Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus
Abstract
1. Introduction
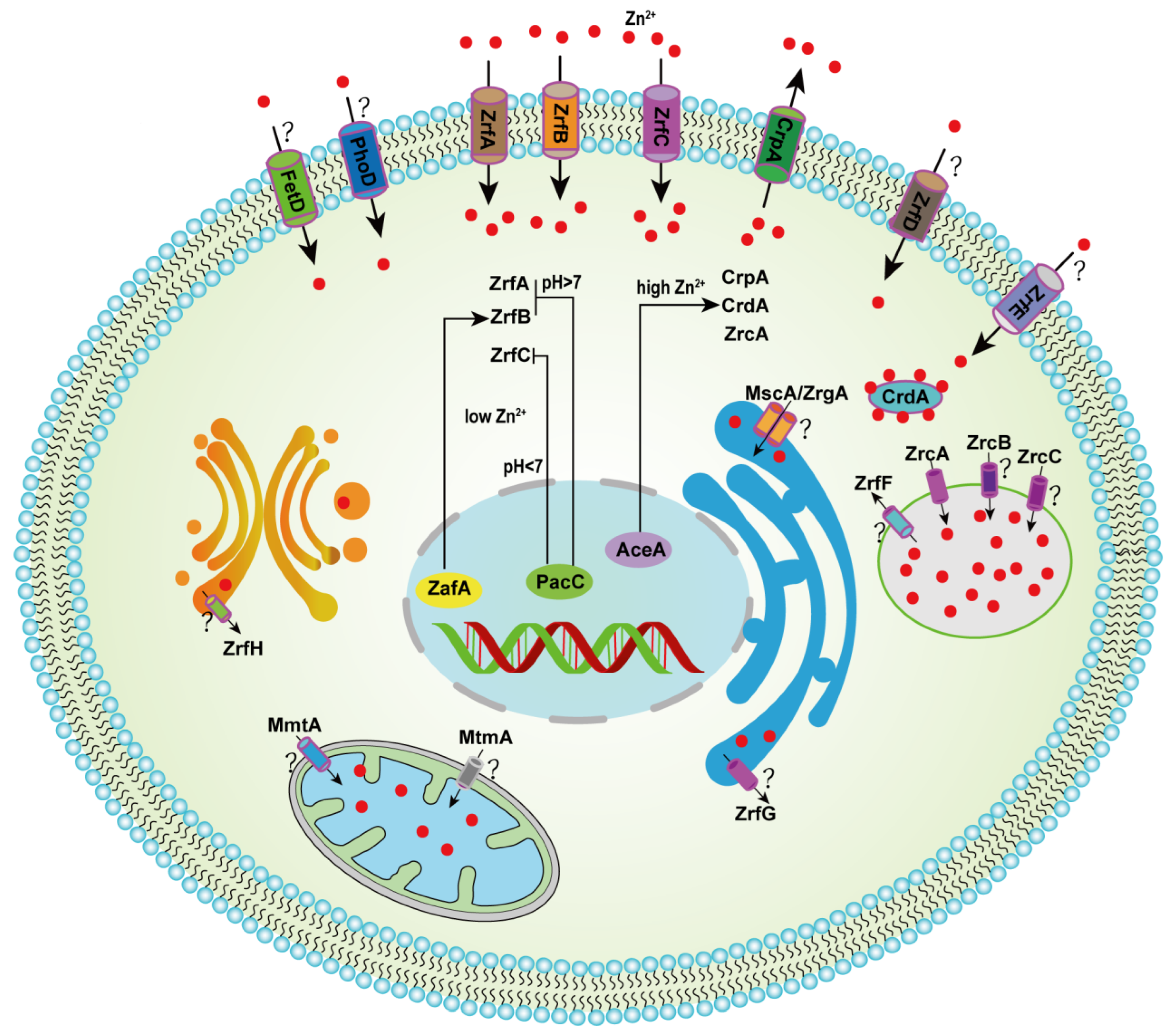
2. Members Involved in Zinc Homeostasis in A. fumigatus
2.1. Identified or Predicted Transporters Involved in Zinc Transport at the Plasma Membrane
2.2. Potential Zinc Transport-Involved and Organelle-Localized Transporters
2.3. Zinc-Responsive Transcription Factors
2.4. Zinc Trafficking-Involved and other Zinc Homeostasis-Related Proteins
3. Zinc Homeostasis for the A. fumigatus Virulence
4. Potential Antifungal Targets of Zinc Homeostasis Regulators and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, G.; Haas, A.; Cornely, O.A. Invasive aspergillosis: Epidemiology, diagnosis and management in immunocompromised patients. Drugs 2007, 67, 1567–1601. [Google Scholar] [CrossRef] [PubMed]
- Balloy, V.; Chignard, M. The innate immune response to Aspergillus fumigatus. Microbes Infect. 2009, 11, 919–927. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.; Loeffler, J.; Ebel, F. Aspergillus fumigatus: Contours of an opportunistic human pathogen. Cell Microbiol. 2010, 12, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Polvi, E.J.; Shekhar-Guturja, T.; Cowen, L.E. Elucidating drug resistance in human fungal pathogens. Future Microbiol. 2014, 9, 523–542. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef]
- Abad, A.; Fernandez-Molina, J.V.; Bikandi, J.; Ramirez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Ponton, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Gifford, A.H.; Klippenstein, J.R.; Moore, M.M. Serum stimulates growth of and proteinase secretion by Aspergillus fumigatus. Infect. Immun. 2002, 70, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, W.; Bruno, V.M.; Phan, Q.T.; Solis, N.V.; Woolford, C.A.; Ehrlich, R.L.; Shetty, A.C.; McCraken, C.; Lin, J.; et al. Determining Aspergillus fumigatus transcription factor expression and function during invasion of the mammalian lung. PLoS Pathog. 2021, 17, e1009235. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.W.; Kathman, S.J.; Sarasua, S.M.; White, M.C. Influence of environmental zinc on the association between environmental and biological measures of lead in children. J. Expo. Anal. Environ. Epidemiol. 2003, 13, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hantke, K. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 2005, 8, 196–202. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I. A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 2012, 111, 150–156. [Google Scholar] [CrossRef]
- Overbeck, S.; Rink, L.; Haase, H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch. Immunol. Ther. Exp. 2008, 56, 15–30. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 175, 598–599. [Google Scholar] [CrossRef]
- Traynor, A.M.; Owens, R.A.; Coughlin, C.M.; Holton, M.C.; Jones, G.W.; Calera, J.A.; Doyle, S. At the metal-metabolite interface in Aspergillus fumigatus: Towards untangling the intersecting roles of zinc and gliotoxin. Microbiology 2021, 167, 001106. [Google Scholar] [CrossRef]
- Seo, H.; Kang, S.; Park, Y.S.; Yun, C.W. The Role of Zinc in Gliotoxin Biosynthesis of Aspergillus fumigatus. Int. J. Mol. Sci. 2019, 20, 6192. [Google Scholar] [CrossRef]
