xylose, and Arabinose. Appl. Environ. Microbiol. 2011, 77, 7886–7895. [Google Scholar] [CrossRef]
- Saltman, P. Hexokinase in Higher Plants. J. Biol. Chem. 1953, 200, 145–154. [Google Scholar] [CrossRef]
- Bessell, E.M.; Foster, A.B.; Westwood, J.H. The use of deoxyfluoro-d-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem. J. 1972, 128, 199–204. [Google Scholar] [CrossRef]
- Conesa, A.; Punt, P.J.; Van Luijk, N.; van den Hondel, C.A.M.J.J. The Secretion Pathway in Filamentous Fungi: A Biotechnoloical View. Fungal Genet. Biol. 2001, 33, 155–171. [Google Scholar] [CrossRef]
- Miura, N.; Ueda, M. Evaluation of Unconventional Protein Secretion by Saccharomyces cerevisiae and other Fungi. Cells 2018, 7, 128. [Google Scholar] [CrossRef]
- Gil-Bona, A.; Llama-Palacios, A.; Parra, C.M.; Vivanco, F.; Nombela, C.; Monteoliva, L.; Gil, C. Proteomics Unravels Extracellular Vesicles as Carriers of Classical Cytoplasmic Proteins in Candida albicans. J. Proteome Res. 2015, 14, 142–153. [Google Scholar] [CrossRef]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.P.; Nosanchuk, J.D.; Cordero, R.J.B.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of Yeast Extracellular Vesicles: Evidence for the Participation of Different Pathways of Cellular Traffic in Vesicle Biogenesis. PLoS ONE 2010, 5, e11113. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Nakayasu, E.S.; Matsuo, A.L.; Sobreira, T.J.P.; Longo, L.V.G.; Ganiko, L.; Almeida, I.C.; Puccia, R. Vesicle and Vesicle-Free Extracellular Proteome of Paracoccidioides brasiliensis: Comparative Analysis with Other Pathogenic Fungi. J. Proteome Res. 2012, 11, 1676–1685. [Google Scholar] [CrossRef]
- Yang, C.-K.; Ewis, H.E.; Zhang, X.; Lu, C.-D.; Hu, H.-J.; Pan, Y.; Abdelal, A.T.; Tai, P.C. Nonclassical Protein Secretion by Bacillus subtilis in the Stationary Phase Is Not Due to Cell Lysis. J. Bacteriol. 2011, 193, 5607–5615. [Google Scholar] [CrossRef]
- Yang, C.-K.; Zhang, X.-Z.; Lu, C.-D.; Tai, P.C. An internal hydrophobic helical domain of Bacillus subtilis enolase is essential but not sufficient as a non-cleavable signal for its secretion. Biochem. Biophys. Res. Commun. 2014, 446, 901–905. [Google Scholar] [CrossRef]
