Exploring the Diversity of Biofilm Formation by the Food Spoiler Brochothrix thermosphacta
Abstract
1. Introduction
2. Materials and Methods
2.1. Brochothrix thermosphacta Strains and Culture Conditions
2.2. Assessment of the Biofilm-Forming Potential of B.thermosphacta Isolates Using the Biofilm Ring Test
2.3. Biofilm Quantification Using the Crystal Violet Assay
2.4. Biofilm Structural Analysis by Microscopy
2.5. Statistical Analyses
3. Results
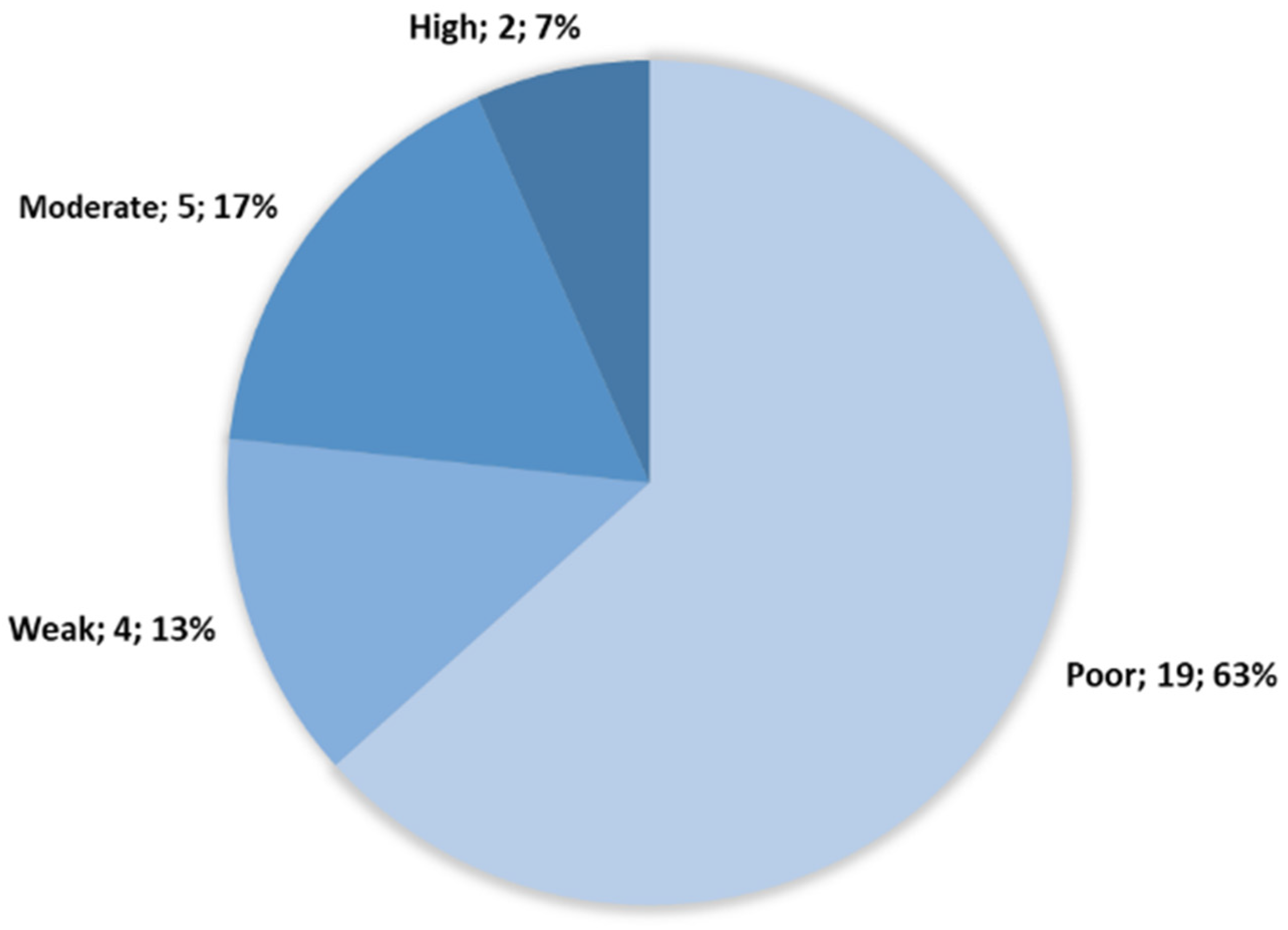
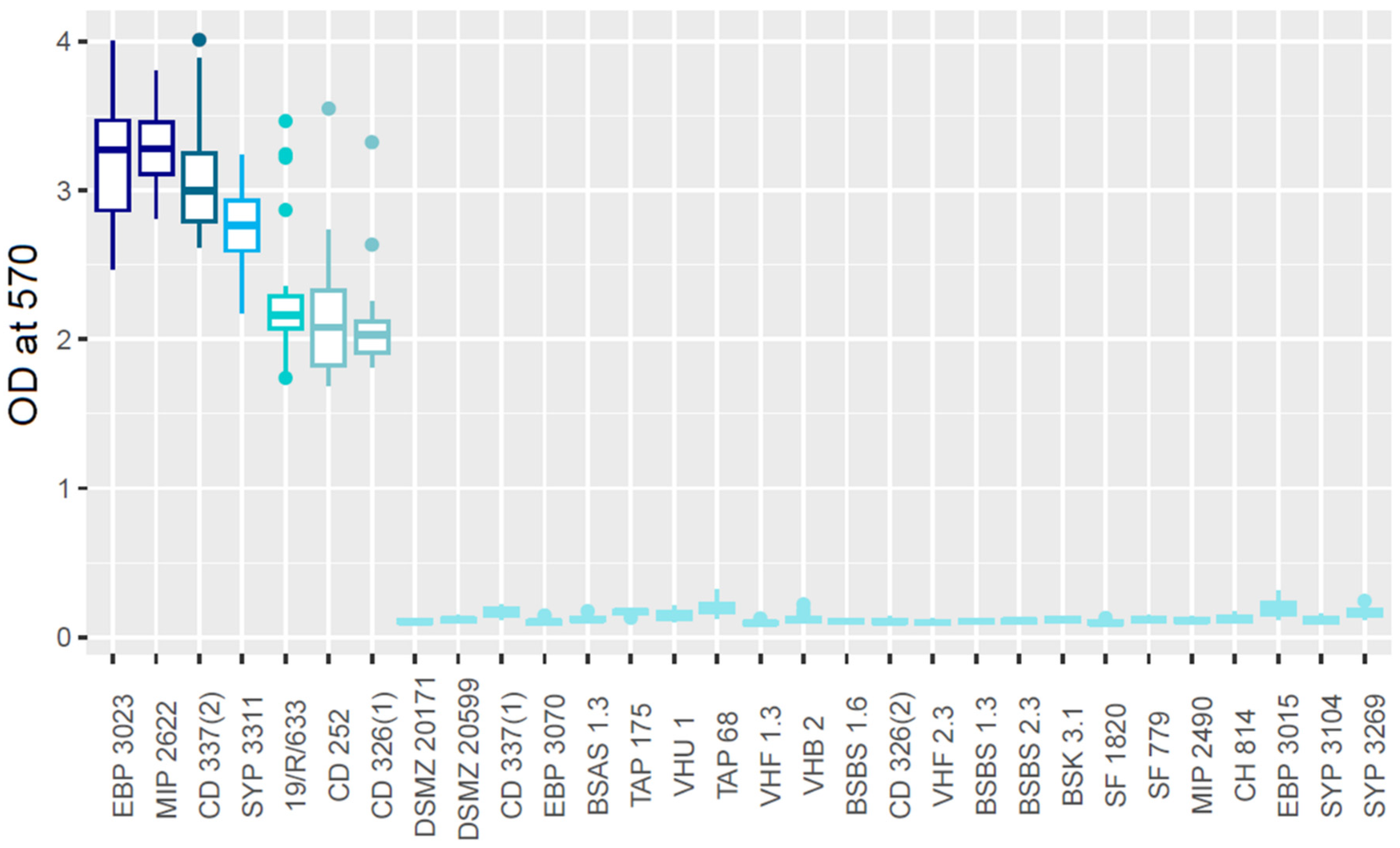
3.1. Assessment of the Biofilm Production Capacity
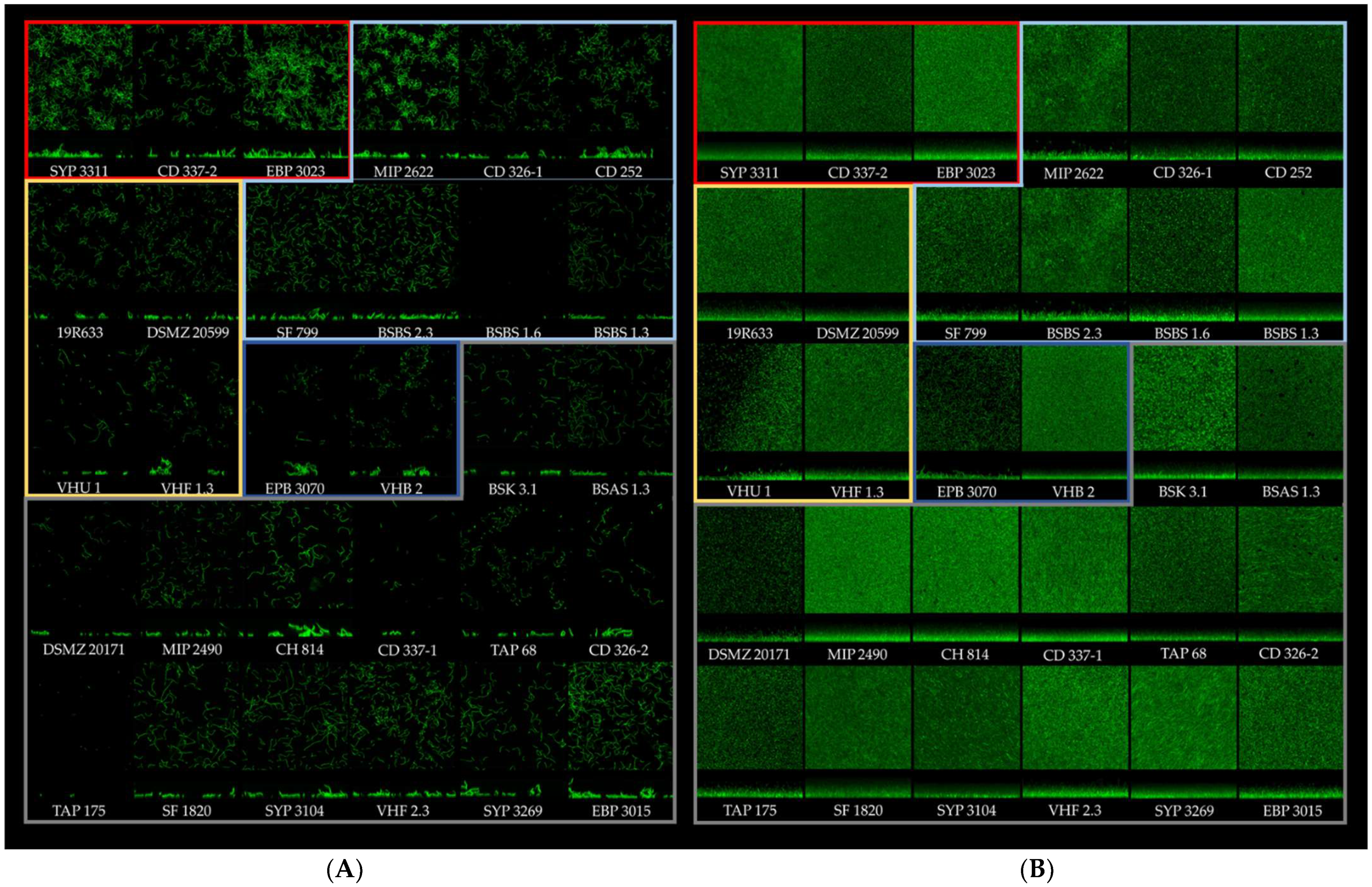
3.2. Analysis of the Biofilm 3D Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borch, E.; Kant-Muermans, M.-L.; Blixt, Y. Bacterial Spoilage of Meat and Cured Meat Products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat Spoilage during Distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Hébert, A.; Abraham, A.-L.; Rasmussen, S.; Monnet, C.; Pons, N.; Delbès, C.; Loux, V.; Batto, J.-M.; Leonard, P.; et al. Construction of a Dairy Microbial Genome Catalog Opens New Perspectives for the Metagenomic Analysis of Dairy Fermented Products. BMC Genom. 2014, 15, 1101. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, S.; Chaulot-Talmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Desmonts, M.H.; Dousset, X.; Feurer, C.; Hamon, E.; et al. Origin and Ecological Selection of Core and Food-Specific Bacterial Communities Associated with Meat and Seafood Spoilage. ISME J. 2015, 9, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Jaffrès, E.; Zagorec, M. Brochothrix thermosphacta. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2018; p. B9780128096338120000. ISBN 978-0-12-809633-8. [Google Scholar]
- Remenant, B.; Jaffrès, E.; Dousset, X.; Pilet, M.-F.; Zagorec, M. Bacterial Spoilers of Food: Behavior, Fitness and Functional Properties. Food Microbiol. 2015, 45, 45–53. [Google Scholar] [CrossRef]
