Why Are Bifidobacteria Important for Infants?
Abstract
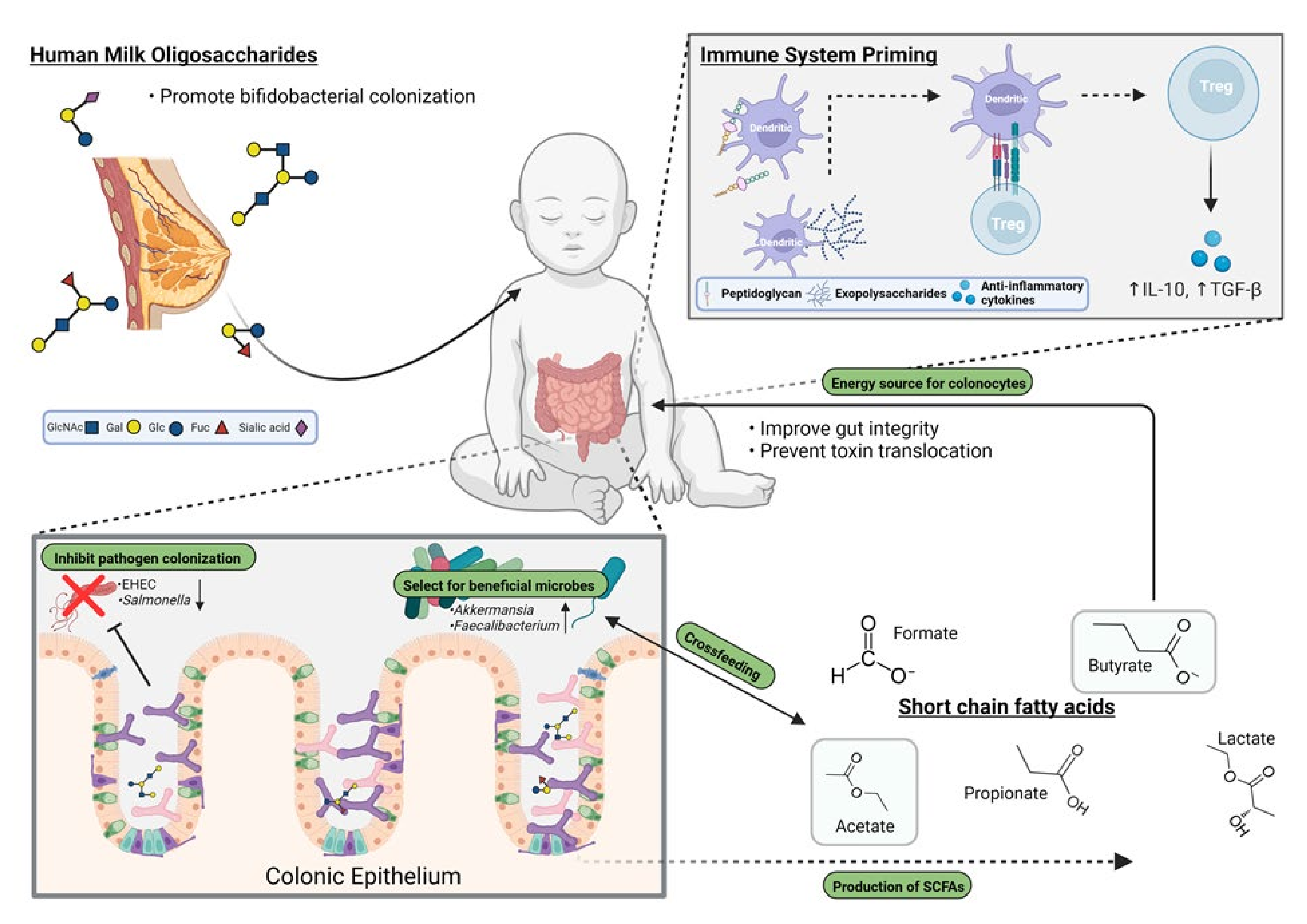
:1. Introduction
2. From Whence They Came
3. Why a Focus on Bifidobacterium?
4. Primary Colonization and Shaping Microbial Composition in the Gut
5. Impact of the Strains on the Host’s Immunity
6. Bifidobacterial Metabolites
7. Further Potential
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hinde, K.; German, J.B. Food in an evolutionary context: Insights from mother’s milk. J. Sci. Food Agric. 2012, 92, 2219–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudley, D.J. The placental microbiome: Yea, nay or maybe? Brit. J. Obstet. Gynecol. 2020, 127, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, W.F.; Ravel, J. Microbiome or no microbiome: Are we looking at the prenatal environment through the right lens? Microbiome 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.; Dixon, J.; Reid, G. Detection of Bifidobacterium species and Gardnerella vaginalis in the vagina using PCR and denaturing gradient gel electrophoresis (DGGE). Int. J. Gynecol. Obstet. 2003, 81, 61–63. [Google Scholar] [CrossRef]
- Sirilun, S.; Takahashi, H.; Boonyaritichaikij, S.; Chaiyasut, C.; Lertruangpanya, P.; Koga, Y.; Mikami, K. Impact of maternal bifidobacteria and the mode of delivery on Bifidobacterium microbiota in infants. Benef. Microbes 2015, 6, 767–774. [Google Scholar] [CrossRef]
- Freitas, A.C.; Hill, J.E. Bifidobacteria isolated from vaginal and gut microbiomes are indistinguishable by comparative genomics. PLoS One 2018, 13, e0196290. [Google Scholar] [CrossRef] [Green Version]
- Turroni, F.; Marchesi, J.R.; Foroni, E.; Gueimonde, M.; Shanahan, F.; Margolles, A.; Van Sinderen, D.; Ventura, M. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J. 2009, 3, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef]
- Stappenbeck, T.S.; Hooper, L.V.; Gordon, J.I. Nonlinear partial differential equations and applications: Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl. Acad. Sci. USA 2002, 99, 15451–15455. [Google Scholar] [CrossRef] [Green Version]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterr. J. Hematol. Infect. Dis. 2016, 8, 2016025. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.D.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, Z.T.; Mills, D.A. Differential establishment of bifidobacteria in the breastfed infant gut. In Global Landscape of Nutrition Challenges in Infants and Children; Karger Medical and Scientific Publishers: Basel, Switzerland, 2017; Volume 88, pp. 149–159. [Google Scholar]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turroni, F.; Milani, C.; Duranti, S.; Ferrario, C.; Lugli, G.A.; Mancabelli, L.; Van Sinderen, D.; Ventura, M. Bifidobacteria and the infant gut: An example of co-evolution and natural selection. Cell. Mol. Life Sci. 2018, 75, 103–118. [Google Scholar] [CrossRef]
- Sakanaka, M.; Gotoh, A.; Yoshida, K.; Odamaki, T.; Koguchi, H.; Xiao, J.-Z.; Kitaoka, M.; Katayama, T. Varied pathways of infant gut-associated Bifidobacterium to assimilate human milk oligosaccharides: Prevalence of the gene set and its correlation with bifidobacteria-rich microbiota formation. Nutrients 2019, 12, 71. [Google Scholar] [CrossRef] [Green Version]
- Devika, N.T.; Raman, K. Deciphering the metabolic capabilities of bifidobacteria using genome-scale metabolic models. Sci. Rep. 2019, 9, 18222. [Google Scholar] [CrossRef] [Green Version]
