Molecular and Pigment Analyses Provide Comparative Results When Reconstructing Historic Cyanobacterial Abundances from Lake Sediment Cores
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sediment Core Sampling
2.2. Cyanobacteria Primers Modification
2.3. Water Content Determination
2.4. DNA Extraction and Droplet Digital PCR
2.5. Pigment Extraction and High-Performance Liquid Chromatography
2.6. Data Analysis
3. Results
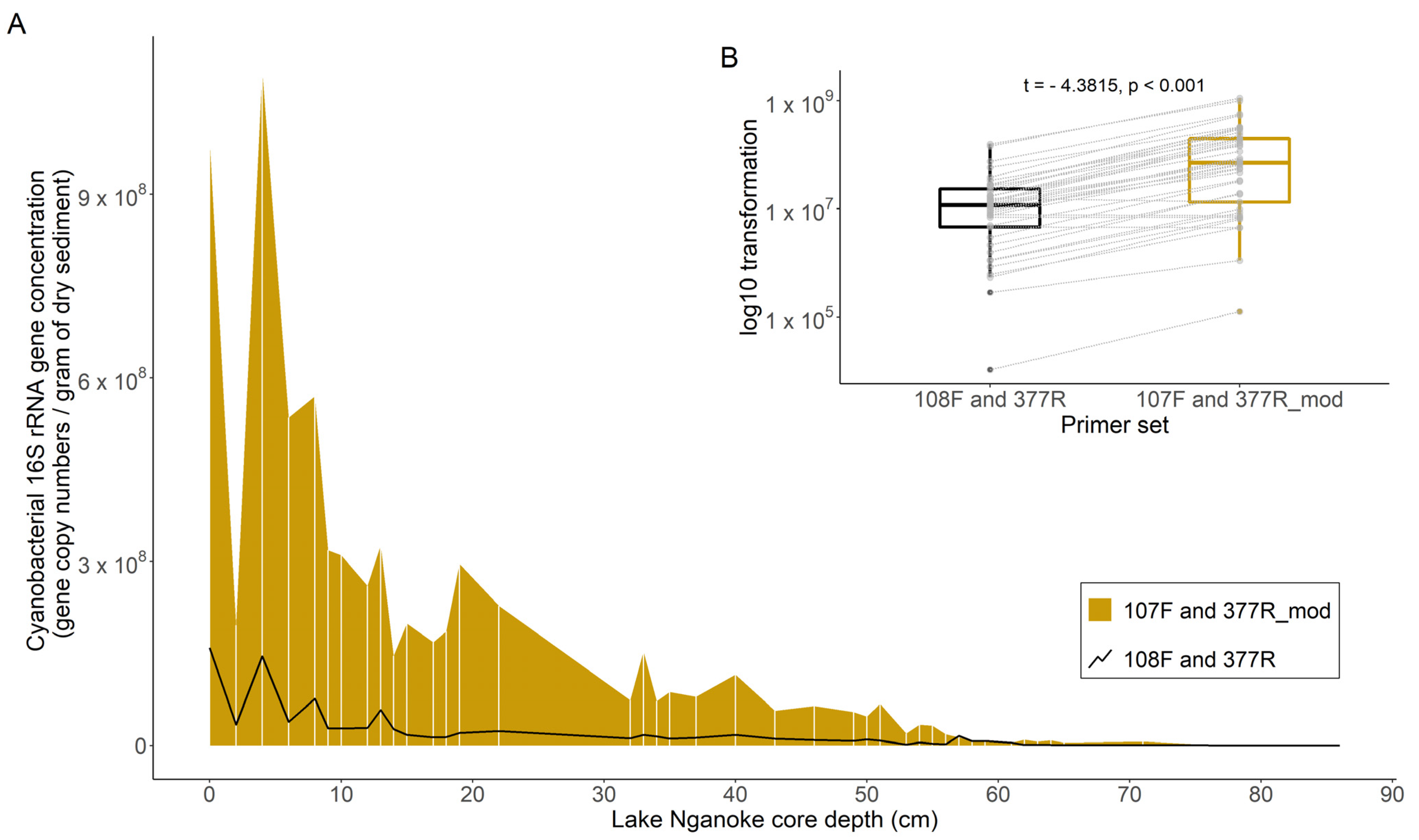
3.1. Cyanobacterial 16S rRNA Gene Primer Comparison
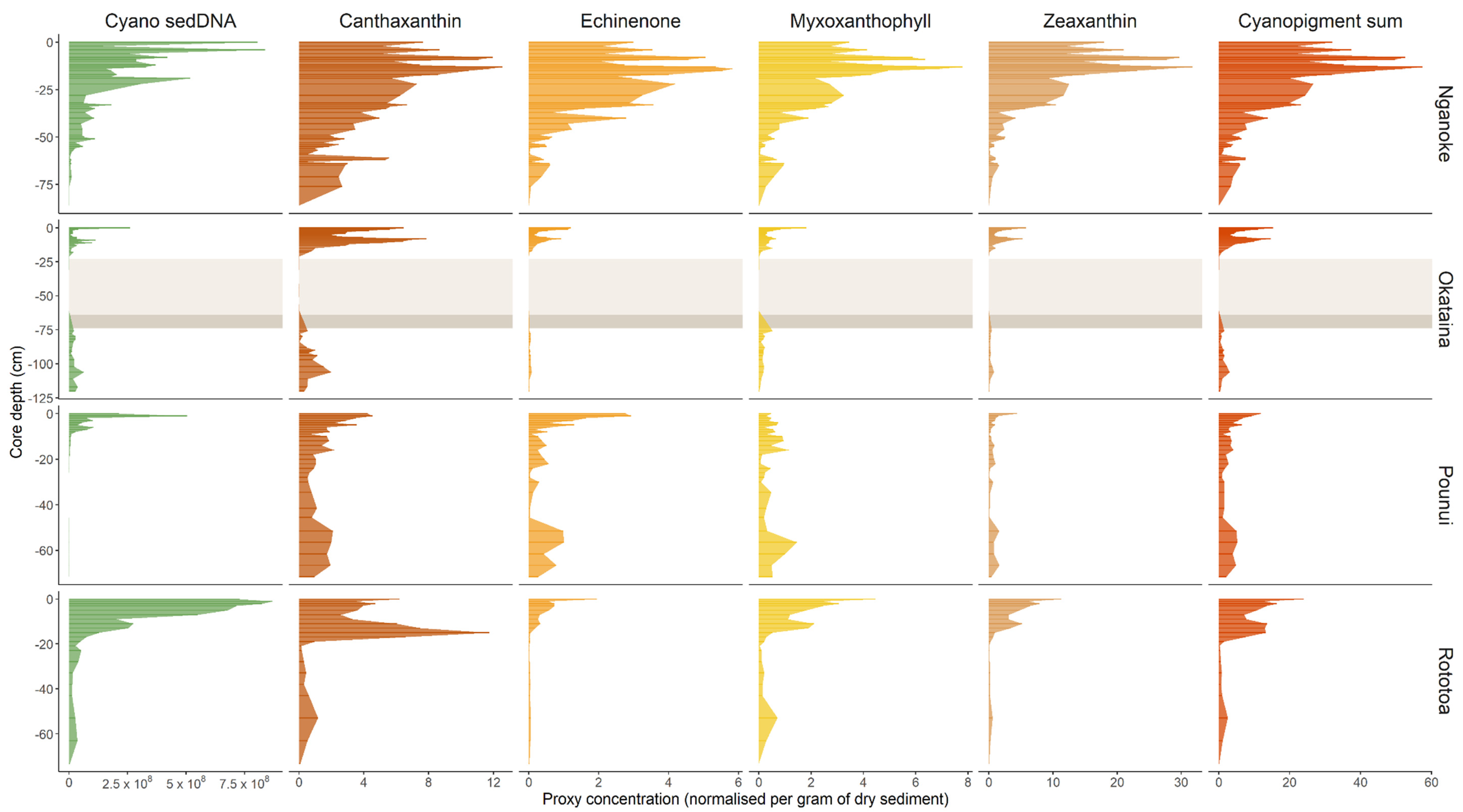
3.2. Cyanobacterial 16S rRNA Genes in Sediment Cores
3.3. Cyanobacterial Pigments
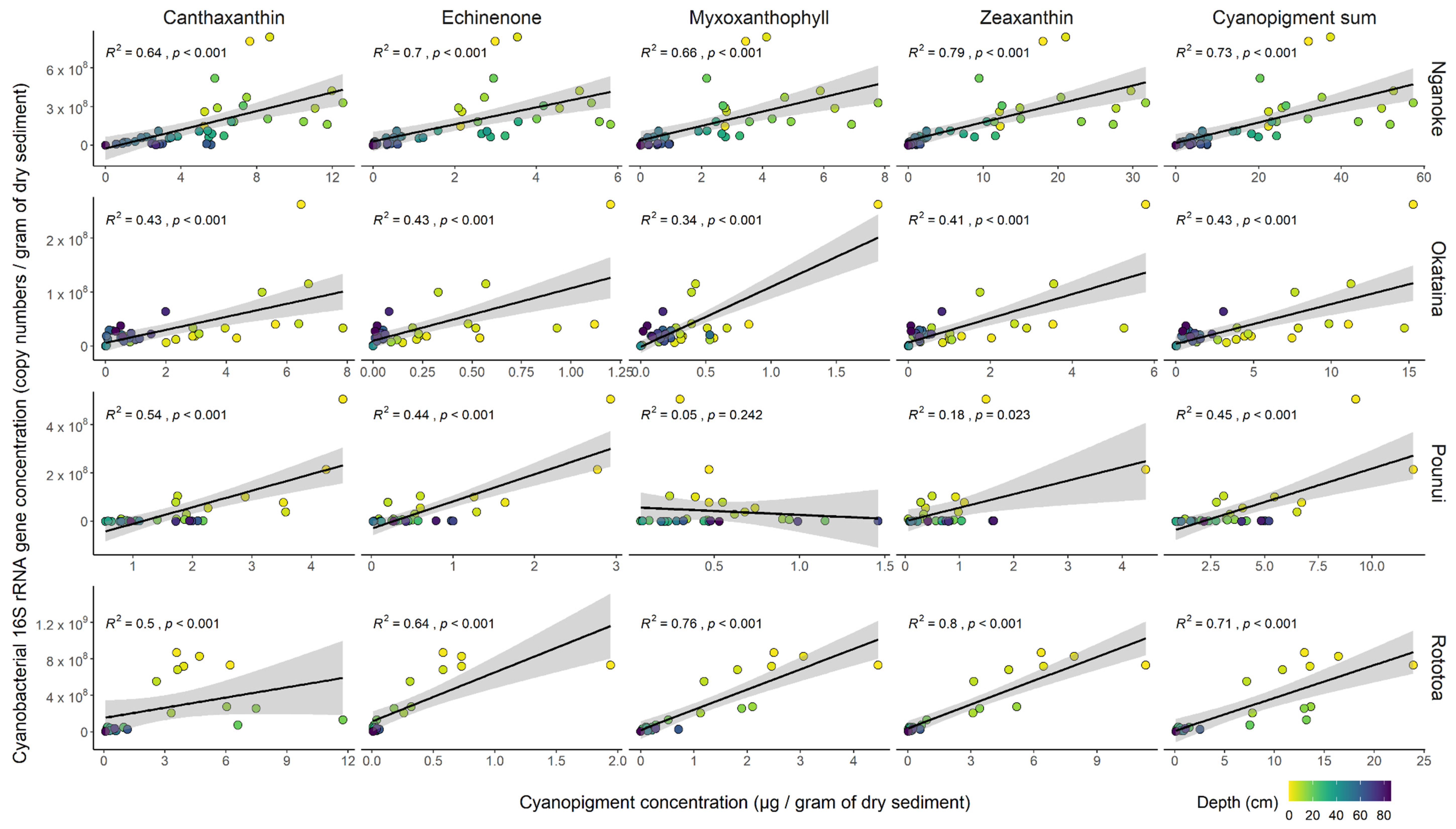
3.4. Proxy Relationships within Each Lake
4. Discussion
4.1. Cyanobacterial-Specific Primer Design and Testing
4.2. Correlation between Cyanobacterial 16S Ribosomal RNA Gene Copies and Cyanopigments
4.3. The Sum of All Cyanopigments—A Better Proxy for Total Cyanobacterial Biomass?
4.4. Reasons for Discrepancies between the Two Methods
4.5. Comparing the Pros and Cons of ddPCR and HPLC to Track Historic Cyanobacteria Abundances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balmford, A.; Bond, W. Trends in the State of Nature and Their Implications for Human Well-Being. Ecol. Lett. 2005, 8, 1218–1234. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Hunt, J.; Mason, P.; Wheater, H.; Wolf, P.; Hunt, J.C.R. Floods in a Changing Climate: A review. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2002, 360, 1531–1543. [Google Scholar] [CrossRef]
- Ellis, E.C.; Kaplan, J.O.; Fuller, D.Q.; Vavrus, S.; Goldewijk, K.K.; Verburg, P.H. Used Planet: A Global History. Proc. Natl. Acad. Sci. USA 2013, 110, 7978–7985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, A.M.; Farnsworth, E.J. Anthropogenic Disturbance of Caribbean Mangrove Ecosystems: Past Impacts, Present Trends, and Future Predictions. Biotropica 1996, 28, 549–565. [Google Scholar] [CrossRef]
