Diversity and Phylogeny of Novel Cord-Forming Fungi from Borneo
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction/PCR/Sequencing
2.3. Phylogenetic Analysis
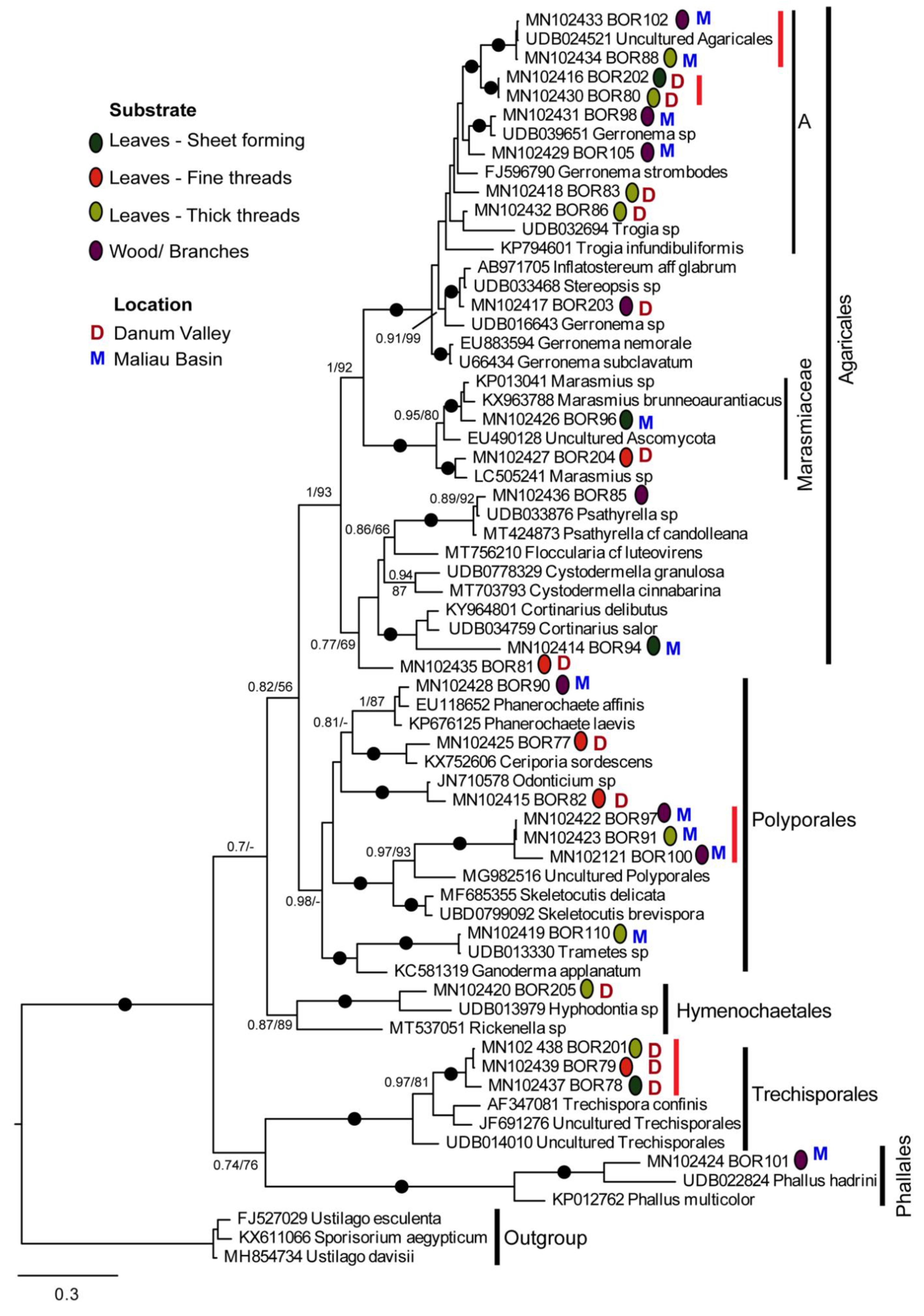
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukasawa, Y.; Savoury, M.; Boddy, L. Ecological memory and relocation decisions in fungal mycelial networks: Responses to quantity and location of new resources. ISME J. 2019, 14, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Cairney, J.W. Basidiomycete mycelia in forest soils: Dimensions, dynamics and roles in nutrient distribution. Mycol. Res. 2005, 109, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L.; Watkinson, S.C. Wood decomposition, higher fungi, and their role in nutrient redistribution. Can. J. Bot. 1995, 73, 1377–1383. [Google Scholar] [CrossRef]
- Snaddon, J.L.; Turner, E.; Fayle, T.; Khen, C.V.; Eggleton, P.; Foster, W.A. Biodiversity hanging by a thread: The importance of fungal litter-trapping systems in tropical rainforests. Biol. Lett. 2011, 8, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Saprotrophic cord-forming fungi: Warfare strategies and other ecological aspects. Mycol. Res. 1993, 97, 641–655. [Google Scholar] [CrossRef]
- Boddy, L. Saprotrophic cord-forming fungi: Meeting the challenge of heterogeneous environments. Mycologia 1999, 91, 13–32. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Koide, R.T.; Fernandez, C.; Malcolm, G. Determining place and process: Functional traits of ectomycorrhizal fungi that affect both community structure and ecosystem function. New Phytol. 2013, 201, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.C.; Cairney, J.W.G. Ectomycorrhizal fungi: Exploring the mycelial frontier. FEMS Microbiol. Rev. 2007, 31, 388–406. [Google Scholar] [CrossRef]
- Tedersoo, L.; Kõljalg, U.; Hallenberg, N.; Larsson, K. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Frolich, J.; Hyde, K.D. Biodiversity of palm fungi in the tropics: Are global fungal diversity estimates realistic? Biodivers. Conserv. 1999, 8, 977–1004. [Google Scholar] [CrossRef]
- Lodge, D.J.; Cantrell, S. Fungal communities in wet tropical forests: Variation in time and space. Can. J. Bot. 1995, 73, 1391–1398. [Google Scholar] [CrossRef]
- Hedger, J.; Lewis, P.; Gitay, H. Litter-trapping by fungi in moist tropical forest. In Aspects of tropical mycology; Cambridge University Press: Cambridge, UK, 1992; pp. 15–35. [Google Scholar]
- Spooner, B.; Roberts, P. Fungi; Collins: London, UK, 2005. [Google Scholar]
- Lodge, D.J.; Asbury, C.E. Basidiomycetes Reduce Export of Organic Matter from Forest Slopes. Mycologia 1988, 80, 888–890. [Google Scholar] [CrossRef]