- Duar, R.M.; Casaburi, G.; Mitchell, R.D.; Scofield, L.N.; Ramirez, C.A.O.; Barile, D.; Henrick, B.M.; Frese, S.A. Comparative genome analysis of Bifidobacterium longum subsp. infantis strains reveals variation in human milk oligosaccharide utilization genes among commercial probiotics. Nutrients 2020, 12, 3247. [Google Scholar] [CrossRef]
- Lawson, M.A.E.; O’neill, I.J.; Kujawska, M.; Javvadi, S.G.; Wijeyesekera, A.; Flegg, Z.; Chalklen, L.; Hall, L.J. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 2019, 14, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taft, D.H.; Liu, J.; Maldonado-Gomez, M.X.; Akre, S.; Huda, M.N.; Ahmad, S.M.; Stephensen, C.B.; Mills, D.A. Bifidobacterial dominance of the gut in early life and acquisition of antimicrobial resistance. mSphere 2018, 3, e00441-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzeskowiak, L.; Collado, M.C.; Mangani, C.; Maleta, K.; Laitinen, K.; Ashorn, P.; Isolauri, E.; Salminen, S. Distinct gut microbiota in southeastern African and northern European infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Ruiz-Moyano, S.; Lemay, D.; Sela, D.A.; German, J.B.; Mills, D.A. Comparative transcriptomics reveals key differences in the response to milk oligosaccharides of infant gut-associated bifidobacteria. Sci. Rep. 2015, 5, 13517. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.A.; Kalanetra, K.M.; Bokulich, N.A.; Lewis, Z.T.; Mirmiran, M.; Tancredi, D.; Mills, D.A. A comparison of two probiotic strains of bifidobacteria in premature infants. J. Pediatr. 2013, 163, 1585–1591.e9. [Google Scholar] [CrossRef] [Green Version]
- De Simone, C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Stuivenberg, G.; Daisley, B.; Akouris, P.; Reid, G. In vitro assessment of histamine and lactate production by a multi-strain synbiotic. J. Food Sci. Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Watson, E.; van der Veer, C.; Chmiel, J.; Carr, C.; Burton, J.; Sumarah, M.; Kort, R.; Reid, G. Interstrain variability of human vaginal Lactobacillus crispatus for metabolism of biogenic amines and antimicrobial activity against urogenital pathogens. Molecules 2021, 26, 4538. [Google Scholar] [CrossRef]
- E Silva, A.C.S.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. 2020, 96 (Suppl. S1), 65–79. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Looijer-van Langen, M.; Madsen, K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar]
- Alessandri, G.; Ossiprandi, M.C.; Mac Sharry, J.; Van Sinderen, D.; Ventura, M. Bifidobacterial dialogue with its human host and consequent modulation of the immune system. Front. Immunol. 2019, 10, 2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Reid, G.; Gadir, A.A.; Barragan, S.P.; Dhir, R. Deconstructing then priming gut microbiota resilience. OBM Hepatol. Gastroenterol. 2021, 5, 9. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [Green Version]
- Eggesbø, M.; Moen, B.; Peddada, S.; Baird, D.; Rugtveit, J.; Midtvedt, T.; Bushel, P.R.; Sekelja, M.; Rudi, K. Development of gut microbiota in infants not exposed to medical interventions. Apmis 2010, 119, 17–35. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Avershina, E.; Lundgård, K.; Sekelja, M.; Dotterud, C.; Storrø, O.; Øien, T.; Johnsen, R.; Rudi, K. Transition from infant- to adult-like gut microbiota. Environ. Microbiol. 2016, 18, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Sutharsan, R.; Mannan, M.; Doi, S.A.; Al Mamun, A. Caesarean delivery and the risk of offspring overweight and obesity over the life course: A systematic review and bias-adjusted meta-analysis. Clin. Obes. 2015, 5, 293–301. [Google Scholar] [CrossRef]