- Hazeleger, W.; van den Hurk, B.J.J.M.; Min, E.; Van Oldenborgh, G.J.; Petersen, A.C.; Stainforth, D.A.; Vasileiadou, E.; Smith, L.A. Tales of Future Weather. Nat. Clim. Chang. 2015, 5, 107–113. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B. On the Use of Statistical Models to Predict Crop Yield Responses to Climate Change. Agric. For. Meteorol. 2010, 150, 1443–1452. [Google Scholar] [CrossRef]
- Douglas, M.S.V. Paleolimnology|Overview of Paleolimnology. In Encyclopedia of Quaternary Science, 2nd ed.; Elias, S.A., Mock, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 259–270. ISBN 978-0-444-53642-6. [Google Scholar]
- Charette, T.; Prepas, E.E. Wildfire Impacts on Phytoplankton Communities of Three Small Lakes on the Boreal Plain, Alberta, Canada: A Paleolimnological Study. Can. J. Fish. Aquat. Sci. 2003, 60, 584–593. [Google Scholar] [CrossRef]
- Levine, S.N.; Lini, A.; Ostrofsky, M.L.; Bunting, L.; Burgess, H.; Leavitt, P.R.; Reuter, D.; Lami, A.; Guilizzoni, P.; Gilles, E. The Eutrophication of Lake Champlain’s Northeastern Arm: Insights from Paleolimnological Analyses. J. Great Lakes Res. 2011, 38, 35–48. [Google Scholar] [CrossRef]
- Riedinger-Whitmore, M.A.; Whitmore, T.J.; Smoak, J.M.; Brenner, M.; Moore, A.; Curtis, J.; Schelske, C.L. Cyanobacterial Proliferation is a Recent Response to Eutrophication in Many Florida Lakes: A Paleolimnological Assessment. Lake Reserv. Manag. 2005, 21, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Sanger, J.E. Fossil Pigments in Paleoecology and Paleolimnology. Palaeogeogr. Palaeoclim. Palaeoecol. 1988, 62, 343–359. [Google Scholar] [CrossRef]
- Waters, M.N.; Schelske, C.L.; Kenney, W.F.; Chapman, A.D. The Use of Sedimentary Algal Pigments to Infer Historic Algal Communities in Lake Apopka, Florida. J. Paleolimnol. 2005, 33, 53–71. [Google Scholar] [CrossRef]