- de Oliveira, A.R.; Oliveira, L.N.; Chaves, E.; Weber, S.S.; Bailão, A.M.; Parente-Rocha, J.A.; Baeza, L.C.; Soares, C.M.D.A.; Borges, C.L. Characterization of extracellular proteins in members of the Paracoccidioides complex. Fungal Biol. 2018, 122, 738–751. [Google Scholar] [CrossRef]
- Nizam, S.; Qiang, X.; Wawra, S.; Nostadt, R.; Getzke, F.; Schwanke, F.; Dreyer, I.; Langen, G.; Zuccaro, A. Serendipita indica E5′ NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep. 2019, 20, 47430. [Google Scholar] [CrossRef]
- Thürich, J.; Meichsner, D.; Furch, A.C.U.; Pfalz, J.; Krüger, T.; Kniemeyer, O.; Brakhage, A.; Oelmüller, R. Arabidopsis thaliana responds to colonisation of Piriformospora indica by secretion of symbiosis-specific proteins. PLoS ONE 2018, 13, e0209658. [Google Scholar] [CrossRef]
- Jammer, A.; Gasperl, A.; Luschin-Ebengreuth, N.; Heyneke, E.; Chu, H.; Cantero-Navarro, E.; Großkinsky, D.K.; Albacete, A.A.; Stabentheiner, E.; Franzaring, J.; et al. Simple and robust determination of the activity signature of key carbohydrate metabolism enzymes for physiological phenotyping in model and crop plants. J. Exp. Bot. 2015, 66, 5531–5542. [Google Scholar] [CrossRef]
- Pontecorvo, G.; Roper, J.; Chemmons, L.; Macdonald, K.; Bufton, A. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 5, 141–238. [Google Scholar] [CrossRef]
- Bonfig, K.B.; Gabler, A.; Simon, U.K.; Luschin-Ebengreuth, N.; Hatz, M.; Berger, S.; Muhammad, N.; Zeier, J.; Sinha, A.K.; Roitsch, T. Post-Translational Derepression of Invertase Activity in Source Leaves via Down-Regulation of Invertase Inhibitor Expression Is Part of the Plant Defense Response. Mol. Plant 2010, 3, 1037–1048. [Google Scholar] [CrossRef]
- Deshmukh, S.; Huckelhoven, R.; Schäfer, P.; Imani, J.; Sharma, M.; Weiss, M.; Waller, F.; Kogel, K.-H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. USA 2006, 103, 18450–18457. [Google Scholar] [CrossRef]
- Slewinski, T.L. Diverse Functional Roles of Monosaccharide Transporters and Their Homologs in Vascular Plants: A Physiological Perspective. Mol. Plant 2011, 4, 641–662. [Google Scholar] [CrossRef]
