Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children
Abstract
:1. Introduction
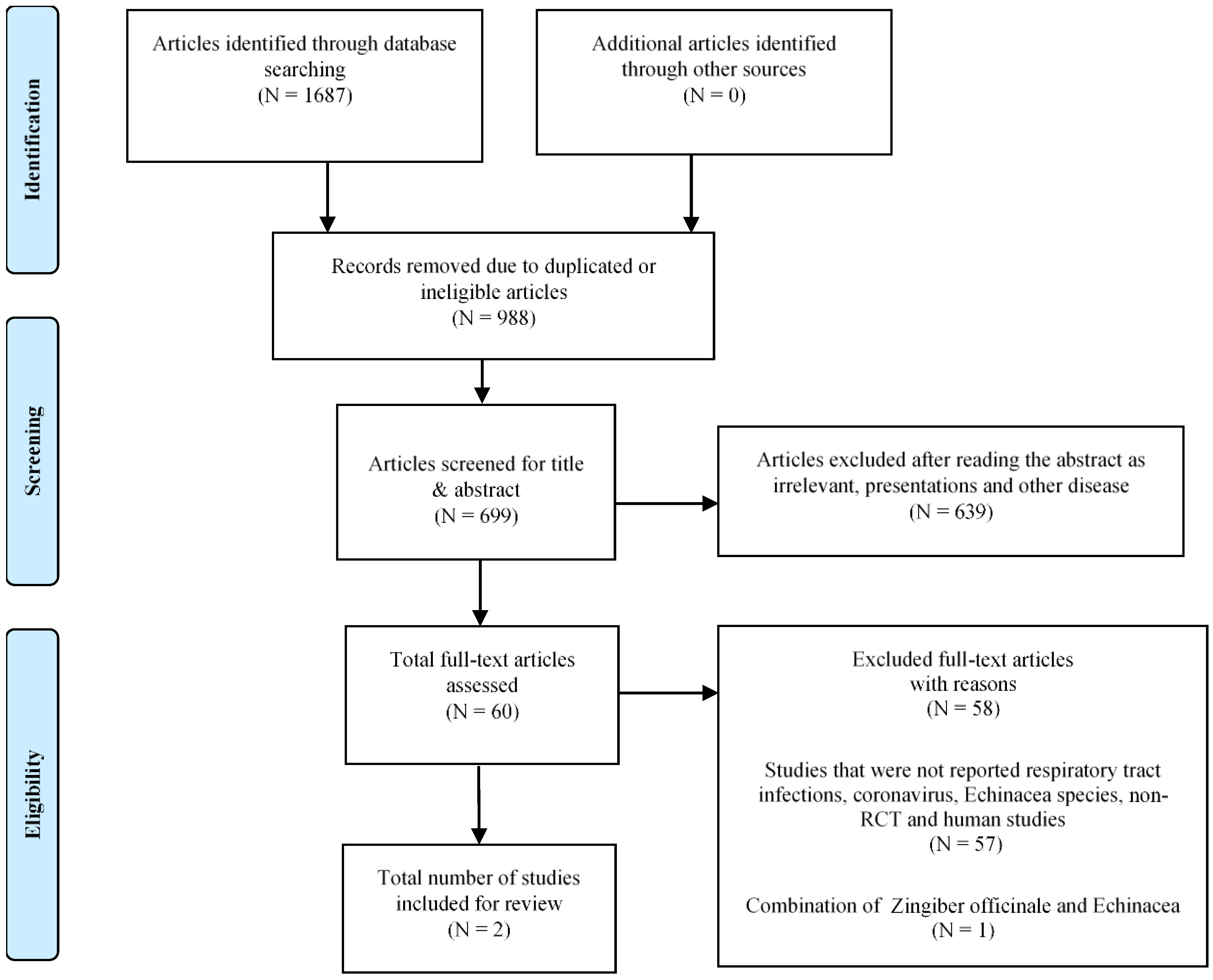
2. Materials and Methods
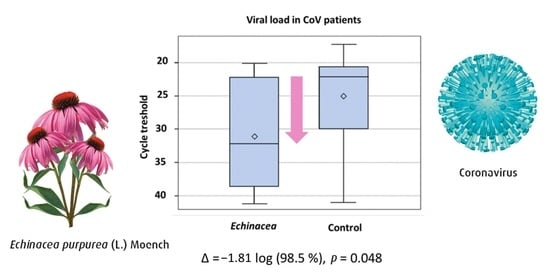
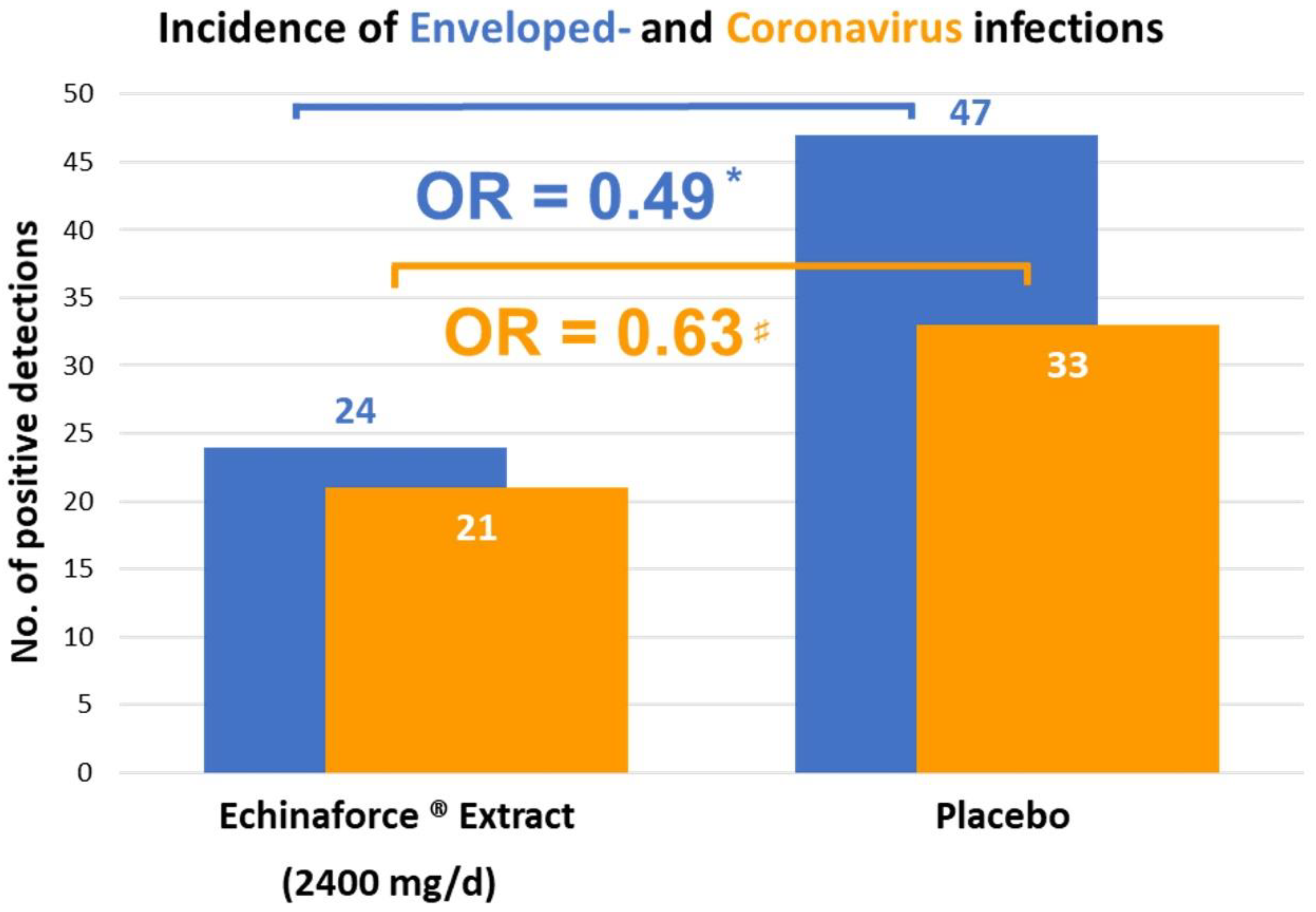
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, J.S.; McIntosh, K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–S227. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Gomaa, M.; Nageh, A.; Shehata, M.M.; Kayed, A.E.; Sabir, J.S.M.; Abiadh, A.; Jrijer, J.; Amr, Z.; Said, M.A.; et al. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) in Dromedary Camels in Africa and Middle East. Viruses 2019, 11, 717. [Google Scholar] [CrossRef] [Green Version]
- Alene, M.; Yismaw, L.; Assemie, M.A.; Ketema, D.B.; Gietaneh, W.; Birhan, T.Y. Serial interval and incubation period of COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Subedi, S.; Davis, J.J.; Hodjat, P.; Walley, D.R.; Kinskey, J.C.; Ojeda Saavedra, M.; Pruitt, L.; et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.; Bhattacharyya, R.; Purkayastha, S.; Zimmermann, L.; Ray, D.; Hazra, A.; Kleinsasser, M.; Mellan, T.; Whittaker, C.; Flaxman, S.; et al. Resurgence of SARS-CoV-2 in India: Potential role of the B.1.617.2 (Delta) variant and delayed interventions. medRxiv 2021. [Google Scholar] [CrossRef]
- Alkhatib, M.; Svicher, V.; Salpini, R.; Ambrosio, F.A.; Bellocchi, M.C.; Carioti, L.; Piermatteo, L.; Scutari, R.; Costa, G.; Artese, A.; et al. SARS-CoV-2 Variants and Their Relevant Mutational Profiles: Update Summer 2021. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021, 600, 21. [Google Scholar] [CrossRef]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. Effect of COVID-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef]
- Stokel-Walker, C. The search for antivirals for COVID-19. BMJ 2021, 374, n2165. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.D.; Parveen, A.; Yadav, D.K. SARS-CoV-2: Emergence of New Variants and Effectiveness of Vaccines. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.R.; Gertsch, J.; Klein, P.; Schoop, R. Effects of Echinaforce® treatment on ex vivo-stimulated blood cells. Phytomedicine 2011, 18, 826–831. [Google Scholar] [CrossRef]