- Whitton, B.A. Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 2, ISBN 978-94-007-3855-3. [Google Scholar]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins Produced in Cyanobacterial Water Blooms—Toxicity and Risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. Int. J. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A Review of the Global Ecology, Genomics, and Biogeography of the Toxic Cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Genet. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W. Mitigating Harmful Cyanobacterial Blooms in a Human- and Climatically-Impacted World. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.A.; Kelly, L.T.; Bouma-Gregson, K.; Humbert, J.-F.; Iv, H.D.L.; Lazorchak, J.; McAllister, T.G.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic Benthic Freshwater Cyanobacterial Proliferations: Challenges and Solutions for Enhancing Knowledge and Improving Monitoring and Mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef]
- Ewing, H.A.; Weathers, K.C.; Cottingham, K.L.; Leavitt, P.R.; Greer, M.L.; Carey, C.C.; Steele, B.G.; Fiorillo, A.U.; Sowles, J.P. “New” Cyanobacterial Blooms are Not New: Two Centuries of Lake Production are Related to Ice Cover and Land Use. Ecosphere 2020, 11, e03170. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Taranu, Z.E.; MacDonald, G.K.; Velghe, K.; Bennett, E.M.; Gregory-Eaves, I. Extrinsic vs. Intrinsic Regimes Shifts in Shallow Lakes: Long-Term Response of Cyanobacterial Blooms to Historical Catchment Phosphorus Loading and Climate Warming. Front. Ecol. Evol. 2017, 5, 146. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Gregory-Eaves, I.; Pick, F.R. Temporal Trends in Cyanobacteria Revealed through DNA and Pigment Analyses of Temperate Lake Sediment Cores. J. Paleolimnol. 2015, 54, 87–101. [Google Scholar] [CrossRef]
