Coordinated Action of RTBV and RTSV Proteins Suppress Host RNA Silencing Machinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
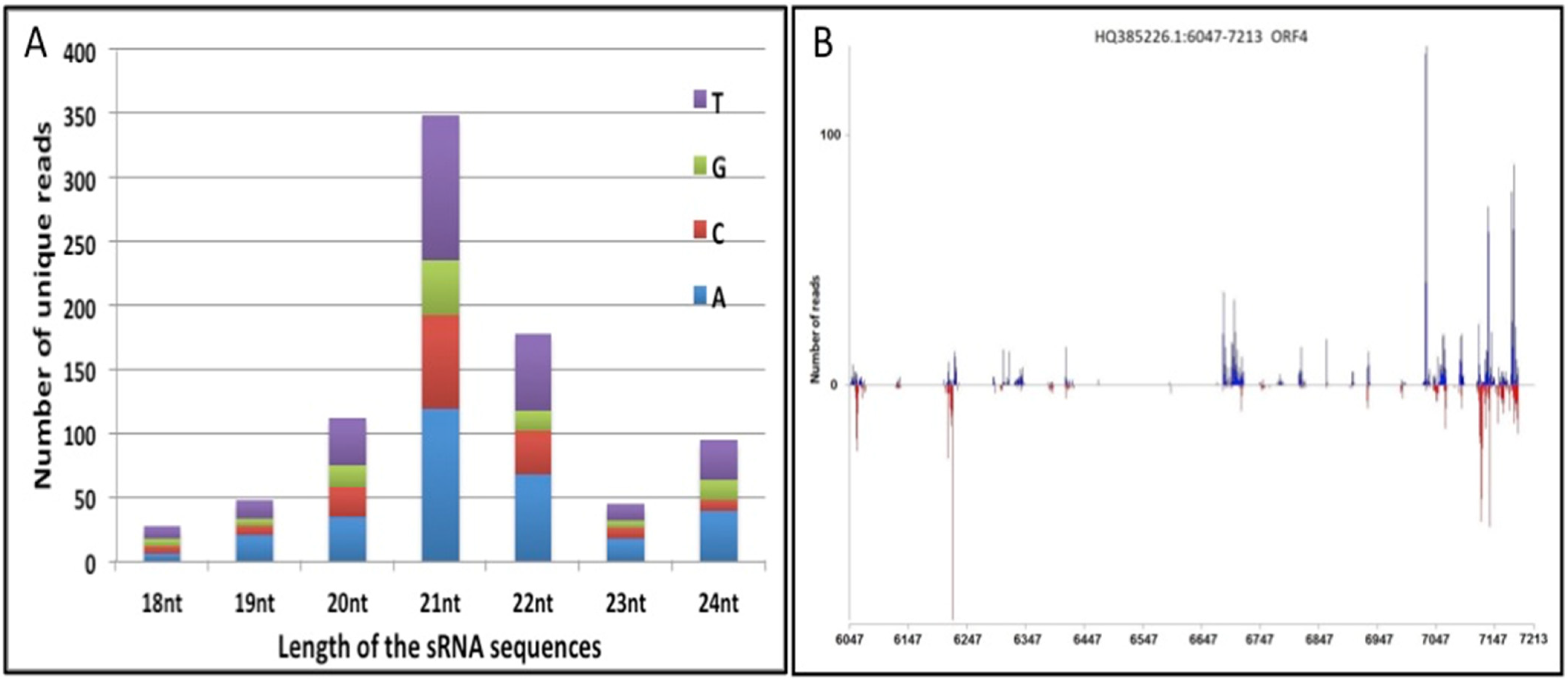
2.2. Small RNA Library Sequencing and Computational Analysis
2.3. Plasmid Constructs
2.4. Generation of GFP Silenced Tobacco Plants and Agro-Infiltration
2.5. cDNA Synthesis
2.6. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
2.7. Northern Blot Analysis
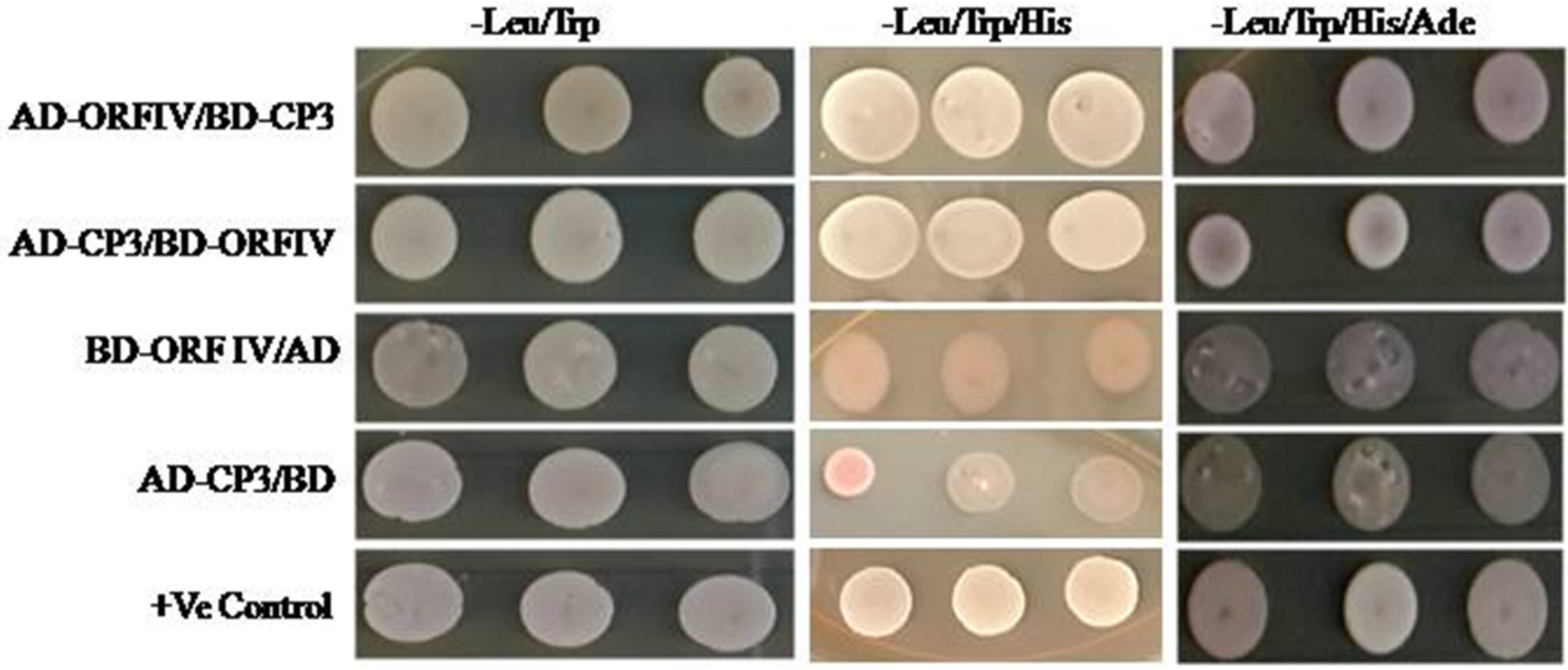
2.8. Yeast Two-Hybrid Analysis
3. Results
3.1. ORF-IV of RTBV Is a Potential Hotspot for siRNA Generation
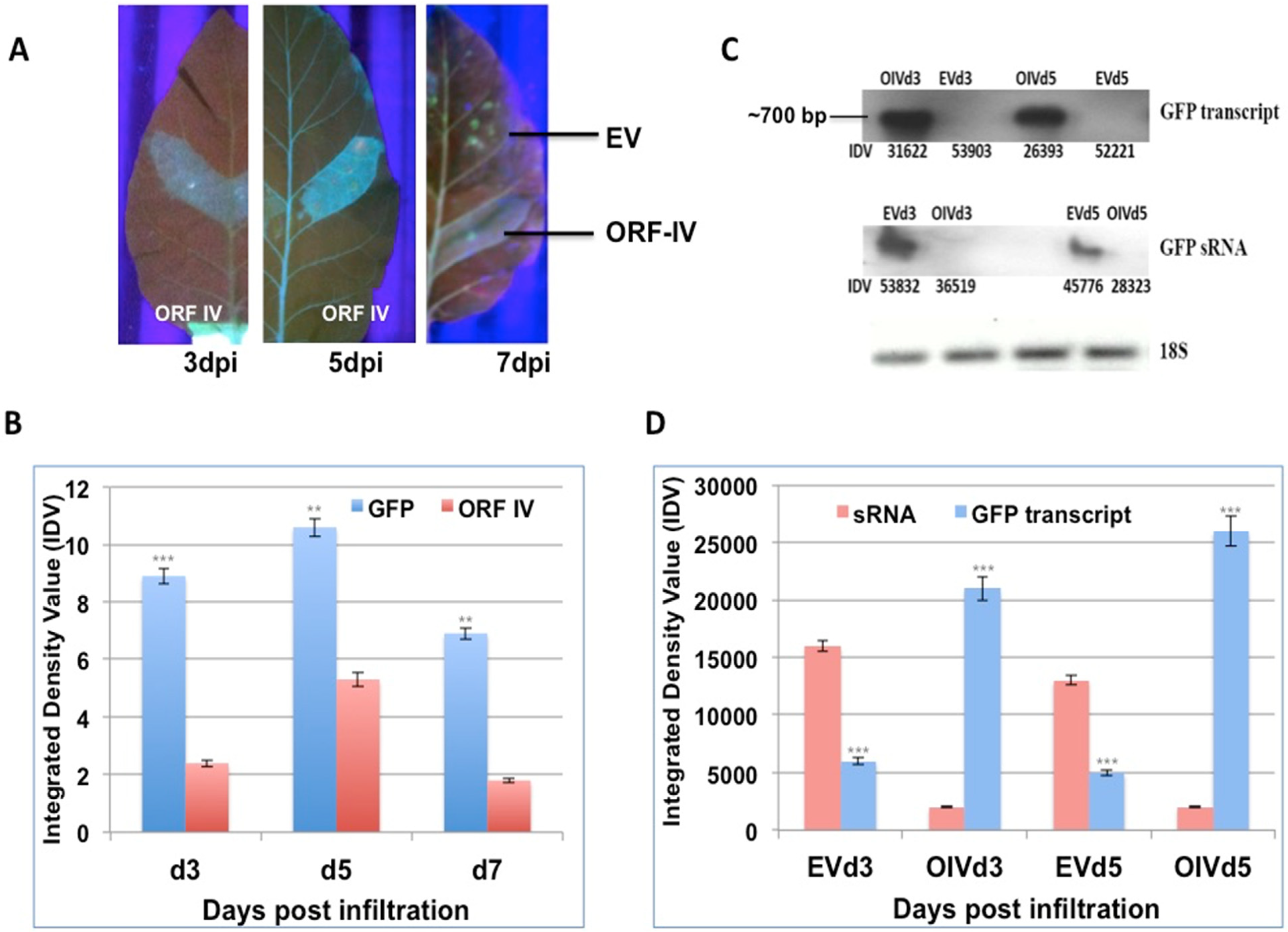
3.2. ORF-IV Can Suppress Pre-Established RNA Silencing
3.3. RTSV Coat Protein Enhances the Suppressor Activity of ORF-IV
3.4. RTBV ORF-IV Interacts with RTSV CP3 Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinney, H.H. Mosaic diseases of the Canary Islands; West Africa and Gibraltar. J. Agri. Res. 1929, 39, 557–578. [Google Scholar]
- Lindbo, J.A.; Silva-Rosales, L.; Proebsting, W.M.; Dougherty, W.G. Induction of a highly specific antiviral state in transgenic plants: Implications for regulation of gene expression and virus resistance. Plant Cell 1993, 5, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Beachy, R.N. Mechanisms and applications of pathogen-derived resistance in transgenic plants. Curr. Opin. Biotechnol. 1997, 8, 215–220. [Google Scholar] [CrossRef]
- Kyrychenko, A.M.; Kovalenko, O.G. Basic engineering strategies for virus-resistant plants. Cytol. Genet. 2018, 52, 213–221. [Google Scholar] [CrossRef]
- Ratcliff, F.; Harrison, B.D.; Baulcombe, D.C. A similarity between viral defense and gene silencing in plants. Science 1997, 276, 1558–1560. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H. Host factors against plant viruses. Mol. Plant Pathol. 2019, 20, 1588–1601. [Google Scholar] [CrossRef]
- Ramesh, S.V.; Yogindran, S.; Gnanasekaran, P.; Chakraborty, S.; Winter, S.; Pappu, H. RVirus and viroid-derived small RNAs as modulators of host gene expression: Molecular insights into pathogenesis. Front. Microbiol. 2021, 11, 614231. [Google Scholar] [CrossRef]
- Teixeira, R.M.; Ferreira, M.A.; Raimundo, G.; Fontes, E. Geminiviral triggers and suppressors of plant antiviral immunity. Microorganisms 2021, 9, 775. [Google Scholar] [CrossRef]
- Ding, S.W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef]