- Joffraud, J.-J.; Cardinal, M.; Cornet, J.; Chasles, J.-S.; Léon, S.; Gigout, F.; Leroi, F. Effect of Bacterial Interactions on the Spoilage of Cold-Smoked Salmon. Int. J. Food Microbiol. 2006, 112, 51–61. [Google Scholar] [CrossRef]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdagué, J.L. Characterisation of Volatile Compounds Produced by Bacteria Isolated from the Spoilage Flora of Cold-Smoked Salmon. Int. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef]
- Stohr, V.; Joffraud, J.J.; Cardinal, M.; Leroi, F. Spoilage Potential and Sensory Profile Associated with Bacteria Isolated from Cold-Smoked Salmon. Food Res. Int. 2001, 34, 797–806. [Google Scholar] [CrossRef]
- Jaffrès, E.; Lalanne, V.; Macé, S.; Cornet, J.; Cardinal, M.; Sérot, T.; Dousset, X.; Joffraud, J.-J. Sensory Characteristics of Spoilage and Volatile Compounds Associated with Bacteria Isolated from Cooked and Peeled Tropical Shrimps Using SPME–GC–MS Analysis. Int. J. Food Microbiol. 2011, 147, 195–202. [Google Scholar] [CrossRef]
- Laursen, B.G.; Leisner, J.J.; Dalgaard, P. Carnobacterium Species: Effect of Metabolic Activity and Interaction with Brochothrix thermosphacta on Sensory Characteristics of Modified Atmosphere Packed Shrimp. J. Agric. Food Chem. 2006, 54, 3604–3611. [Google Scholar] [CrossRef]
- Mejlholm, O.; Bøknaes, N.; Dalgaard, P. Shelf Life and Safety Aspects of Chilled Cooked and Peeled Shrimps (Pandalus borealis) in Modified Atmosphere Packaging. J. Appl. Microbiol. 2005, 99, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; De Filippis, F.; Villani, F.; Ercolini, D. Activities of Strains of Brochothrix thermosphacta in Vitro and in Meat. Food Res. Int. 2014, 62, 366–374. [Google Scholar] [CrossRef]
- Dainty, R.H.; Mackey, B.M. The Relationship between the Phenotypic Properties of Bacteria from Chill-Stored Meat and Spoilage Processes. J. Appl. Bacteriol. 1992, 73, 103s–114s. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality: Residential Bacteria in Food Industry. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Rossero, A.; Chauvet, R.; Courcoux, P.; Pilet, M.-F.; Charrier, T.; Jaffrès, E.; Zagorec, M. Genotypic and Phenotypic Characterization of the Food Spoilage Bacterium Brochothrix thermosphacta. Food Microbiol. 