- Monchamp, M.-È.; Bruel, R.; Frossard, V.; McGowan, S.; Lavrieux, M.; Muschick, M.; Perga, M.-É.; Dubois, N. Paleoecological Evidence for a Multi-Trophic Regime Shift in a Perialpine Lake (Lake Joux, Switzerland). Anthropocene 2021, 35, 100301. [Google Scholar] [CrossRef]
- Hobbs, W.O.; Dreher, T.W.; Davis, E.W.; Vinebrooke, R.D.; Wong, S.; Weissman, T.; Dawson, M. Using a Lake Sediment Record to Infer the Long-Term History of Cyanobacteria and the Recent Rise of an Anatoxin Producing Dolichospermum sp. Harmful Algae 2021, 101, 101971. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, B.N.; Tremblay, R.; Pienitz, R.; Vincent, W.F. Sedimentary Pigments as Indicators of Cyanobacterial Dynamics in a Hypereutrophic Lake. J. Paleolimnol. 2014, 52, 171–184. [Google Scholar] [CrossRef]
- Zastepa, A.; Taranu, Z.E.; Kimpe, L.E.; Blais, J.M.; Gregory-Eaves, I.; Zurawell, R.W.; Pick, F.R. Reconstructing a Long-Term Record of Microcystins from the Analysis of Lake Sediments. Sci. Total Environ. 2016, 579, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Coolen, M.J.L.; Talbot, H.M.; Abbas, B.A.; Ward, C.; Schouten, S.; Volkman, J.K.; Sinninghe Damsté, J.S. Sources for Sedimentary Bacteriohopanepolyols as Revealed by 16S RDNA Stratigraphy. Environ. Microbiol. 2008, 10, 1783–1803. [Google Scholar] [CrossRef]
- Monchamp, M.-E.; Spaak, P.; Domaizon, I.; Dubois, N.; Bouffard, D.; Pomati, F. Homogenization of Lake Cyanobacterial Communities over a Century of Climate Change and Eutrophication. Nat. Ecol. Evol. 2018, 2, 317. [Google Scholar] [CrossRef] [Green Version]
- Monchamp, M.-E.; Walser, J.-C.; Pomati, F.; Spaak, P. Sedimentary DNA Reveals Cyanobacterial Community Diversity over 200 Years in Two Perialpine Lakes. Appl. Environ. Microbiol. 2016, 82, 6472–6482. [Google Scholar] [CrossRef] [Green Version]