- Saleh, A.A.; Jones, G.W.; Tinley, F.C.; Delaney, S.F.; Alabbadi, S.H.; Fenlon, K.; Doyle, S.; Owens, R.A. Systems impact of zinc chelation by the epipolythiodioxopiperazine dithiol gliotoxin in Aspergillus fumigatus: A new direction in natural product functionality. Metallomics 2018, 10, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, W.; Solis, N.V.; Woolford, C.; Mitchell, A.P.; Filler, S.G. Functional convergence of gliP and aspf1 in Aspergillus fumigatus pathogenicity. Virulence 2018, 9, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- du Pré, S.; Dogra, T.; van de Sande, W.W.J. The putative role of zinc homeostasis in grain formation by Madurella mycetomatis during mycetoma infection. Fungal Biol. Rev. 2022, 39, 73–82. [Google Scholar] [CrossRef]
- Korndorfer, I.P.; Brueckner, F.; Skerra, A. The crystal structure of the human (S100A8/S100A9)2 heterotetramer, calprotectin, illustrates how conformational changes of interacting alpha-helices can determine specific association of two EF-hand proteins. J. Mol. Biol. 2007, 370, 887–898. [Google Scholar] [CrossRef]
- Corbin, B.D.; Seeley, E.H.; Raab, A.; Feldmann, J.; Miller, M.R.; Torres, V.J.; Anderson, K.L.; Dattilo, B.M.; Dunman, P.M.; Gerads, R.; et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 2008, 319, 962–965. [Google Scholar] [CrossRef]
- Hayden, J.A.; Brophy, M.B.; Cunden, L.S.; Nolan, E.M. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J. Am. Chem. Soc. 2013, 135, 775–787. [Google Scholar] [CrossRef]
- Sandrin, T.R.; Maier, R.M. Impact of metals on the biodegradation of organic pollutants. Environ. Health Perspect. 2003, 111, 1093–1101. [Google Scholar] [CrossRef]
- Foote, J.W.; Delves, H.T. Albumin bound and alpha 2-macroglobulin bound zinc concentrations in the sera of healthy adults. J. Clin. Pathol. 1984, 37, 1050–1054. [Google Scholar] [CrossRef]
- Iyengar, V.; Woittiez, J. Trace elements in human clinical specimens: Evaluation of literature data to identify reference values. Clin. Chem. 1988, 34, 474–481. [Google Scholar] [CrossRef]
- Amich, J.; Vicentefranqueira, R.; Mellado, E.; Ruiz-Carmuega, A.; Leal, F.; Calera, J.A. The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol. 2014, 16, 548–564. [Google Scholar] [CrossRef]
- Clohessy, P.A.; Golden, B.E. Calprotectin-mediated zinc chelation as a biostatic mechanism in host defence. Scand. J. Immunol. 1995, 42, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Vermilyea, D.M.; Crocker, A.W.; Gifford, A.H.; Hogan, D.A. Calprotectin-Mediated Zinc Chelation Inhibits Pseudomonas aeruginosa Protease Activity in Cystic Fibrosis Sputum. J. Bacteriol. 2021, 203, e0010021. [Google Scholar] [CrossRef] [PubMed]
- Garcia Silva-Bailao, M.; Lobato Potenciano da Silva, K.; Raniere Borges Dos Anjos, L.; de Sousa Lima, P.; de Melo Teixeira, M.; Maria de Almeida Soares, C.; Melo Bailao, A. Mechanisms of copper and zinc homeostasis in pathogenic black fungi. Fungal Biol. 2018, 122, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Mislin, G.L.A.; Latge, J.P.; Beauvais, A. Interactions between Aspergillus fumigatus and Pulmonary Bacteria: Current State of the Field, New Data, and Future Perspective. J. Fungi 2019, 5, 28. [Google Scholar] [CrossRef]
- Bird, A.J.; Wilson, S. Zinc homeostasis in the secretory pathway in yeast. Curr. Opin. Chem. Biol. 2020, 55, 145–150. [Google Scholar] [CrossRef]