- Korpela, K.; Zijlmans, M.A.C.; Kuitunen, M.; Kukkonen, K.; Savilahti, E.; Salonen, A.; De Weerth, C.; De Vos, W.M. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate- and acetate-based cross-feeding interactions between selected strains of lactobacilli, bifidobacteria and colon bacteria in the presence of inulin-type fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Özcan, E.; Sela, D.A. Inefficient metabolism of the human milk oligosaccharides Lacto-N-tetraose and Lacto-N-neotetraose shifts Bifidobacterium longum subsp. infantis physiology. Front. Nutr. 2018, 5, 46. [Google Scholar] [CrossRef]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Ibrahim, R.; Lambert, K. The gut microbiota profile of adults with kidney disease and kidney stones: A systematic review of the literature. BMC Nephrol. 2020, 21, 215. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Clayton, D.B.; Pope, J.C. The increasing pediatric stone disease problem. Ther. Adv. Urol. 2011, 3, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Kelly, S.M.; Munoz-Munoz, J.; van Sinderen, D. Plant glycan metabolism by bifidobacteria. Front. Microbiol. 2021, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Turroni, F.; Özcan, E.; Milani, C.; Mancabelli, L.; Viappiani, A.; van Sinderen, D.; Sela, D.; Ventura, M. Glycan cross-feeding activities between bifidobacteria under in vitro conditions. Front. Microbiol. 2015, 6, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, M.; Motherway, M.O.; Kilcoyne, M.; Kane, M.; Joshi, L.; Ventura, M.; Van Sinderen, D. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol. 2014, 14, 282. [Google Scholar] [CrossRef] [Green Version]
- Bunesova, V.; Lacroix, C.; Schwab, C. Mucin cross-feeding of infant bifidobacteria and Eubacterium hallii. Microb. Ecol. 2018, 75, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de Los Reyes-Gavilan, C. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boger, M.C.L.; van Bueren, A.L.; Dijkhuizen, L. Cross-feeding among probiotic bacterial strains on prebiotic inulin involves the extracellular exo-inulinase of Lactobacillus paracasei strain W20. Appl. Environ. Microbiol. 2018, 84, e01539-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.C.; Duar, R.M.; Lin, X.; Perez-Munoz, M.E.; Tollenaar, S.; Oh, J.-H.; van Pijkeren, J.-P.; Li, F.; van Sinderen, D.; Gänzle, M.G.; et al. Ecological importance of cross-feeding of the intermediate metabolite 1,2-propanediol between bacterial gut symbionts. Appl. Environ. Microbiol. 2020, 86, e00190-20. [Google Scholar] [CrossRef]
- Munoz, J.; James, K.; Bottacini, F.; Van Sinderen, D. Biochemical analysis of cross-feeding behaviour between two common gut commensals when cultivated on plant-derived arabinogalactan. Microb. Biotechnol. 2020, 13, 1733–1747. [Google Scholar] [CrossRef]
- Neu, J.; Rushing, J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Tian, L.; Luo, J.; Yu, J. Ongoing supplementation of probiotics to caesarean-born neonates during the first month of life may impact the gut microbial. Am. J. Perinatol. 2021, 38, 1181–1191. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motherway, M.O.; Houston, A.; O’Callaghan, G.; Reunanen, J.; O’brien, F.; O’driscoll, T.; Casey, P.G.; De Vos, W.M.; Van Sinderen, D.; Shanahan, F. A bifidobacterial pilus-associated protein promotes colonic epithelial proliferation. Mol. Microbiol. 2019, 111, 287–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werlang, I.C.R.; Mueller, N.T.; Pizoni, A.; Wisintainer, H.; Matte, U.; de Almeida Martins Costa, S.H.; Ramos, J.G.L.; Goldani, M.Z.; Dominguez-Bello, M.G.; Goldani, H.A.S. Associations of birth mode with cord blood cytokines, white blood cells, and newborn intestinal bifidobacteria. PLoS One 2018, 13, e0205962. [Google Scholar] [CrossRef]