- Prasad, A.; Sharma, N.; Muthamilarasan, M.; Rana, S.; Prasad, M. Recent advances in small RNA mediated plant-virus interactions. Crit. Rev. Biotechnol. 2019, 39, 587–601. [Google Scholar] [CrossRef]
- Sanan-Mishra, N.; Jailani, A.A.K.; Mandal, B.; Mukherjee, S.K. Secondary siRNAs in plants: Biosynthesis; various functions; applications in virology. Front. Plant Sci. 2021, 12, 610283. [Google Scholar] [CrossRef]
- Sanan-Mishra, N.; Mukherjee, S.K. A peep into Plant miRNA world. Open Plant Sci. J. 2007, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Pelaez, P.; Sanchez, F. Small RNAs in plant defense responses during viral and bacterial interactions: Similarities and differences. Front. Plant Sci. 2013, 4, 343. [Google Scholar] [CrossRef]
- Sinha, V.; Anand, A.; Mukherjee, S.K.; Sanan-Mishra, N. RNAi based strategies for enhancing plant resistance to virus infection. In Advances in Biotechnology; Datta, A., Fakruddin, M., Iqbal, H.M.N., Abraham, J., Eds.; Open Access eBooks: Las Vegas, NV, USA, 2017; Volume 4. [Google Scholar]
- Qu, F.; Morris, T.J. Suppressors of RNA silencing encoded by plant viruses and their role in viral infections. FEBS Lett. 2005, 579, 5958–5964. [Google Scholar] [CrossRef]
- Anand, A.; Mukherjee, S.K.; Sanan-Mishra, N. Tools for pathogenicity: Virus encoded RNA silencing suppressors. In Recent Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Microbiology Book Series; Mendez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 751–763. [Google Scholar]
- Sanan-Mishra, N.; Chakraborty, S.; Gupta, D.; Mukherjee, S.K. RNAi suppressors: Biology and mechanisms. In Plant Epigenetics; RNA Technologies; Rajewsky, N., Jurga, S., Barciszewski, J., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2017; pp. 199–230. [Google Scholar]
- Carrington, J.C.; Kasschau, K.D.; Johansen, L.K. Activation and suppression of RNA silencing by plant viruses. Virology 2001, 281, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, D.; Molnar, A.; Lucioli, A.; Szittya, G.; Hornyik, C.; Tavazza, M.; Burgyan, J. A viral protein suppresses RNA silencing and binds silencing-generated; 21- to 25-nucleotide double-stranded RNAs. EMBO J. 2002, 21, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mishra, S.K.; Rehman, J.; Taneja, J.; Sundaresan, G.; Sanan-Mishra, N.; Mukherjee, S.K. Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology 2015, 486, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Veluthambi, K.; Sunitha, S. Targets and mechanisms of geminivirus silencing suppressor protein AC2. Front. Microbiol. 2021, 12, 645419. [Google Scholar] [CrossRef]