- Seifert, G.; Barber, C.; Wells, B.; Dolan, L.; Roberts, K. Galactose Biosynthesis in Arabidopsis: Genetic Evidence for Substrate Channeling from UDP-D-Galactose into Cell Wall Polymers. Curr. Biol. 2002, 12, 1840–1845. [Google Scholar] [CrossRef]
- Friese, C.; Allen, M. Tracking the fates of exotic and local VA mycorrhizal fungi: Methods and patterns. Agric. Ecosyst. Environ. 1991, 34, 87–96. [Google Scholar] [CrossRef]
- Morton, J.B.; Koske, R.E.; Stürmer, S.L.; Bentivenga, S.P. Mutualistic Arbuscular Endomycorrhizal Fungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier: Amsterdam, The Netherlands, 2004; pp. 317–336. [Google Scholar]
- Giardina, B.J.; Stanley, B.A.; Chiang, H.-L. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci. 2014, 12, 9. [Google Scholar] [CrossRef]
- Stein, K.; Chiang, H.L. Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces Cerevisiae. Membranes 2014, 4, 608–629. [Google Scholar] [CrossRef]
- Büttner, M. The Arabidopsis sugar transporter (AtSTP) family: An update. Plant Biol. 2010, 12, 35–41. [Google Scholar] [CrossRef]
- Rottmann, T.M.; Klebl, F.; Schneider, S.; Kischka, D.; Rüscher, D.; Sauer, N.; Stadler, R. Sugar Transporter STP7 Specificity for l-Arabinose and d-Xylose Contrasts with the Typical Hexose Transporters STP8 and STP12. Plant Physiol. 2018, 176, 2330–2350. [Google Scholar] [CrossRef]
- O’Leary, B.; Neale, H.C.; Geilfus, C.-M.; Jackson, R.; Arnold, D.L.; Preston, G.M. Early changes in apoplast composition associated with defence and disease in interactions Phaseolus Vulgaris and the Halo Blight Pathogen Pseudomonas Syringae Pv. Phaseolicola. Plant Cell Environ. 2016, 39, 2172–2184. [Google Scholar] [CrossRef]
- Lee, E.-J.; Matsumura, Y.; Soga, K.; Hoson, T.; Koizumi, N. Glycosyl Hydrolases of Cell Wall are Induced by Sugar Starvation in Arabidopsis. Plant Cell Physiol. 2007, 48, 405–413. [Google Scholar] [CrossRef]
- Quirino, B.F.; Reiter, W.-D.; Amasino, R.D. One of two tandem Arabidopsis genes homologous to monosaccharide transporters is senescence-associated. Plant Mol. Biol. 2001, 46, 447–457. [Google Scholar] [CrossRef]