- Eide, D.J. Multiple regulatory mechanisms maintain zinc homeostasis in Saccharomyces cerevisiae. J. Nutr. 2003, 133 (Suppl. 1), 1532S–1535S. [Google Scholar] [CrossRef]
- MacDiarmid, C.W.; Gaither, L.A.; Eide, D. Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 2000, 19, 2845–2855. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. Eukaryotic zinc transporters and their regulation. Biometals 2001, 14, 251–270. [Google Scholar] [CrossRef]
- Wu, X.; Su, N.; Yue, X.; Fang, B.; Zou, J.; Chen, Y.; Shen, Z.; Cui, J. IRT1 and ZIP2 were involved in exogenous hydrogen-rich water-reduced cadmium accumulation in Brassica chinensis and Arabidopsis thaliana. J. Hazard. Mater. 2021, 407, 124599. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Ricachenevsky, F.K.; Punshon, T.; Tappero, R.; Salt, D.E.; Guerinot, M.L. Redundant roles of four ZIP family members in zinc homeostasis and seed development in Arabidopsis thaliana. Plant. J. 2021, 108, 1162–1173. [Google Scholar] [CrossRef]
- Amich, J.; Calera, J.A. Zinc acquisition: A key aspect in Aspergillus fumigatus virulence. Mycopathologia 2014, 178, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta 2000, 1465, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gitan, R.S.; Shababi, M.; Kramer, M.; Eide, D.J. A cytosolic domain of the yeast Zrt1 zinc transporter is required for its post-translational inactivation in response to zinc and cadmium. J. Biol. Chem. 2003, 278, 39558–39564. [Google Scholar] [CrossRef] [PubMed]
- Gitan, R.S.; Eide, D.J. Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem. J. 2000, 346 Pt 2, 329–336. [Google Scholar] [CrossRef]
- Zhao, H.; Eide, D. The ZRT2 gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 23203–23210. [Google Scholar] [CrossRef]
- Vicentefranqueira, R.; Moreno, M.A.; Leal, F.; Calera, J.A. The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot. Cell 2005, 4, 837–848. [Google Scholar] [CrossRef]
- Amich, J.; Vicentefranqueira, R.; Leal, F.; Calera, J.A. Aspergillus fumigatus survival in alkaline and extreme zinc-limiting environments relies on the induction of a zinc homeostasis system encoded by the zrfC and aspf2 genes. Eukaryot. Cell 2010, 9, 424–437. [Google Scholar] [CrossRef]
- Vicentefranqueira, R.; Amich, J.; Laskaris, P.; Ibrahim-Granet, O.; Latge, J.P.; Toledo, H.; Leal, F.; Calera, J.A. Targeting zinc homeostasis to combat Aspergillus fumigatus infections. Front. Microbiol. 2015, 6, 160. [Google Scholar] [CrossRef]
- Hassett, R.; Dix, D.R.; Eide, D.J.; Kosman, D.J. The Fe(II) permease Fet4p functions as a low affinity copper transporter and supports normal copper trafficking in Saccharomyces cerevisiae. Biochem. J. 2000, 351 Pt 2, 477–484. [Google Scholar] [CrossRef]
- Waters, B.M.; Eide, D.J. Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J. Biol. Chem. 2002, 277, 33749–33757. [Google Scholar] [CrossRef]
- Levy, S.; Kafri, M.; Carmi, M.; Barkai, N. The competitive advantage of a dual-transporter system. Science 2011, 334, 1408–1412. [Google Scholar] [CrossRef]
- Cai, Z.; Du, W.; Zhang, Z.; Guan, L.; Zeng, Q.; Chai, Y.; Dai, C.; Lu, L. The Aspergillus fumigatus transcription factor AceA is involved not only in Cu but also in Zn detoxification through regulating transporters CrpA and ZrcA. Cell Microbiol. 2018, 20, e12864. [Google Scholar] [CrossRef] [PubMed]