- Anandalakshmi, R.; Pruss, G.J.; Ge, X.; Marathe, R.; Mallory, A.C.; Smith, T.H.; Vance, V.B. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 13079–13084. [Google Scholar] [CrossRef]
- Pruss, G.; Ge, X.; Shi, X.M.; Carrington, J.C.; Vance, V.B. Plant viral synergism: The potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 1997, 9, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Lin, S.S.; Chen, K.C.; Yeh, S.D.; Chua, N.H. Discriminating mutations of HC-Pro of Zucchini yellow mosaic virus with differential effects on small RNA pathways involved in viral pathogenicity and symptom development. Mol. Plant-Microbe Interact. 2010, 23, 17–28. [Google Scholar] [CrossRef]
- Palauqui, J.C.; Elmayan, T.; Pollien, J.M.; Vaucheret, H. Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 1997, 16, 4738–4745. [Google Scholar] [CrossRef] [PubMed]
- Voinnet, O. Induction and suppression of RNA silencing: Insights from viral infections. Nat. Rev. Genet. 2005, 6, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Karjee, S.; Islam, M.N.; Mukherjee, S.K. Screening and identification of virus-encoded RNA silencing suppressors. Methods Mol. Biol. 2008, 442, 187–203. [Google Scholar] [PubMed]
- Azzam, O.; Chancellor, T.B. The biology; epidemiology; management of rice tungro disease in Asia. Plant Dis. 2002, 86, 88–100. [Google Scholar] [CrossRef]
- Cabauatan, P.; Hibino, H. Transmission of rice tungro bacilliform and spherical viruses by Nephotettix virescens (Distant). Philipp. Phytopathol. 1985, 21, 103–109. [Google Scholar]
- Jones, M.; Gough, K.; Dasgupta, I.; Rao, B.S.; Cliffe, J.; Qu, R.; Shen, P.; Kaniewska, M.; Blakebrough, M.; Davies, J. Rice tungro disease is caused by an RNA and a DNA virus. J. Gen. Virol. 1991, 72, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Sanfaçon, H.; Wellink, J.; Le Gall, O.; Karasev, A.; van der Vlugt, R.; Wetzel, T. Secoviridae: A proposed family of plant viruses within the order Picornavirales that combines the families Sequiviridae and Comoviridae; the unassigned genera Cheravirus and Sadwavirus; the proposed genus Torradovirus. Arch. Virol. 2009, 154, 899–907. [Google Scholar] [CrossRef]
- Shen, P.; Kaniewska, M.; Smith, C.; Beachy, R. Nucleotide sequence and genomic organization of rice tungro spherical virus. Virology 1993, 193, 621–630. [Google Scholar] [CrossRef]