2019, 81, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Maillet, A.; Denojean, P.; Bouju-Albert, A.; Scaon, E.; Leuillet, S.; Dousset, X.; Jaffrès, E.; Combrisson, J.; Prévost, H. Characterization of Bacterial Communities of Cold-Smoked Salmon during Storage. Foods 2021, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Korber, D.R.; Greer, G.G.; Wolfaardt, G.M.; Kohlman, S. Efficacy Enhancement of Trisodium Phosphate against Spoilage and Pathogenic Bacteria in Model Biofilms and on Adipose Tissue. J. Food Prot. 2002, 65, 627–635. [Google Scholar] [CrossRef]
- Karaynir, A.; Salih, H.; Bozdoğan, B.; Güçlü, Ö.; Keskin, D. Isolation and Characterization of Brochothrix Phage ADU4. Virus Res. 2022, 321, 198902. [Google Scholar] [CrossRef]
- Patange, A.; Boehm, D.; Bueno-Ferrer, C.; Cullen, P.J.; Bourke, P. Controlling Brochothrix thermosphacta as a Spoilage Risk Using In-Package Atmospheric Cold Plasma. Food Microbiol. 2017, 66, 48–54. [Google Scholar] [CrossRef]
- Wagner, E.M.; Pracser, N.; Thalguter, S.; Fischel, K.; Rammer, N.; Pospíšilová, L.; Alispahic, M.; Wagner, M.; Rychli, K. Identification of Biofilm Hotspots in a Meat Processing Environment: Detection of Spoilage Bacteria in Multi-Species Biofilms. Int. J. Food Microbiol. 2020, 328, 108668. [Google Scholar] [CrossRef]
- Wagner, E.M.; Fischel, K.; Rammer, N.; Beer, C.; Palmetzhofer, A.L.; Conrady, B.; Roch, F.-F.; Hanson, B.T.; Wagner, M.; Rychli, K. Bacteria of Eleven Different Species Isolated from Biofilms in a Meat Processing Environment Have Diverse Biofilm Forming Abilities. Int. J. Food Microbiol. 2021, 349, 109232. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Klopp, C.; Roulet, A.; Bouchez, O.; Marsaud, N.; Jaffrès, E.; Zagorec, M. One Complete and Three Draft Genome Sequences of Four Brochothrix thermosphacta Strains, CD 337, TAP 175, BSAS1 3 and EBP 3070. Stand. Genom. Sci. 2018, 13, 22. [Google Scholar] [CrossRef]