- Morais, L.H.; Golubeva, A.V.; Moloney, G.M.; Moya-Pérez, A.; Ventura-Silva, A.P.; Arboleya, S.; Bastiaanssen, T.F.; O’sullivan, O.; Rea, K.; Borre, Y.; et al. Enduring behavioral effects induced by birth by caesarean section in the mouse. Curr. Biol. 2020, 30, 3761–3774.e6. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- He, F.; Ouwehand, A.C.; Isolauri, E.; Hashimoto, H.; Benno, Y.; Salminen, S. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol. Med. Microbiol. 2001, 30, 43–47. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 177, 27509–27515. [Google Scholar] [CrossRef]
- Mansfield, J.A.; Bergin, S.W.; Cooper, J.R.; Olsen, C.H. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: A systematic review and meta-analysis. Mil. Med. 2014, 179, 580–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.Z.; Kondo, S.; Yanagisawa, N.; Takahashi, N.; Odamaki, T.; Iwabuchi, N.; Iwatsuki, K.; Kokubo, S.; Togashi, H.; Enomoto, K.; et al. Effect of probiotic Bifidobacterium longum BB536 [corrected] in relieving clinical symptoms and modulating plasma cytokine levels of Japanese cedar pollinosis during the pollen season. A randomized double-blind, placebo-controlled trial. J. Investig. Allergol. Clin. Immunol. 2006, 16, 86–93. [Google Scholar] [PubMed]
- Reid, G.; Gaudier, E.; Guarner, F.; Huffnagle, G.B.; Macklaim, J.M.; Munoz, A.M.; Martini, M.; Ringel-Kulka, T.; Sartor, B.R.; Unal, R.R.; et al. Responders and non-responders to probiotic interventions: How can we improve the odds? Gut Microbes 2010, 1, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojima, M.N.; Gotoh, A.; Takada, H.; Odamaki, T.; Xiao, J.-Z.; Katoh, T.; Katayama, T. Bifidobacterium bifidum suppresses gut inflammation caused by repeated antibiotic disturbance without recovering gut microbiome diversity in mice. Front. Microbiol. 2020, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, O.; Amaretti, A.; Puddu, P.E.; Raimondi, S.; Abello, F.; Cagliero, P.; Rossi, M. Bifidobacteria supplementation: Effects on plasma lipid profiles in dyslipidemic children. Nutrition 2014, 30, 831–836. [Google Scholar] [CrossRef]
- Van den Akker, C.H.; van Goudoever, J.B.; Shamir, R.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Canani, R.B.; Bronsky, J.; et al. Probiotics and preterm infants: A position paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar]
- Cheng, L.; Kiewiet, M.B.G.; Logtenberg, M.J.; Groeneveld, A.; Nauta, A.; Schols, H.A.; Walvoort, M.T.C.; Harmsen, H.J.M.; De Vos, P. Effects of different human milk oligosaccharides on growth of bifidobacteria in monoculture and co-culture with Faecalibacterium prausnitzii. Front. Microbiol. 2020, 11, 569700. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 160, 461–478. [Google Scholar] [CrossRef]
- Townsend, S.; Caubillabarron, J.; Loc-Carrillo, C.; Forsythe, S. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiol. 2007, 24, 67–74. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Chen, J.; Wang, X.; Gu, Q.; Yin, Y. Effects of bifidobacteria-produced exopolysaccharides on human gut microbiota in vitro. Appl. Microbiol. Biotechnol. 2019, 103, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Reid, G. Distant site effects of ingested prebiotics. Nutrition 2016, 8, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.-Z.; Tao, S.