- Schüßler, A.; Martin, H.; Cohen, D.; Fitz, M.; Wipf, D. Arbuscular MycorrhizaMycorrhiza—Studies on the Geosiphon Symbiosis Lead to the Characterization of the First Glomeromycotan Sugar Trans-porter. Plant Signal. Behav. 2007, 2, 431–434. [Google Scholar] [CrossRef]
- Helber, N.; Wippel, K.; Sauer, N.; Schaarschmidt, S.; Hause, B.; Requena, N. A Versatile Monosaccharide Transporter That Operates in the Arbuscular Mycorrhizal Fungus Glomus sp Is Crucial for the Symbiotic Relationship with Plants. Plant Cell 2011, 23, 3812–3823. [Google Scholar] [CrossRef]
- Martchenko, M.; Levitin, A.; Hogues, H.; Nantel, A.; Whiteway, M. Transcriptional Rewiring of Fungal Galactose-Metabolism Circuitry. Curr. Biol. 2007, 17, 1007–1013. [Google Scholar] [CrossRef][Green Version]
- Bacon, C.W.; White, J.F. Biotechnology of Endophytic Fungi of Grasses; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-07877-1. [Google Scholar]
- Hadacek, F. Plant root carbohydrates affect growth behaviour of endophytic microfungi. FEMS Microbiol. Ecol. 2002, 41, 161–170. [Google Scholar] [CrossRef]
- Boldt, K.; Pörs, Y.; Haupt, B.; Bitterlich, M.; Kühn, C.; Grimm, B.; Franken, P. Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J. Plant Physiol. 2011, 168, 1256–1263. [Google Scholar] [CrossRef]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. The sucrose transporter Sl SUT 2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. 2014, 78, 877–889. [Google Scholar] [CrossRef]
- Seiboth, B.; Pakdaman, B.S.; Hartl, L.; Kubicek, C.P. Lactose Metabolism in Filamentous Fungi: How to Deal with an Unknown Substrate. Fungal Biol. Rev. 2007, 21, 42–48. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Quinton, L. Essentials of Carbohydrate Chemistry and Biochemistry. Carbohydr. Polym. 2002, 47, 87. [Google Scholar] [CrossRef]
- de Queiroz, C.B.; Santana, M.F. Prediction of the secretomes of endophytic and nonendophytic fungi reveals similarities in host plant infection and colonization strategies. Mycologia 2020, 112, 491–503. [Google Scholar] [CrossRef]