- Solomon, E.B.; Niemira, B.A.; Sapers, G.M.; Annous, B.A. Biofilm Formation, Cellulose Production, and Curli Biosynthesis by Salmonella Originating from Produce, Animal, and Clinical Sources. J. Food Prot. 2005, 68, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Nguyen, L.-H.T.; Paoli, G.C.; Irwin, P.L. The Complex Multicellular Morphology of the Food Spoilage Bacteria Brochothrix thermosphacta Strains Isolated from Ground Chicken. Can. J. Microbiol. 2020, 66, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.-J. How Bacteria Stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of Biofilm by Candida and Non-Candida Spp. Isolates Causing Fungemia: Comparison of Biomass Production and Metabolic Activity and Development of Cut-off Points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Gomes, I.B.; Meireles, A.; Gonçalves, A.L.; Goeres, D.M.; Sjollema, J.; Simões, L.C.; Simões, M. Standardized Reactors for the Study of Medical Biofilms: A Review of the Principles and Latest Modifications. Crit. Rev. Biotechnol. 2018, 38, 657–670. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A Steam-Based Method to Investigate Biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, E.G.; Toma, L.; Provot, C.; Ascenzioni, F.; Sperduti, I.; Prignano, G.; Gallo, M.T.; Pimpinelli, F.; Bordignon, V.; Bernardi, T.; et al. Development of an in Vitro Assay, Based on the BioFilm Ring Test®, for Rapid Profiling of Biofilm-Growing Bacteria. Front. Microbiol. 2016, 7, 1429. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.R.; den Besten, H.M.W.; van der Veen, S.; Zwietering, M.H.; Moezelaar, R.; Abee, T. Diversity Assessment of Listeria monocytogenes Biofilm Formation: Impact of Growth Condition, Serotype and Strain Origin. Int. J. Food Microbiol. 2013, 165, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Suo, Y.; Deng, X.; Cheng, M.; Shi, C.; Shi, X. Prevalence, Serotype Diversity, Biofilm-Forming Ability and Eradication of Listeria monocytogenes Isolated from Diverse Foods in Shanghai, China. Food Control. 2017, 73, 1068–1073. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Røder, H.L.; Raghupathi, P.K.; Herschend, J.; Brejnrod, A.; Knøchel, S.; Sørensen, S.J.; Burmølle, M. Interspecies Interactions Result in Enhanced Biofilm Formation by Co-Cultures of Bacteria Isolated from a Food Processing Environment. Food Microbiol. 2015, 51, 18–24. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Chen, G.; Li, B.; Chen, D.; Li, L.; Xu, Z. Pathogenic Features and Characteristics of Food Borne Pathogens Biofilm: Biomass, Viability and Matrix. Microb. Pathog. 2017, 111, 285–291. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of Biofilm Formation in Pseudomonas fluorescens WCS365 Proceeds via Multiple, Convergent Signalling Pathways: A Genetic Analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Cavallo, I.; Guembe, M.; Prignano, G.; Gallo, M.T.; Bordignon, V.; D’Agosto, G.; Sperduti, I.; Toma, L.; Ensoli, F. The Clinical Biofilm Ring Test: A Promising Tool for the Clinical Assessment of Biofilm-Producing Candida Species. FEMS Yeast Res. 2018, 18, foy025. [Google Scholar] [CrossRef]
- Chavant, P.; Gaillard-Martinie, B.; Talon, R.; Hébraud, M.; Bernardi, T. A New Device for Rapid Evaluation of Biofilm Formation Potential by Bacteria. J. Microbiol. Methods 2007, 68, 605–612. [Google Scholar] [CrossRef]
- Mountcastle, S.E.; Vyas, N.; Villapun, V.M.; Cox, S.C.; Jabbari, S.; Sammons, R.L.; Shelton, R.M.; Walmsley, A.D.; Kuehne, S.A. Biofilm Viability Checker: An Open-Source Tool for Automated Biofilm Viability Analysis from Confocal Microscopy Images. Npj Biofilms Microbiomes 2021, 7, 44. [Google Scholar] [CrossRef]
- Hartmann, R.; Jeckel, H.; Jelli, E.; Singh, P.K.; Vaidya, S.; Bayer, M.; Rode, D.K.H.; Vidakovic, L.; Díaz-Pascual, F.; Fong, J.C.N.; et al. Quantitative Image Analysis of Microbial Communities with BiofilmQ. Nat. Microbiol. 2021, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Sneath, H.A.; Jones, D. Brochothrix, a New Genus Tentatively Placed in the Family Lactobacillaceae. J. Syst. Evol. Microbiol. 1976, 26, 102–104. [Google Scholar]
- Jaffrès, E.; Sohier, D.; Leroi, F.; Pilet, M.F.; Prévost, H.; Joffraud, J.J.; Dousset, X. Study of the Bacterial Ecosystem in Tropical Cooked and Peeled Shrimps Using a Polyphasic Approach. Int. J. Food Microbiol. 2009, 131, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, A. Écosystèmes Microbiens Des Poissons Tropicaux, Thunnusalbacareset sciaenopsocellatus, Après Abattage et Incidence Sur La Qualité Des Produits. PhD Thesis, University of Antilles, Saint-Claude, France, 2016. [Google Scholar]