- Declerck, K.; Novo, C.P.; Grielens, L.; Van Camp, G.; Suter, A.; Vanden Berghe, W. Echinacea purpurea (L.) Moench treatment of monocytes promotes tonic interferon signaling, increased innate immunity gene expression and DNA repeat hypermethylated silencing of endogenous retroviral sequences. BMC Complement. Med. Ther. 2021, 21, 141. [Google Scholar] [CrossRef]
- Gertsch, J.; Schoop, R.; Kuenzle, U.; Suter, A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 2004, 577, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelkart, K.; Xu, W.; Pei, Y.; Makriyannis, A.; Picone, R.P.; Bauer, R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Med. 2005, 71, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarkatti, P.; Miranda, K.; Nagarkatti, M. Use of Cannabinoids to Treat Acute Respiratory Distress Syndrome and Cytokine Storm Associated with Coronavirus Disease-2019. Front. Pharmacol. 2020, 11, 1677. [Google Scholar] [CrossRef]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.-H.; Schoop, R.; Gertsch, J. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef]
- Nicolussi, S.; Gertsch, J. Endocannabinoid transport revisited. Vitam. Horm. 2015, 98, 441–485. [Google Scholar]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.C.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Safety and Efficacy Profile of Echinacea purpurea to Prevent Common Cold Episodes: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid.-Based. Complement. Altern. Med. 2012, 2012, 841315. [Google Scholar] [CrossRef] [Green Version]
- Ogal, M.; Johnston, S.L.; Klein, P.; Schoop, R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: A randomized, blinded, controlled clinical trial. Eur. J. Med. Res. 2021, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.G.; Dowling, H.F.; Spiesman, I.G.; Boand, A.V. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch. Intern. Med. 1958, 101, 267–278. [Google Scholar] [CrossRef]