- Hull, R. Molecular biology of rice tungro viruses. Annu. Rev. Phytopathol. 1996, 34, 275–297. [Google Scholar] [CrossRef]
- Mangrauthia, S.K.; Malathi, P.; Agarwal, S.; Sailaja, B.; Singh, J.; Ramkumar, G.; Krishnaveni, D.; Balachandran, S.M. The molecular diversity and evolution of Rice tungro bacilliform virus from Indian perspective. Virus Genes 2012, 45, 126–138. [Google Scholar] [CrossRef]
- Kannan, M.; Saad, M.M.; Talip, N.; Baharum, S.N.; Bunawan, H. Complete genome sequence of Rice Tungro Bacilliform Virus infecting asian rice (Oryza sativa) in Malaysia. Microbiol. Resour. Announc. 2019, 8, e00262-19. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.K.; Sharma, S.; Kant, R.; Johnson, A.; Saigopal, D.V.R.; Dasgupta, I. Bacilliform DNA-containing plant viruses in the tropics: Commonalities within a genetically diverse group. Mol. Plant Pathol. 2013, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Hull, R.; Eastop, S.; Poggi-Pollini, C.; Blakebrough, M.; Boulton, M.I.; Davies, J.W. Rice tungro bacilliform virus DNA independently infects rice after Agrobacterium-mediated transfer. J. Gen. Virol. 1991, 72, 1215–1221. [Google Scholar] [CrossRef]
- Srilatha, P.; Yousuf, F.; Methre, R.; Vishnukiran, T.; Agarwal, S.; Poli, Y.; Raghurami Reddy, M.; Vidyasagar, B.; Shanker, C.; Krishnaveni, D.; et al. Physical interaction of RTBV ORFI with D1 protein of Oryza sativa and Fe/Zn homeostasis play a key role in symptoms development during rice tungro disease to facilitate the insect mediated virus transmission. Virology 2019, 526, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, H.; Rajasubramaniam, S.; Rajam, M.V.; Dasgupta, I. RNA-interference in rice against Rice tungro bacilliform virus results in its decreased accumulation in inoculated rice plants. Transgenic Res. 2008, 17, 897–904. [Google Scholar] [CrossRef]
- Rajeswaran, R.; Golyaev, V.; Seguin, J.; Zvereva, A.S.; Farinelli, L.; Pooggin, M.M. Interactions of Rice Tungro Bacilliform Pararetrovirus and Its Protein P4 with Plant RNA-Silencing Machinery. Mol. Plant-Microbe Interact. 2014, 27, 1370–1378. [Google Scholar] [CrossRef]
- Mangrauthia, S.K.; Malathi, P.; Krishnaveni, D.; Reddy, C.S.; Viraktamath, B.C.; Balachandran, S.M.; Neeraja, C.N.; Biswal, S.K. Rapid detection of rice tungro spherical virus by RT-PCR and dot-blot hybridization. J. Mycol. Plant Pathol. 2010, 40, 445–449. [Google Scholar]