- Mejbel, H.S.; Dodsworth, W.; Baud, A.; Gregory-Eaves, I.; Pick, F.R. Comparing Quantitative Methods for Analyzing Sediment DNA Records of Cyanobacteria in Experimental and Reference Lakes. Front. Microbiol. 2021, 12, 1532. [Google Scholar] [CrossRef]
- Capo, E.; Giguet-Covex, C.; Rouillard, A.; Nota, K.; Heintzman, P.D.; Vuillemin, A.; Ariztegui, D.; Arnaud, F.; Belle, S.; Bertilsson, S.; et al. Lake Sedimentary DNA Research on Past Terrestrial and Aquatic Biodiversity: Overview and Recommendations. Quaternary 2021, 4, 6. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Beolchini, F.; Dell’anno, A. Damage and Degradation Rates of Extracellular DNA in Marine Sediments: Implications for the Preservation of Gene Sequences. Mol. Ecol. 2008, 17, 3939–3951. [Google Scholar] [CrossRef]
- Domaizon, I.; Winegardner, A.; Capo, E.; Gauthier, J.; Gregory-Eaves, I. DNA-Based Methods in Paleolimnology: New Opportunities for Investigating Long-Term Dynamics of Lacustrine Biodiversity. J. Paleolimnol. 2017, 1, 1–21. [Google Scholar] [CrossRef]
- Wood, S.A.; Pochon, X.; Laroche, O.; von Ammon, U.; Adamson, J.; Zaiko, A. A Comparison of Droplet Digital Polymerase Chain Reaction (PCR), Quantitative PCR and Metabarcoding for Species-Specific Detection in Environmental DNA. Mol. Ecol. Resour. 2019, 19, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, S.; Liaaen-Jensen, S.; Siegelman, H.W. The Carotenoids of Blue-Green Algae. Phytochemistry 1971, 10, 3121–3127. [Google Scholar] [CrossRef]
- Puddick, J.; Naeher, S.; Pearman, J.K.; Page, C.D.; Romanazzi, D.; Schallenberg, L.A.; Howarth, J.D.; Vandergoes, M.J.; Wood, S.A. Carotenoid Ratios Effectively Differentiate between Picocyanobacteria and Bloom-Forming Cyanobacteria in Sediment Cores. J. Paleolimnol. 2021, to be submitted.
- Parrish, J.E. A Paleolimnological Investigation of Agricultural Intensification, Water Quality and Ecosystem Change at Lake Nganoke, Southern Wairarapa, NZ; Victoria University of Wellington: Wellington, New Zealand, 2020. [Google Scholar]