- Schapowal, A. Efficacy and safety of Echinaforce® in respiratory tract infections. Wien. Med. Wochenschr. 2013, 163, 102–105. [Google Scholar] [CrossRef]
- Taylor, J.A.; Weber, W.J.; Martin, E.T.; McCarty, R.L.; Englund, J.A. Development of a symptom score for clinical studies to identify children with a documented viral upper respiratory tract infection. Pediatr. Res. 2010, 68, 252–257. [Google Scholar] [CrossRef]
- Sharma, M.; Schoop, R.; Hudson, J.B. Echinacea as an antiinflammatory agent: The influence of physiologically relevant parameters. Phytother. Res. 2009, 23, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, R.; Hildebert, W. Echinacea-Ein Handbuch für Ärzte, Apotheker und andere Naturwissenschaftler; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1990; ISBN 9783804709997. [Google Scholar]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Vimalanathan, S.; Shehata, M.; Sadasivam, K.; Delbue, S.; Dolci, M.; Pariani, E.; D’Alessandro, S.; Pleschka, S. Broad antiviral effects of Echinacea purpurea against SARS-CoV-2 variants of concern and potential mechanism of action. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kolev, E.; Mircheva, L.; Edwards, M.; Johnston, S.L.; Kalinov, K.; Stange, R.; Gancitano, G.; Berghe, W.V.; Kreft, S. Echinacea purpurea for the Long-term Prevention of Viral Respiratory Tract Infections during COVID-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Bjorkman, K.K.; Saldi, T.K.; Lasda, E.; Bauer, L.C.; Kovarik, J.; Gonzales, P.K.; Fink, M.R.; Tat, K.L.; Hager, C.R.; Davis, J.C.; et al. Higher Viral Load Drives Infrequent Severe Acute Respiratory Syndrome Coronavirus 2 Transmission Between Asymptomatic Residence Hall Roommates. J. Infect. Dis. 2021, 224, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Little, P.; Read, R.C.; Amlôt, R.; Chadborn, T.; Rice, C.; Bostock, J.; Yardley, L. Reducing risks from coronavirus transmission in the home—The role of viral load. BMJ 2020, 369. [Google Scholar] [CrossRef]