- Malathi, P.; Mangrauthia, S.K. Deciphering the multiplication behaviour of Rice tungro bacilliform virus by absolute quantitation through real-time PCR. Arch. Phytopathol. Plant Protect. 2013, 46, 2366–2375. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid; transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Das, S.S.; Sanan-Mishra, N. Comparative analysis of RNAi suppression activity of proteins from two disparate viruses. Am. J. Plant Sci. 2014, 5, 1789–1798. [Google Scholar] [CrossRef][Green Version]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1989; Volume 1. [Google Scholar]
- Song, X.; Li, P.; Zhai, J.; Zhou, M.; Ma, L.; Liu, B.; Jeong, D.H.; Nakano, M.; Cao, S.; Liu, C. Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J. 2012, 69, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, X.; Ding, S.W. Viral suppressors of RNA-based viral immunity: Host targets. Cell Host Microbe 2010, 8, 12–15. [Google Scholar] [CrossRef]
- Wei, L.; Gu, L.; Song, X.; Cui, X.; Lu, Z.; Zhou, M.; Wang, L.; Hu, F.; Zhai, J.; Meyers, B.C. Dicer-like 3 produces transposable element-associated 24-nt siRNAs that control agricultural traits in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 3877–3882. [Google Scholar] [CrossRef]
- Llave, C. Virus-derived small interfering RNAs at the core of plant–virus interactions. Trends Plant Sci. 2010, 15, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, V. Plant RNA silencing in viral defence. Adv. Expt. Med. Biol. 2011, 722, 39–58. [Google Scholar]
- Rajeswaran, R.; Pooggin, M.M. RDR6-mediated synthesis of complementary RNA is terminated by miRNA stably bound to template RNA. Nuc. Acids Res. 2012, 40, 594–599. [Google Scholar] [CrossRef]
- Szittya, G.; Burgyán, J. RNA interference-mediated intrinsic antiviral immunity in plants. Curr. Top. Microbiol. Immunol. 2013, 371, 153–181. [Google Scholar] [PubMed]
- Hull, R. Plant Virology; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Aregger, M.; Borah, B.K.; Seguin, J.; Rajeswaran, R.; Gubaeva, E.G.; Zvereva, A.S.; Windels, D.; Vazquez, F.; Blevins, T.; Farinelli, L.; et al. Primary and secondary siRNAs in geminivirus-induced gene silencing. PLoS Pathog. 2012, 8, e1002941. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, R.; Aregger, M.; Zvereva, A.S.; Borah, B.K.; Gubaeva, E.G.; Pooggin, M.M. Sequencing of RDR6-dependent double-stranded RNAs reveals novel features of plant siRNA biogenesis. Nuc. Acids Res. 2012, 40, 6241–6254. [Google Scholar] [CrossRef]