- Caird, S. The Bio-Physial and Ecological Response of Lake Okataina, Bay of Plenty, New Zealand Following the Natural Disturbance of the 1886 Eruption of Mt Tarawera; Victoria University of Wellington: Wellington, New Zealand, 2021. [Google Scholar]
- Pearman, J.K.; Biessy, L.; Howarth, J.D.; Vandergoes, M.J.; Rees, A.; Wood, S.A. Deciphering the Molecular Signal from Past and Alive Bacterial Communities in Aquatic Sedimentary Archives. Mol. Ecol. Resour. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Ouellette, A.J.A.; Boyer, G.L.; Twiss, M.R.; Bridgeman, T.B.; Wilhelm, S.W. Quantification of Toxic Microcystis Spp. during the 2003 and 2004 Blooms in Western Lake Erie Using Quantitative Real-Time PCR. J. Environ. Sci. Technol. 2005, 39, 4198–4205. [Google Scholar] [CrossRef]
- Picard, M.; Pochon, X.; Atalah, J.; Pearman, J.K.; Rees, A.; Howarth, J.D.; Moy, C.M.; Vandergoes, M.J.; Hawes, I.; Khan, S.; et al. Using Metabarcoding and Droplet Digital PCR to Investigate Drivers of Historical Shifts in Cyanobacteria in Six Contrasting Lakes. Sci. Rep. 2021, to be submitted.
- Roy, S.; Llewellyn, C.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, MA, USA, 2011; ISBN 978-0-511-73226-3. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 3 October 2021).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 3 October 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Mihali, T.K.; Pomati, F.; Neilan, B.A. Detection of Saxitoxin-Producing Cyanobacteria and Anabaena Circinalis in Environmental Water Blooms by Quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7836–7842. [Google Scholar] [CrossRef] [Green Version]
- Droplet DigitalTM PCR Application Guide; Bio-Rad Laboratories: Hercules, CA, USA, 2018; p. 145. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf (accessed on 3 October 2021).