- Maury-Lechon, G.; Curtet, L. Biogeography and evolutionary systematics of Dipterocarpaceae. In A Review of Dipterocarps Taxonomy, Ecology and Silviculture; Appanah, S., TurnbullU, M.J., Eds.; CIFOR: Jakarta, Indonesia, 1998; pp. 5–44. [Google Scholar]
- Smith, M.E.; Henkel, T.W.; Uehling, J.K.; Fremier, A.K.; Clarke, H.D.; Vilgalys, R. The Ectomycorrhizal Fungal Community in a Neotropical Forest Dominated by the Endemic Dipterocarp Pakaraimaea dipterocarpacea. PLoS ONE 2013, 8, e55160. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Davies, S.J.; Tan, S.; Bruns, T.D. Potential link between plant and fungal distributions in a dipter-ocarp rainforest: Community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone. New Phytol. 2010, 185, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.B.; Elias, D.; Johnson, D.; Both, S.; Riutta, T.; Goodall, T.; Majalap, N.; McNamara, N.P.; Griffiths, R.; Ostle, N. Soil Fungal Community Characteristics and Mycelial Production Across a Disturbance Gradient in Lowland Dipterocarp Rainforest in Borneo. Front. For. Glob. Chang. 2020, 3, 64. [Google Scholar] [CrossRef]
- Kerfahi, D.; Tripathi, B.M.; Lee, J.; Edwards, D.P.; Adams, J.M. The Impact of Selective-Logging and Forest Clearance for Oil Palm on Fungal Communities in Borneo. PLoS ONE 2014, 9, e111525. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.L.; D’Angelo, H.; Brearley, F.Q.; Gedallovich, S.M.; Babar, N.; Yang, N.; Gillikin, C.M.; Gradoville, R.; Bateman, C.; Turner, B.; et al. Responses of Soil Fungi to Logging and Oil Palm Agriculture in Southeast Asian Tropical Forests. Microb. Ecol. 2014, 69, 733–747. [Google Scholar] [CrossRef]
- Fiedler, P.L. Conservation Biology: The Theory and Practice of Nature Conservation Preservation and Management; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Stubbe, D.; Nuytinck, J.; Verbeken, A. Lactarius subgenus Plinthogalus of Malaysia. Fungal Divers. 2008, 32, 125–156. [Google Scholar]
- Tan, Y.-S.; Desjardin, D.E.; Perry, B.A.; Vikineswary, S.; Noorlidah, A. Marasmius sensu stricto in Peninsular Malaysia. Fungal Divers. 2009, 37, 9–100. [Google Scholar]
- Demoulin, V. The study of larger basidiomycetes, especially polypores, in the Malesian region and the role of the Singapore Botanic Gardens. Gard. Bull. Singap. 2011, 63, 175–188. [Google Scholar]
- Corner, E.J.H. Boletus in Malaysia; Singapore Botanic Gardens: Singapore, 1972; pp. 1–263. [Google Scholar]