- Olivares, E.; Tasse, J.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T. Clinical Biofilm Ring Test® Reveals the Potential Role of β-Lactams in the Induction of Biofilm Formation by P. aeruginosa in Cystic Fibrosis Patients. Pathogens 2020, 9, 1065. [Google Scholar] [CrossRef]
- MIGALE. Migale Bioinformatics Facility; Migale Bioinformatics Faculty: Jouy-en-Josas, France, 2018. [Google Scholar] [CrossRef]
- Lee, B.-H.; Hébraud, M.; Bernardi, T. Increased Adhesion of Listeria monocytogenes Strains to Abiotic Surfaces under Cold Stress. Front. Microbiol. 2017, 8, 2221. [Google Scholar] [CrossRef]
- Novais, Â.; Vuotto, C.; Pires, J.; Montenegro, C.; Donelli, G.; Coque, T.M.; Peixe, L. Diversity and Biofilm-Production Ability among Isolates of Escherichia coli Phylogroup D Belonging to ST69, ST393 and ST405 Clonal Groups. BMC Microbiol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Cherif-Antar, A.; Moussa–Boudjemâa, B.; Didouh, N.; Medjahdi, K.; Mayo, B.; Flórez, A.B. Diversity and Biofilm-Forming Capability of Bacteria Recovered from Stainless Steel Pipes of a Milk-Processing Dairy Plant. Dairy Sci. Technol. 2016, 96, 27–38. [Google Scholar] [CrossRef]
- Guilbaud, M.; Piveteau, P.; Desvaux, M.; Brisse, S.; Briandet, R. Exploring the Diversity of Listeria monocytogenes Biofilm Architecture by High-Throughput Confocal Laser Scanning Microscopy and the Predominance of the Honeycomb-Like Morphotype. Appl. Environ. Microbiol. 2015, 81, 1813–1819. [Google Scholar] [CrossRef]
- Chávez de Paz, L.E.; Hamilton, I.R.; Svensäter, G. Oral Bacteria in Biofilms Exhibit Slow Reactivation from Nutrient Deprivation. Microbiology 2008, 154, 1927–1938. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The Biofilm Matrix: Multitasking in a Shared Space. Nat. Rev. Microbiol. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Xie, J.; Zhao, L.-J.; Qian, Y.-F.; Zhao, Y.; Liu, X. Biofilm Formation Characteristics of Pseudomonas lundensis Isolated from Meat: Pseudomonas lundensis Biofilms. J. Food Sci. 2015, 80, M2904–M2910. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F.; Kathariou, S. Competition of Listeria monocytogenes Serotype 1/2a and 4b Strains in Mixed-Culture Biofilms. Appl. Environ. Microbiol. 2009, 75, 5846–5852. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.M.; Miranda, C.D.; De la Fuente, M.; Alarcón, M.; Aroca, G.; Sossa, K.; Ruiz, P.; Urrutia, H. Formation of Biofilms of the Salmon Pathogen Flavobacterium psychrophilum in Differents Surfaces Using the CDC Biofilm Reactor. Aquaculture 2020, 514, 734459. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm Formation and Persistence on Abiotic Surfaces in the Context of Food and Medical Environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Larsen, M.H.; Dalmasso, M.; Ingmer, H.; Langsrud, S.; Malakauskas, M.; Mader, A.; Møretrø, T.; Možina, S.S.; Rychli, K.; Wagner, M.; et al. Persistence of Foodborne Pathogens and Their Control in Primary and Secondary Food Production Chains. Food Control. 2014, 44, 92–109. [Google Scholar] [CrossRef]
- Vestby, L.K.; Møretrø, T.; Langsrud, S.; Heir, E.; Nesse, L.L. Biofilm Forming Abilities of Salmonella are Correlated with Persistence in Fish Meal- and Feed Factories. BMC Vet. Res. 2009, 5, 20. [Google Scholar] [CrossRef]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of Bacterial Biofilms to Disinfectants: A Review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef]

| Scheme. | Ecological Source | Strain Origin | References |
|---|---|---|---|
| DSMZT20171 | Fresh pork sausage | DSM/ATCC | [44] |
| DSMZ20599 | Bacon | DSM/ATCC | |
| CD 337(1) | Peeled shrimp | INRAE-SECALIM | [45] |
| CD 337(2) | Peeled shrimp | INRAE-SECALIM | |
| CD326 (1) | Peeled shrimp | INRAE-SECALIM | |
| CD326 (2) | Peeled shrimp | INRAE-SECALIM | |
| CD252 | Peeled shrimp | INRAE-SECALIM | |
| EBP3070 | Smoked salmon | INRAE-SECALIM | [16] |
| MIP2622 | Salmon | INRAE-SECALIM | |
| MIP2490 | Salmon | INRAE-SECALIM | |
| BSAS1.3 | Bovine slaughterhouse animal skin | INRAE-SECALIM | |
| VHU1 | Chopped steak | INRAE-SECALIM | |
| VHF1.3 | Chopped steak | INRAE-SECALIM | |