- Blevins, T.; Rajeswaran, R.; Shivaprasad, P.V.; Beknazariants, D.; Si-Ammour, A.; Park, H.S.; Vazquez, F.; Robertson, D.; Meins, F., Jr.; Hohn, T.; et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nuc. Acids Res. 2006, 34, 6233–6246. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Voinnet, O.; Chappell, L.; Baulcombe, D. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002, 21, 4671–4679. [Google Scholar] [CrossRef]
- Himber, C.; Dunoyer, P.; Moissiard, G.; Ritzenthaler, C.; Voinnet, O. Transitivity-dependent and independent cell-to-cell movement of RNA silencing. EMBO J. 2003, 22, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Dunoyer, P.; Schott, G.; Himber, C.; Meyer, D.; Takeda, A.; Carrington, J.C.; Voinnet, O. Small RNA duplexes function as mobile silencing signals between plant cells. Science 2010, 328, 912–916. [Google Scholar] [CrossRef]
- Molnar, A.; Melnyk, C.W.; Bassett, A.; Hardcastle, T.J.; Dunn, R.; Baulcombe, D.C. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010, 328, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Fütterer, J.; Potrykus, I.; Brau, M.P.V.; Dasgupta, I.; Hull, R.; Hohn, T. Splicing in a plant Pararetrovirus. Virology 1994, 198, 663–670. [Google Scholar] [CrossRef]
- Wang, X.B.; Jovel, J.; Udomporn, P.; Wang, Y.; Wu, Q.; Li, W.X.; Gasciolli, V.; Vaucheret, H.; Ding, S.W. The 21-nucleotide; but not 22-nucleotide; viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell 2011, 23, 1625–1638. [Google Scholar] [CrossRef]
- Blevins, T.; Rajeswaran, R.; Aregger, M.; Borah, B.K.; Schepetilnikov, M.; Baerlocher, L.; Farinelli, L.; Meins, F.; Hohn, T.; Pooggin, M.M. Massive production of small RNAs from a non-coding region of Cauliflower mosaic virus in plant defense and viral counter-defense. Nuc. Acids Res. 2011, 39, 5003–5014. [Google Scholar] [CrossRef]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a novel RNA silencing suppressor; NSs protein of Tomato spotted wilt virus. FEBS Letts. 2002, 532, 75–79. [Google Scholar] [CrossRef]
- Fusaro, A.F.; Matthew, L.; Smith, N.A.; Curtin, S.J.; Dedic-Hagan, J.; Ellacott, G.A.; Watson, J.M.; Wang, M.B.; Brosnan, C.; Carroll, B.J. RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep. 2006, 7, 1168–1175. [Google Scholar] [CrossRef]
- Mallory, A.C.; Vaucheret, H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006, 38, S31–S36. [Google Scholar] [CrossRef]