- Wilson, A.W.; Desjardin, D.E. Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 2005, 97, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Yamashita, S.; Lee, S.-S. Diversity and conservation of wood-inhabiting polypores and other aphyllophoraceous fungi in Malaysia. Biodivers. Conserv. 2012, 21, 2375–2396. [Google Scholar] [CrossRef]
- Geml, J.; Morgado, L.N.; Semenova-Nelsen, T.A.; Schilthuizen, M. Changes in richness and community composition of ecto-mycorrhizal fungi among altitudinal vegetation types on Mount Kinabalu in Borneo. New Phytol. 2017, 215, 454–468. [Google Scholar] [CrossRef]
- Mulyani, R.B.; Sastrahidayat, I.R.; Abadi1, A.L.; Djauhari, S. Exploring ectomycorrhiza in peat swamp forest of Nyaru Menteng Palangka Raya Central Borneo. J. Biodivers. Environ. Sci. 2014, 5, 133–145. [Google Scholar]
- Yamashita, S.; Hattori, T.; Ohkubo, T.; Nakashizuka, T. Spatial distribution of the basidiocarps of aphyllophoraceous fungi in a tropical rainforest on Borneo Island, Malaysia. Mycol. Res. 2009, 113, 1200–1207. [Google Scholar] [CrossRef]
- Yamashita, S.; Hattori, T.; Lee, S.S.; Okabe, K. Estimating the diversity of wood-decaying polypores in tropical lowland rain forests in Malaysia: The effect of sampling strategy. Biodivers. Conserv. 2014, 24, 393–406. [Google Scholar] [CrossRef]
- Peay, K.G.; Kennedy, P.G.; Bruns, T.D. Fungal Community Ecology: A Hybrid Beast with a Molecular Master. BioScience 2008, 58, 799–810. [Google Scholar] [CrossRef]
- Allmer R, J.; Vasiliauskas, R.; Ihrmark, K.; Stenlid, J.; Dahlberg, A. Wood-inhabiting fungal communities in woody debris of Norway spruce (Picea abies (L.) Karst.), as reflected by sporocarps, mycelial isolations and T-RFLP identification. FEMS Microbiol. Ecol. 2006, 55, 57–67. [Google Scholar] [CrossRef][Green Version]
- Bent, E.; Taylor, D.L. Direct amplification of DNA from fresh and preserved ectomycorrhizal root tips. J. Microbiol. Methods 2010, 80, 206–208. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2013, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Hibbett, D.A. Phylogenetic Overview of the Agaricomycotina. Mycologia 2006, 98, 917–925. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Hibbett, D.; Bauer, R.; Binder, M.; Giachini, A.; Hosaka, K.; Justo, A.; Larsson, E.; Larsson, K.; Lawrey, J.D.; Miettinen, O.; et al. Agaricomycetes. In Systematics and Evolution: The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Springer: Berlin/Heidelberg, Germany, 2014; Volume 7A. [Google Scholar] [CrossRef]
- Huckfeldt, T.; Schmidt, O. Identification key for European strand-forming house-rot fungi. Mycologist 2006, 20, 42–56. [Google Scholar] [CrossRef]
- Miettinen, O.; Niemelä, T. Two New Temperate Polypore Species of Skeletocutis (Polyporales, Basidiomycota). Ann. Bot. Fenn. 2018, 55, 195–206. [Google Scholar] [CrossRef]