- Ehlert, C.; Plassard, C.; Cookson, S.J.; Tardieu, F.; Simonneau, T. Do pH changes in the leaf apoplast contribute to rapid inhibition of leaf elongation rate by water stress? Comparison of stress responses induced by polyethylene glycol and down-regulation of root hydraulic conductivity. Plant Cell Environ. 2011, 34, 1258–1266. [Google Scholar] [CrossRef]
- Grignon, C.; Sentenac, H. PH and Ionic Conditions in the Apoplast. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 98, 451–463. [Google Scholar] [CrossRef]
- Williams, A.M.; MacLean, D.J.; Scott, K.J. Cellular Location and Properties of Invertase in Mycelium of Puccinia Graminis. New Phytol. 1984, 98, 451–463. [Google Scholar] [CrossRef]
- Nelson, J.M.; Anderson, R.S. Glucose and Fructose Retardation of Invertase Action. J. Biol. Chem. 1926, 69, 443–448. [Google Scholar] [CrossRef]
- Dynesen, J.; Smits, H.P.; Olsson, L.; Nielsen, J. Carbon catabolite repression of invertase during batch cultivations of Saccharomyces cerevisiae: The role of glucose, fructose, and mannose. Appl. Microbiol. Biotechnol. 1998, 50, 579–582. [Google Scholar] [CrossRef]
- Zhang, D. Post-translational inhibitory regulation of acid invertase induced by fructose and glucose in developing apple fruit. Sci. China Ser. C Life Sci. 2002, 45, 309–321. [Google Scholar] [CrossRef]
- Longo, L.V.G.; Da Cunha, J.P.C.; Sobreira, T.J.P.; Puccia, R. Proteome of cell wall-extracts from pathogenic Paracoccidioides brasiliensis: Comparison among morphological phases, isolates, and reported fungal extracellular vesicle proteins. EuPA Open Proteom. 2014, 3, 216–228. [Google Scholar] [CrossRef]
- Weisser, P.; Krämer, R.; A Sprenger, G. Expression of the Escherichia coli pmi gene, encoding phosphomannose-isomerase in Zymomonas mobilis, leads to utilization of mannose as a novel growth substrate, which can be used as a selective marker. Appl. Environ. Microbiol. 1996, 62, 4155–4161. [Google Scholar] [CrossRef]
- Schnarrenberger, C. Characterization and compartmentation, in green leaves, of hexokinases with different specificities for glucose, fructose, and mannose and for nucleoside triphosphates. Planta 1990, 181, 249–255. [Google Scholar] [CrossRef]