- Chung, E.; Chow, E.J.; Wilcox, N.C.; Burstein, R.; Brandstetter, E.; Han, P.D.; Fay, K.; Pfau, B.; Adler, A.; Lacombe, K.; et al. Comparison of Symptoms and RNA Levels in Children and Adults with SARS-CoV-2 Infection in the Community Setting. JAMA Pediatr. 2021, 175. [Google Scholar] [CrossRef]
- Schapowal, A.; Klein, P.; Johnston, S.L. Echinacea reduces the risk of recurrent respiratory tract infections and complications: A meta-analysis of randomized controlled trials. Adv. Ther. 2015, 32, 187–200. [Google Scholar] [CrossRef]

| Study | Population | Country | Jadad Score | Plant Species | Preparation | Dosage | Control | Sample Source | Analysis Method | Symptomatic Assessments |
|---|---|---|---|---|---|---|---|---|---|---|
| Jawad M, et al. 2012 [25] | Adults > 18 years, N = 755 | UK | 5 | Echinacea purpurea | Alcoholic extract of freshly harvested herb/roots | 3 × 20 drops/d | Placebo | Naso- pharynx | RT-PCR | Individual and TSS, AUC |
| Ogal M, et al. 2021 [26] | Children 4–12 years, N = 203 | CH | 4 | Echinacea purpurea | Alcoholic extract of freshly harvested herb/roots | 3 × 1 tablet/d | Vitamin C | Naso- pharynx | RT-PCR, Ct-values reported | Individual and TSS, AUC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolussi, S.; Ardjomand-Woelkart, K.; Stange, R.; Gancitano, G.; Klein, P.; Ogal, M. Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms 2022, 10, 211. https://doi.org/10.3390/microorganisms10020211
Nicolussi S, Ardjomand-Woelkart K, Stange R, Gancitano G, Klein P, Ogal M. Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms. 2022; 10(2):211. https://doi.org/10.3390/microorganisms10020211
Chicago/Turabian StyleNicolussi, Simon, Karin Ardjomand-Woelkart, Rainer Stange, Giuseppe Gancitano, Peter Klein, and Mercedes Ogal. 2022. "Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children" Microorganisms 10, no. 2: 211. https://doi.org/10.3390/microorganisms10020211
APA StyleNicolussi, S., Ardjomand-Woelkart, K., Stange, R., Gancitano, G., Klein, P., & Ogal, M. (2022). Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms, 10(2), 211. https://doi.org/10.3390/microorganisms10020211