- Antsotegi-Uskola, M.; Markina-Inarrairaegui, A.; Ugalde, U. Copper Resistance in Aspergillus nidulans Relies on the PI-Type ATPase CrpA, Regulated by the Transcription Factor AceA. Front. Microbiol. 2017, 8, 912. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Perevitsky, A.; Lim, F.Y.; Shadkchan, Y.; Knox, B.P.; Landero Figueora, J.A.; Choera, T.; Niu, M.; Steinberger, A.J.; Wuthrich, M.; et al. Aspergillus fumigatus Copper Export Machinery and Reactive Oxygen Intermediate Defense Counter Host Copper-Mediated Oxidative Antimicrobial Offense. Cell Rep. 2017, 19, 2174–2176. [Google Scholar] [CrossRef] [PubMed]
- Kumanovics, A.; Poruk, K.E.; Osborn, K.A.; Ward, D.M.; Kaplan, J. YKE4 (YIL023C) encodes a bidirectional zinc transporter in the endoplasmic reticulum of Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 22566–22574. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.W.A.; Kinskovski, U.P.; Diehl, C.; Reuwsaat, J.C.V.; Motta de Souza, H.; Pinto, H.B.; Trentin, D.D.S.; de Oliveira, H.C.; Rodrigues, M.L.; Becker, E.M.; et al. Participation of Zip3, a ZIP domain-containing protein, in stress response and virulence in Cryptococcus gattii. Fungal Genet. Biol. 2020, 144, 103438. [Google Scholar] [CrossRef]
- Lin, S.J.; Culotta, V.C. Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, which encodes a manganese-trafficking protein that localizes to Golgi-like vesicles. Mol. Cell Biol. 1996, 16, 6303–6312. [Google Scholar] [CrossRef]
- Kolaj-Robin, O.; Russell, D.; Hayes, K.A.; Pembroke, J.T.; Soulimane, T. Cation Diffusion Facilitator family: Structure and function. FEBS Lett. 2015, 589, 1283–1295. [Google Scholar] [CrossRef]
- Porcheron, G.; Garenaux, A.; Proulx, J.; Sabri, M.; Dozois, C.M. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: Correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front. Cell Infect. Microbiol. 2013, 3, 90. [Google Scholar] [CrossRef]
- DalCorso, G.; Martini, F.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Enhancement of Zn tolerance and accumulation in plants mediated by the expression of Saccharomyces cerevisiae vacuolar transporter ZRC1. Planta 2021, 253, 117. [Google Scholar] [CrossRef]
- Miyabe, S.; Izawa, S.; Inoue, Y. Expression of ZRC1 coding for suppressor of zinc toxicity is induced by zinc-starvation stress in Zap1-dependent fashion in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2000, 276, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, S.; Izawa, S.; Inoue, Y. The Zrc1 is involved in zinc transport system between vacuole and cytosol in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2001, 282, 79–83. [Google Scholar] [CrossRef]
- Lin, H.; Kumanovics, A.; Nelson, J.M.; Warner, D.E.; Ward, D.M.; Kaplan, J. A single amino acid change in the yeast vacuolar metal transporters ZRC1 and COT1 alters their substrate specificity. J. Biol. Chem. 2008, 283, 33865–33873. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Abt, B.; Schrettl, M.; Moussa, T.A.; Werner, E.R.; Haas, H. The interplay between iron and zinc metabolism in Aspergillus fumigatus. Fungal Genet. Biol. 2009, 46, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kaplan, J. The yeast gene MSC2, a member of the cation diffusion facilitator family, affects the cellular distribution of zinc. J. Biol. Chem. 2001, 276, 5036–5043. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.D.; Wang, F.; MacDiarmid, C.W.; Clark, S.; Lyons, T.; Eide, D.J. Zinc and the Msc2 zinc transporter protein are required for endoplasmic reticulum function. J. Cell Biol. 2004, 166, 325–335. [Google Scholar] [CrossRef]