- Domsch, W.K.; Gams, T.A. Compendium of Soil Fungi. Institute of Soil Biology. Fed. Agric. Res. Cent. 1993. [Google Scholar] [CrossRef]
- Adomako, D.; Kaye, A.G.; Lewis, D.H. Carbohydrate Metabolism in Chaetomium Globosum. II. Some Properties of Kinases and Isomerases Initiating the Utilization of Sugars. New Phytol. 1971, 70, 699–712. [Google Scholar] [CrossRef]
- Kaffarnik, F.A.R.; Jones, A.; Rathjen, J.; Peck, S.C. Effector Proteins of the Bacterial Pathogen Pseudomonas syringae Alter the Extracellular Proteome of the Host Plant, Arabidopsis thaliana. Mol. Cell. Proteom. 2009, 8, 145–156. [Google Scholar] [CrossRef]
- Floerl, S.; Majcherczyk, A.; Possienke, M.; Feussner, K.; Tappe, H.; Gatz, C.; Feussner, I.; Kües, U.; Polle, A. Verticillium longisporum Infection Affects the Leaf Apoplastic Proteome, Metabolome, and Cell Wall Properties in Arabidopsis thaliana. PLoS ONE 2012, 7, e31435. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Blackburn, K.; Lin, Y.-M.; Goshe, M.B.; Williamson, J.D. Absolute Protein Quantification by LC/MSE for Global Analysis of Salicylic Acid-Induced Plant Protein Secretion Responses. J. Proteome Res. 2009, 8, 82–93. [Google Scholar] [CrossRef]
- Gould, S.J.; Keller, G.-A.; Schneider, M.; Howell, S.H.; Garrard, L.J.; Goodman, J.M.; Distel, B.; Tabak, H. Subramani Peroxisomal Protein Import Is Conserved between Yeast, Plants, Insects and Mammals. Trends Genet. 1990, 6, 73. [Google Scholar] [CrossRef]
- Gould, S.J.; Keller, G.A.; Subramani, S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J. Cell Biol. 1988, 107, 897–905. [Google Scholar] [CrossRef]
- Gould, S.J.; Keller, G.-A.; Hosken, N.; Wilkinson, J.; Subramani, S. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 1989, 108, 1657–1664. [Google Scholar] [CrossRef]
- McCommis, K.S.; Finck, B.N. Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem. J. 2015, 466, 443–454. [Google Scholar] [CrossRef]
- Kwon, Y.; Oh, J.E.; Noh, H.; Hong, S.-W.; Bhoo, S.H.; Lee, H. The ethylene signaling pathway has a negative impact on sucrose-induced anthocyanin accumulation in Arabidopsis. J. Plant Res. 2010, 124, 193–200. [Google Scholar] [CrossRef]