-B.; Ma, L.; Fu, P. Roles of short-chain fatty acids in kidney diseases. Chin. Med. J. 2019, 132, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Koenig, D.; Engelbrecht, K.; Doney, L.; Hards, K.; Al, K.F.; Reid, G.; Burton, J.P. Emerging connections between gut microbiome bioenergetics and chronic metabolic diseases. Cell Rep. 2021, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Daisley, B.A.; Chanyi, R.M.; Abdur-Rashid, K.; Al, K.F.; Gibbons, S.; Chmiel, J.A.; Wilcox, H.; Reid, G.; Anderson, A.; Dewar, M.; et al. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat. Commun. 2020, 11, 4822. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Barcenilla, A.; Stewart, C.S.; Flint, H.J. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 2002, 52, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Shetty, S.A.; Boeren, S.; Bui, T.P.N.; Smidt, H.; De Vos, W.M. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ. Microbiol. 2020, 22, 4863–4875. [Google Scholar] [CrossRef] [PubMed]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Wang, G.; Wang, D.; Huang, L.; Song, Y.; Chen, Z.; Du, M. Enhanced production of volatile fatty acids by adding a kind of sulfate reducing bacteria under alkaline pH. Colloids Surf. B Biointerfaces 2020, 195, 111249. [Google Scholar] [CrossRef]
- Sagheddu, V.; Patrone, V.; Miragoli, F.; Morelli, L. Abundance and diversity of hydrogenotrophic microorganisms in the infant gut before the weaning period assessed by denaturing gradient gel electrophoresis and quantitative PCR. Front. Nutr. 2017, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Infante, D.; Segarra, O.; Le Luyer, B. Dietary treatment of colic caused by excess gas in infants: Biochemical evidence. World J. Gastroenterol. 2011, 17, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Taylor, T.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Pérez, A.; Peterson, V.; et al. Microbiota-related changes in bileaAcid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 2013, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; De Magistris, L.; et al. Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef]
- Pascucci, T.; Colamartino, M.; Fiori, E.; Sacco, R.; Coviello, A.; Ventura, R.; Puglisi-Allegra, S.; Turriziani, L.; Persico, A.M. p-cresol alters brain dopamine metabolism and exacerbates autism-like behaviors in the BTBR mouse. Brain Sci. 2020, 10, 233. [Google Scholar] [CrossRef]
- Harambat, J.; Van Stralen, K.J.; Kim, J.J.; Tizard, E.J. Epidemiology of chronic kidney disease in children. Pediatr. Nephrol. 2012, 27, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Nada, A.; Bonachea, E.M.; Askenazi, D.J. Acute kidney injury in the fetus and neonate. Semin. Fetal Neonatal Med. 2017, 22, 90–97. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuivenberg, G.A.; Burton, J.P.; Bron, P.A.; Reid, G. Why Are Bifidobacteria Important for Infants? Microorganisms 2022, 10, 278. https://doi.org/10.3390/microorganisms10020278
Stuivenberg GA, Burton JP, Bron PA, Reid G. Why Are Bifidobacteria Important for Infants? Microorganisms. 2022; 10(2):278. https://doi.org/10.3390/microorganisms10020278
Chicago/Turabian StyleStuivenberg, Gerrit A., Jeremy P. Burton, Peter A. Bron, and Gregor Reid. 2022. "Why Are Bifidobacteria Important for Infants?" Microorganisms 10, no. 2: 278. https://doi.org/10.3390/microorganisms10020278
APA StyleStuivenberg, G. A., Burton, J. P., Bron, P. A., & Reid, G. (2022). Why Are Bifidobacteria Important for Infants? Microorganisms, 10(2), 278. https://doi.org/10.3390/microorganisms10020278