- Zhou, L.-W.; Qin, W.-M. A new species of Skeletocutis (Polyporaceae) on bamboo in tropical China. Mycotaxon 2012, 119, 345–350. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, D.V.; Saxena, R.K.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I.; Kõljalg, U. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol. 2008, 180, 479–490. [Google Scholar] [CrossRef]
- Nouhra, E.; Urcelay, C.; Longo, S.; Tedersoo, L. Ectomycorrhizal fungal communities associated to Nothofagus species in Northern Patagonia. Mycorrhiza 2013, 23, 487–496. [Google Scholar] [CrossRef]
- Larsson, K.-H.; Parmasto, E.; Fischer, M.; Langer, E.; Nakasone, K.K.; Redhead, S.A. Hymenochaetales: A molecular phylogeny for the hymenochaetoid clade. Mycologia 2006, 98, 926–936. [Google Scholar] [CrossRef]
- Koch, R.A.; Liu, J.; Brann, M.; Jumbam, B.; Siegel, N.; Aime, M.C. Marasmioid rhizomorphs in bird nests: Species diversity, functional specificity, and new species from the tropics. Mycologia 2020, 112, 1086–1103. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K. Preliminary list of phallales (phallomycetidae, basidiomycota) in Thailand. Mem. Natl. Mus. Nat. Sci. 2012, 48, 81–89. [Google Scholar]
- Yafetto, L. The structure of mycelial cords and rhizomorphs of fungi: A mini-review. Mycosphere 2018, 9, 984–998. [Google Scholar] [CrossRef]
- Bass, D.; Richards, T.A. Three reasons to re-evaluate fungal diversity ‘on Earth and in the ocean. Fungal Biol. Rev. 2011, 25, 159–164. [Google Scholar] [CrossRef]
- Meiser, A.; Bálint, M.; Schmitt, I. Meta-analysis of deep-sequenced fungal communities indicates limited taxon sharing between studies and the presence of biogeographic patterns. New Phytol. 2013, 201, 623–635. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, R.; Hartikainen, H.; Hall, A.; Bass, D. Diversity and Phylogeny of Novel Cord-Forming Fungi from Borneo. Microorganisms 2022, 10, 239. https://doi.org/10.3390/microorganisms10020239
Foster R, Hartikainen H, Hall A, Bass D. Diversity and Phylogeny of Novel Cord-Forming Fungi from Borneo. Microorganisms. 2022; 10(2):239. https://doi.org/10.3390/microorganisms10020239
Chicago/Turabian StyleFoster, Rachel, Hanna Hartikainen, Andie Hall, and David Bass. 2022. "Diversity and Phylogeny of Novel Cord-Forming Fungi from Borneo" Microorganisms 10, no. 2: 239. https://doi.org/10.3390/microorganisms10020239
APA StyleFoster, R., Hartikainen, H., Hall, A., & Bass, D. (2022). Diversity and Phylogeny of Novel Cord-Forming Fungi from Borneo. Microorganisms, 10(2), 239. https://doi.org/10.3390/microorganisms10020239