- Li, L.; Miao, R.; Jia, X.; Ward, D.M.; Kaplan, J. Expression of the yeast cation diffusion facilitators Mmt1 and Mmt2 affects mitochondrial and cellular iron homeostasis: Evidence for mitochondrial iron export. J. Biol. Chem. 2014, 289, 17132–17141. [Google Scholar] [CrossRef]
- Li, L.; Bertram, S.; Kaplan, J.; Jia, X.; Ward, D.M. The mitochondrial iron exporter genes MMT1 and MMT2 in yeast are transcriptionally regulated by Aft1 and Yap1. J. Biol. Chem. 2020, 295, 1716–1726. [Google Scholar] [CrossRef]
- Wu, C.Y.; Bird, A.J.; Chung, L.M.; Newton, M.A.; Winge, D.R.; Eide, D.J. Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genom. 2008, 9, 370. [Google Scholar] [CrossRef]
- Zhao, H.; Butler, E.; Rodgers, J.; Spizzo, T.; Duesterhoeft, S.; Eide, D. Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements. J. Biol. Chem. 1998, 273, 28713–28720. [Google Scholar] [CrossRef]
- Moreno, M.A.; Ibrahim-Granet, O.; Vicentefranqueira, R.; Amich, J.; Ave, P.; Leal, F.; Latge, J.P.; Calera, J.A. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol. Microbiol. 2007, 64, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Vicentefranqueira, R.; Amich, J.; Marin, L.; Sanchez, C.I.; Leal, F.; Calera, J.A. The Transcription Factor ZafA Regulates the Homeostatic and Adaptive Response to Zinc Starvation in Aspergillus fumigatus. Genes 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Bignell, E.; Negrete-Urtasun, S.; Calcagno, A.M.; Haynes, K.; Arst, H.N., Jr.; Rogers, T. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol. Microbiol. 2005, 55, 1072–1084. [Google Scholar] [CrossRef]
- Amich, J.; Leal, F.; Calera, J.A. Repression of the acid ZrfA/ZrfB zinc-uptake system of Aspergillus fumigatus mediated by PacC under neutral, zinc-limiting conditions. Int. Microbiol. 2009, 12, 39–47. [Google Scholar] [PubMed]
- Dasari, P.; Shopova, I.A.; Stroe, M.; Wartenberg, D.; Martin-Dahse, H.; Beyersdorf, N.; Hortschansky, P.; Dietrich, S.; Cseresnyes, Z.; Figge, M.T.; et al. Aspf2 From Aspergillus fumigatus Recruits Human Immune Regulators for Immune Evasion and Cell Damage. Front. Immunol. 2018, 9, 1635. [Google Scholar] [CrossRef] [PubMed]
- Garstka, K.; Hecel, A.; Kozlowski, H.; Rowinska-Zyrek, M. Specific Zn(II)-binding site in the C-terminus of Aspf2, a zincophore from Aspergillus fumigatus. Metallomics 2022, 14, mfac042. [Google Scholar] [CrossRef]
- Luk, E.; Carroll, M.; Baker, M.; Culotta, V.C. Manganese activation of superoxide dismutase 2 in Saccharomyces cerevisiae requires MTM1, a member of the mitochondrial carrier family. Proc. Natl. Acad. Sci. USA 2003, 100, 10353–10357. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Wang, L.; Wu, J.; Simth, N.; Zhang, L.; Wang, Y.; Wu, X. MTM1 plays an important role in the regulation of zinc tolerance in Saccharomyces cerevisiae. J. Trace Elem. Med. Biol. 2021, 66, 126759. [Google Scholar] [CrossRef]