- Moissiard, G.; Parizotto, E.A.; Himber, C.; Voinnet, O. Transitivity in Arabidopsis can be primed; requires the redundant action of the antiviral Dicer-like 4 and Dicer-like 2, is compromised by viral-encoded suppressor proteins. RNA 2007, 13, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Di Serio, F.; Schöb, H.; Iglesias, A.; Tarina, C.; Bouldoires, E.; Meins, F. Sense-and antisense-mediated gene silencing in tobacco is inhibited by the same viral suppressors and is associated with accumulation of small RNAs. Proc. Natl. Acad. Sci. USA 2001, 98, 6506–6510. [Google Scholar] [CrossRef]
- Zvereva, A.S.; Pooggin, M.M. Silencing and innate immunity in plant defense against viral and non-viral pathogens. Viruses 2012, 4, 2578–2597. [Google Scholar] [CrossRef] [PubMed]
- Malathi, P.; Muzammil, S.A.; Krishnaveni, D.; Balachandran, S.M.; Mangrauthia, S.K. Coat protein 3 of Rice tungro spherical virus is the key target gene for development of RNAi mediated tungro disease resistance in rice. Agri Gene 2019, 12, 100084. [Google Scholar] [CrossRef]
- Mangrauthia, S.K.; Malathi, P.; Balachandran, S.M.; Reddy, C.S.; Viraktamath, B.C. Global analysis of Rice tungro spherical virus coat proteins reveals new roles in evolutionary consequences. J. Plant Biochem. Biotechnol. 2010, 19, 263–266. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, A.; Pinninti, M.; Tripathi, A.; Mangrauthia, S.K.; Sanan-Mishra, N. Coordinated Action of RTBV and RTSV Proteins Suppress Host RNA Silencing Machinery. Microorganisms 2022, 10, 197. https://doi.org/10.3390/microorganisms10020197
Anand A, Pinninti M, Tripathi A, Mangrauthia SK, Sanan-Mishra N. Coordinated Action of RTBV and RTSV Proteins Suppress Host RNA Silencing Machinery. Microorganisms. 2022; 10(2):197. https://doi.org/10.3390/microorganisms10020197
Chicago/Turabian StyleAnand, Abhishek, Malathi Pinninti, Anita Tripathi, Satendra Kumar Mangrauthia, and Neeti Sanan-Mishra. 2022. "Coordinated Action of RTBV and RTSV Proteins Suppress Host RNA Silencing Machinery" Microorganisms 10, no. 2: 197. https://doi.org/10.3390/microorganisms10020197
APA StyleAnand, A., Pinninti, M., Tripathi, A., Mangrauthia, S. K., & Sanan-Mishra, N. (2022). Coordinated Action of RTBV and RTSV Proteins Suppress Host RNA Silencing Machinery. Microorganisms, 10(2), 197. https://doi.org/10.3390/microorganisms10020197