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Yokoyama, R.; Nishitani, K. A Proteomic Approach to Apoplastic Proteins Involved in Cell Wall Regeneration in Protoplasts of Arabidopsis Suspension-cultured Cells. Plant Cell Physiol. 2005, 46, 843–857. [Google Scholar] [CrossRef]
- Kehr, J.; Kragler, F. Long distance RNA movement. New Phytol. 2018, 218, 29–40. [Google Scholar] [CrossRef]
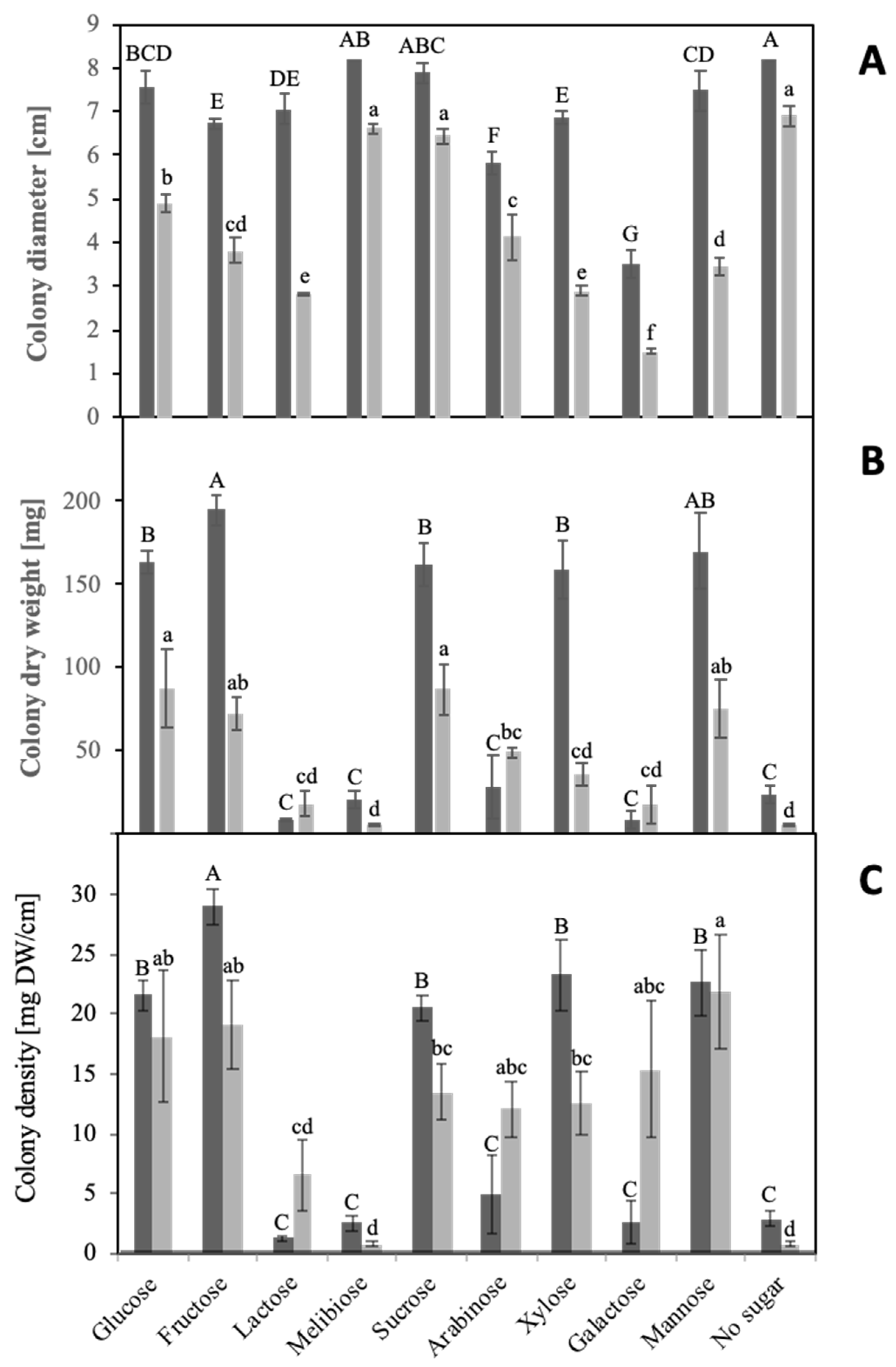
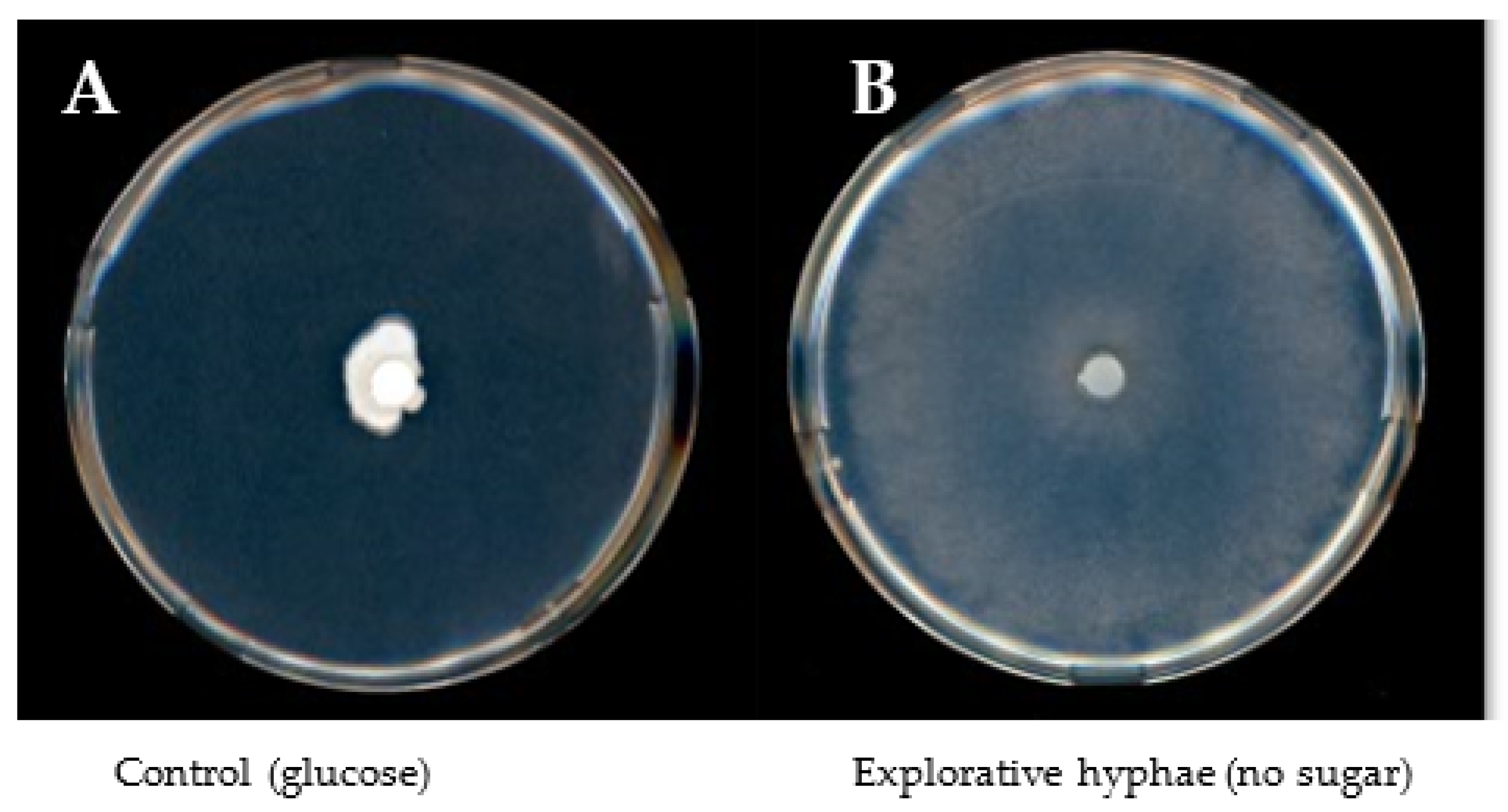
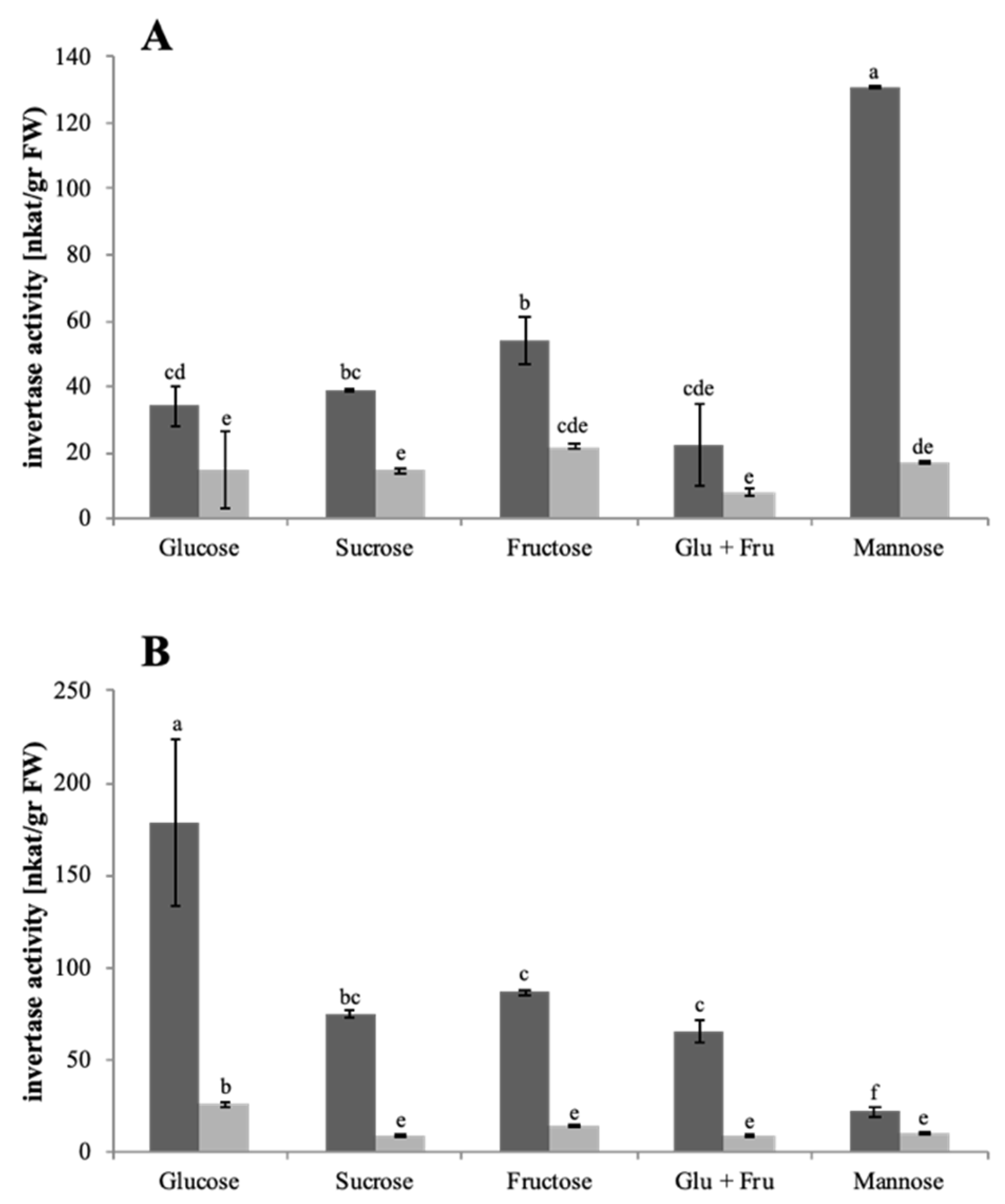
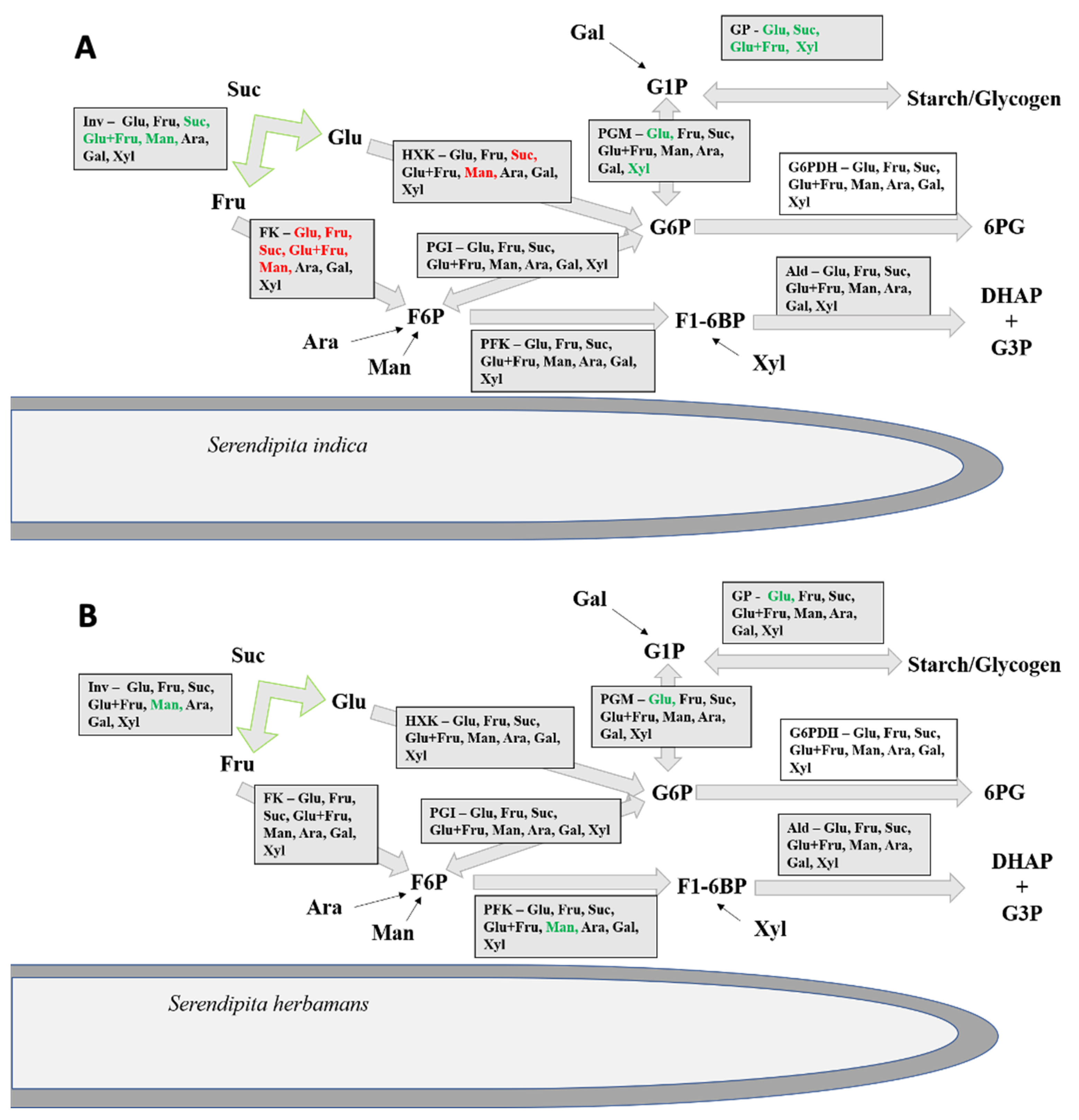
| Sample Fraction | Enzyme | |
|---|---|---|
| Supernatant | Centrifuged raw extract | aldolase, phosphofructokinase, glucose-6-phosphate dehydrogenase, phosphoglucose isomerase, phospho-glucose mutase, UDP-glucose pyrophosphorylase |
| Dialyzed | vacuolar (acidic) invertase, cytosolic (neutral) invertase, hexokinase and fructokinase | |
| S. indica | Glu | Fru | Suc | Glu + Fru | Man |
|---|---|---|---|---|---|
| Aldolase | −0.35 | 1.42 | −0.03 | −0.35 | 1.3 |
| Fructokinase | 1.86 | 3.08 | 2.36 | 1.82 | 3.58 |
| GP | −1.32 | 0.79 | −3.91 | −0.91 | 0.85 |
| Hexokinase | −0.16 | 0.68 | −0.03 | −0.35 | 1.55 |
| Invertase | −0.56 | 0.1 | −0.37 | −1.17 | 1.38 |
| PFK | −1.58 | 2.12 | 0.85 | 0.42 | 4.39 |
| PGI | −5.45 | −6.03 | −4.91 | −5.23 | −6.26 |
| PGM | 0.07 | −0.46 | 0.04 | −0.31 | −1.07 |
| UGPase | 1.14 | 2.7 | 1.85 | 1.85 | 2.81 |
| S. herbamans | Glu | Fru | Suc | Glu + Fru | Man |
|---|---|---|---|---|---|
| Aldolase | −2.26 | −3.35 | −2.65 | −3.34 | −2.18 |
| Fructokinase | 3.95 | 3.23 | 3.58 | 3.46 | 3.88 |
| GP | −0.34 | −2.24 | 1.98 | −2.26 | −0.54 |
| Hexokinase | 0.38 | −0.15 | 0.32 | −0.14 | 0.13 |
| Invertase | −1.79 | −3.05 | −2.84 | −3.24 | −4.82 |
| PFK | −2.55 | −2.47 | −2.31 | −3.44 | −3.34 |
| PGI | −7.35 | −6.54 | −6.59 | −7.01 | −9.90 |
| PGM | 0.02 | 0.72 | 0.53 | 0.26 | −0.95 |
| UGPase | 2.90 | 1.80 | 5.05 | 3.73 | 5.07 |
| S. indica | Glu | Fru | Suc | Glu + Fru | Man | Ara | Gal | Xyl |
|---|---|---|---|---|---|---|---|---|
| Aldolase | −0.60 | −1.39 | −1.72 | −2.16 | 0.26 | −0.18 | −0.55 | −0.49 |