- Zhai, P.; Ma, Y.; Xu, H.; Lu, L. Molecular Characterization and the Essential Biological Function of the Metal Chaperone Protein MtmA in Aspergillus fumigatus. Appl. Environ. Microbiol. 2022, 88, e0018222. [Google Scholar] [CrossRef]
- Haas, C.E.; Rodionov, D.A.; Kropat, J.; Malasarn, D.; Merchant, S.S.; de Crecy-Lagard, V. A subset of the diverse COG0523 family of putative metal chaperones is linked to zinc homeostasis in all kingdoms of life. BMC Genom. 2009, 10, 470. [Google Scholar] [CrossRef]
- Botella, H.; Peyron, P.; Levillain, F.; Poincloux, R.; Poquet, Y.; Brandli, I.; Wang, C.; Tailleux, L.; Tilleul, S.; Charriere, G.M.; et al. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe 2011, 10, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E.; Coyne, A.G.; Hudson, S.A.; Abell, C. Fragment-based approaches in drug discovery and chemical biology. Biochemistry 2012, 51, 4990–5003. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.S.; Mills, J.E.; Williams, G.P.; Brannigan, J.A.; Wilkinson, A.J.; Parkinson, T.; Leatherbarrow, R.J.; Tate, E.W.; Holder, A.A.; Smith, D.F. Selective inhibitors of protozoan protein N-myristoyltransferases as starting points for tropical disease medicinal chemistry programs. PLoS Negl. Trop. Dis. 2012, 6, e1625. [Google Scholar] [CrossRef] [PubMed]
- Rackham, M.D.; Brannigan, J.A.; Rangachari, K.; Meister, S.; Wilkinson, A.J.; Holder, A.A.; Leatherbarrow, R.J.; Tate, E.W. Design and synthesis of high affinity inhibitors of Plasmodium falciparum and Plasmodium vivax N-myristoyltransferases directed by ligand efficiency dependent lipophilicity (LELP). J. Med. Chem. 2014, 57, 2773–2788. [Google Scholar] [CrossRef] [PubMed]
| Systematic Name | Gene Name * | Description | Location | References |
|---|---|---|---|---|
| AFUB_079250 | zrfA(zrt1) | Plasma membrane zinc transporter | Plasma membrane | [46] |
| AFUB_020930 | zrfB(zrt2) | Plasma membrane zinc transporter | Plasma membrane | [46] |
| AFUB_066680 | zrfC | Plasma membrane zinc transporter | Plasma membrane | [30,47] |
| AFUB_097050 | zrfD | Putative zinc importer | Plasma membrane (Putative) | [41] |
| AFUB_083560 | zrfE | Putative zinc importer | Plasma membrane (Putative) | [41] |
| AFUB_024650 | zrfF(zrt3) | Putative zinc importer | Vacuole (Putative) | Predicted in this study |
| AFUB_018540 | zrfG(yke4) | Putative zinc importer | Endoplasmic reticulum (Putative) | Predicted in this study |
| AFUB_027750 | zrfH(atx2) | Putative zinc importer | Golgi (Putative) | Predicted in this study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, P.; Chai, Y.; Lu, L. Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus. Microorganisms 2022, 10, 2469. https://doi.org/10.3390/microorganisms10122469
Zhai P, Chai Y, Lu L. Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus. Microorganisms. 2022; 10(12):2469. https://doi.org/10.3390/microorganisms10122469
Chicago/Turabian StyleZhai, Pengfei, Yanfei Chai, and Ling Lu. 2022. "Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus" Microorganisms 10, no. 12: 2469. https://doi.org/10.3390/microorganisms10122469
APA StyleZhai, P., Chai, Y., & Lu, L. (2022). Fungal Zinc Homeostasis and Its Potential as an Antifungal Target: A Focus on the Human Pathogen Aspergillus fumigatus. Microorganisms, 10(12), 2469. https://doi.org/10.3390/microorganisms10122469