- Schirrmeister, B.E.; Dalquen, D.A.; Anisimova, M.; Bagheri, H.C. Gene Copy Number Variation and Its Significance in Cyanobacterial Phylogeny. BMC Microbiol. 2012, 12, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Větrovský, T.; Baldrian, P. The Variability of the 16S RRNA Gene in Bacterial Genomes and Its Consequences for Bacterial Community Analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serive, B.; Nicolau, E.; Bérard, J.-B.; Kaas, R.; Pasquet, V.; Picot, L.; Cadoret, J.-P. Community Analysis of Pigment Patterns from 37 Microalgae Strains Reveals New Carotenoids and Porphyrins Characteristic of Distinct Strains and Taxonomic Groups. PLoS ONE 2017, 12, e0171872. [Google Scholar] [CrossRef] [PubMed]
- Callieri, C. Picophytoplankton in Freshwater Ecosystems: The Importance of Small-Sized Phototrophs. Freshw. Rev. 2008, 1, 1–28. [Google Scholar] [CrossRef]
- Caroppo, C. Ecology and Biodiversity of Picoplanktonic Cyanobacteria in Coastal and Brackish Environments. Biodivers Conserv. 2015, 24, 949–971. [Google Scholar] [CrossRef]
- Rydberg, J.; Cooke, C.A.; Tolu, J.; Wolfe, A.P.; Vinebrooke, R.D. An Assessment of Chlorophyll Preservation in Lake Sediments Using Multiple Analytical Techniques Applied to the Annually Laminated Lake Sediments of Nylandssjön. J. Paleolimnol 2020, 64, 379–388. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Pinheiro, L.B.; Coleman, V.A.; Hindson, C.M.; Herrmann, J.; Hindson, B.J.; Bhat, S.; Emslie, K.R. Evaluation of a Droplet Digital Polymerase Chain Reaction Format for DNA Copy Number Quantification. Anal. Chem. 2012, 84, 1003–1011. [Google Scholar] [CrossRef]
| Primer | Sequence | Source |
|---|---|---|
| CYAN108F | 5′-ACGGGTGAGTAACRCGTRA-3′ | [41] |
| CYAN107F | 5′-GACGGGTGAGTAACRCGTRRG-3’ | This paper |
| CYAN377R | 5′-CCATGGCGGAAAATTCCCC-3′ | [41] |
| CYAN377R_mod | 5’-CCATTGCGGAAAATTCCCC-3’ | This paper |
| Pigment | RT | Wavelength | Equivalence Factor |
|---|---|---|---|
| Lutein | 9.6 | 435–455 | - |
| Zeaxanthin | 10.3 | 435–455 | 1.56 |
| Myxoxanthophyll | 12.7 | 460–480 | 1.26 |
| Canthaxanthin | 16.1 | 460–480 | 1.06 |
| Echinenone | 32.9 | 435–455 | 0.63 |
| Method | Pros | Cons |
|---|---|---|
| HPLC |
|
|
| ddPCR |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picard, M.; Wood, S.A.; Pochon, X.; Vandergoes, M.J.; Reyes, L.; Howarth, J.D.; Hawes, I.; Puddick, J. Molecular and Pigment Analyses Provide Comparative Results When Reconstructing Historic Cyanobacterial Abundances from Lake Sediment Cores. Microorganisms 2022, 10, 279. https://doi.org/10.3390/microorganisms10020279
Picard M, Wood SA, Pochon X, Vandergoes MJ, Reyes L, Howarth JD, Hawes I, Puddick J. Molecular and Pigment Analyses Provide Comparative Results When Reconstructing Historic Cyanobacterial Abundances from Lake Sediment Cores. Microorganisms. 2022; 10(2):279. https://doi.org/10.3390/microorganisms10020279
Chicago/Turabian StylePicard, Maïlys, Susanna A. Wood, Xavier Pochon, Marcus J. Vandergoes, Lizette Reyes, Jamie D. Howarth, Ian Hawes, and Jonathan Puddick. 2022. "Molecular and Pigment Analyses Provide Comparative Results When Reconstructing Historic Cyanobacterial Abundances from Lake Sediment Cores" Microorganisms 10, no. 2: 279. https://doi.org/10.3390/microorganisms10020279
APA StylePicard, M., Wood, S. A., Pochon, X., Vandergoes, M. J., Reyes, L., Howarth, J. D., Hawes, I., & Puddick, J. (2022). Molecular and Pigment Analyses Provide Comparative Results When Reconstructing Historic Cyanobacterial Abundances from Lake Sediment Cores. Microorganisms, 10(2), 279. https://doi.org/10.3390/microorganisms10020279