| VHB2 | Butcher’s chopped steak | INRAE-SECALIM | |
| VHF2.3 | Chopped steak-Férial | INRAE-SECALIM | |
| TAP175 | Chicken thigh (MAP) | INRAE-SECALIM | |
| TAP68 | Chicken thigh (MAP) | INRAE-SECALIM | |
| BSBS1.6 | Beef slaughterhouse environment | INRAE-SECALIM | |
| BSBS1.3 | Beef slaughterhouse environment | INRAE-SECALIM | |
| BSBS2.3 | Beef slaughterhouse environment | INRAE-SECALIM | |
| BSK3.1 | Bovine slaughterhouse knife | INRAE-SECALIM | |
| SF1820 | Smoked salmon | INRAE-SECALIM | [17] |
| SF779 | Smoked salmon | INRAE-SECALIM | |
| 19/R/633 | Salmon-filleting machine after cleaning | INRAE-SECALIM | |
| CH814 | Saint Nectaire cheese | UMRF Aurillac-France | [3] |
| EBP3015 | Cod (MAP) | IFREMER Nantes | [46] |
| EBP3023 | Cod (MAP) | IFREMER Nantes | |
| SYP3104 | Fresh tuna | IFREMER Nantes | |
| SYP3269 | Fresh red drum | IFREMER Nantes | |
| SYP3311 | Red drum (MAP) | IFREMER Nantes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaillac, A.; Briandet, R.; Delahaye, E.; Deschamps, J.; Vigneau, E.; Courcoux, P.; Jaffrès, E.; Prévost, H. Exploring the Diversity of Biofilm Formation by the Food Spoiler Brochothrix thermosphacta. Microorganisms 2022, 10, 2474. https://doi.org/10.3390/microorganisms10122474
Gaillac A, Briandet R, Delahaye E, Deschamps J, Vigneau E, Courcoux P, Jaffrès E, Prévost H. Exploring the Diversity of Biofilm Formation by the Food Spoiler Brochothrix thermosphacta. Microorganisms. 2022; 10(12):2474. https://doi.org/10.3390/microorganisms10122474
Chicago/Turabian StyleGaillac, Antoine, Romain Briandet, Elodie Delahaye, Julien Deschamps, Evelyne Vigneau, Philippe Courcoux, Emmanuel Jaffrès, and Hervé Prévost. 2022. "Exploring the Diversity of Biofilm Formation by the Food Spoiler Brochothrix thermosphacta" Microorganisms 10, no. 12: 2474. https://doi.org/10.3390/microorganisms10122474
APA StyleGaillac, A., Briandet, R., Delahaye, E., Deschamps, J., Vigneau, E., Courcoux, P., Jaffrès, E., & Prévost, H. (2022). Exploring the Diversity of Biofilm Formation by the Food Spoiler Brochothrix thermosphacta. Microorganisms, 10(12), 2474. https://doi.org/10.3390/microorganisms10122474