| Fructokinase | −1.38 | −2.03 | −1.91 | −1.71 | −2.48 | −0.59 | −0.75 | −0.62 |
| G6PDH | −1.10 | −1.30 | −1.23 | −0.99 | −1.00 | −0.99 | −0.04 | −1.01 |
| GP | 16.28 | n.d. | 11.81 | 13.81 | n.d. | n.d. | n.d. | 16.33 |
| Hexokinase | −1.45 | −1.21 | −1.80 | −1.66 | −1.95 | −0.29 | −0.07 | −0.32 |
| Invertase | 1.79 | 1.75 | 3.33 | 3.73 | 3.34 | −1.07 | −0.17 | −0.20 |
| PFK | −0.95 | −1.87 | −2.00 | −2.10 | −0.52 | −0.52 | −0.35 | −0.63 |
| PGI | −3.07 | −1.57 | −1.59 | −2.32 | −0.53 | 0.63 | 0.03 | −1.96 |
| PGM | 2.49 | −0.75 | −0.69 | 0.54 | 0.95 | 0.07 | −1.49 | 2.43 |
| S. herbamans | Glu | Fru | Suc | Glu + Fru | Man | Ara | Gal | Xyl |
|---|---|---|---|---|---|---|---|---|
| Aldolase | 1.93 | 2.16 | 1.90 | 1.86 | 2.45 | 1.30 | 1.18 | 1.67 |
| Fructokinase | 1.15 | 0.90 | 0.70 | 0.91 | 1.04 | 0.92 | 0.56 | 0.71 |
| G6PDH | 0.26 | 0.81 | −0.62 | −0.74 | 0.63 | −0.86 | −1.74 | −1.32 |
| GP | 6.17 | 2.00 | 4.31 | 4.57 | 3.67 | 0.49 | 1.32 | 4.83 |
| Hexokinase | 0.85 | 1.07 | 0.34 | 0.39 | 1.34 | 0.68 | 0.32 | 0.92 |
| Invertase | −0.41 | 1.15 | 1.01 | 1.05 | 1.33 | −0.28 | −0.06 | 0.65 |
| PFK | 1.42 | 1.22 | 1.18 | 1.09 | 1.87 | 0.79 | 0.41 | 0.96 |
| PGI | −0.69 | 0.88 | −0.28 | −0.37 | 0.69 | −0.24 | −0.37 | −0.67 |
| PGM | 4.28 | 2.64 | 3.02 | 2.75 | 2.55 | 1.58 | 0.81 | 3.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rocchis, V.; Roitsch, T.; Franken, P. Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars. Microorganisms 2022, 10, 320. https://doi.org/10.3390/microorganisms10020320
De Rocchis V, Roitsch T, Franken P. Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars. Microorganisms. 2022; 10(2):320. https://doi.org/10.3390/microorganisms10020320
Chicago/Turabian StyleDe Rocchis, Vincenzo, Thomas Roitsch, and Philipp Franken. 2022. "Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars" Microorganisms 10, no. 2: 320. https://doi.org/10.3390/microorganisms10020320
APA StyleDe Rocchis, V., Roitsch, T., & Franken, P. (2022). Extracellular Glycolytic Activities in Root Endophytic Serendipitaceae and Their Regulation by Plant Sugars. Microorganisms, 10(2), 320. https://doi.org/10.3390/microorganisms10020320





