Comparative Analysis of the Liver Transcriptome of Beijing You Chickens and Guang Ming Broilers under Salmonella enterica Serovar Typhimurium Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Population and Design
2.3. Salmonella enterica Serovar Typhimurium Infection
2.4. Phenotype Determination
2.5. Total RNA Isolation, cDNA Library Construction, and Sequencing
2.6. Screening of DEGs
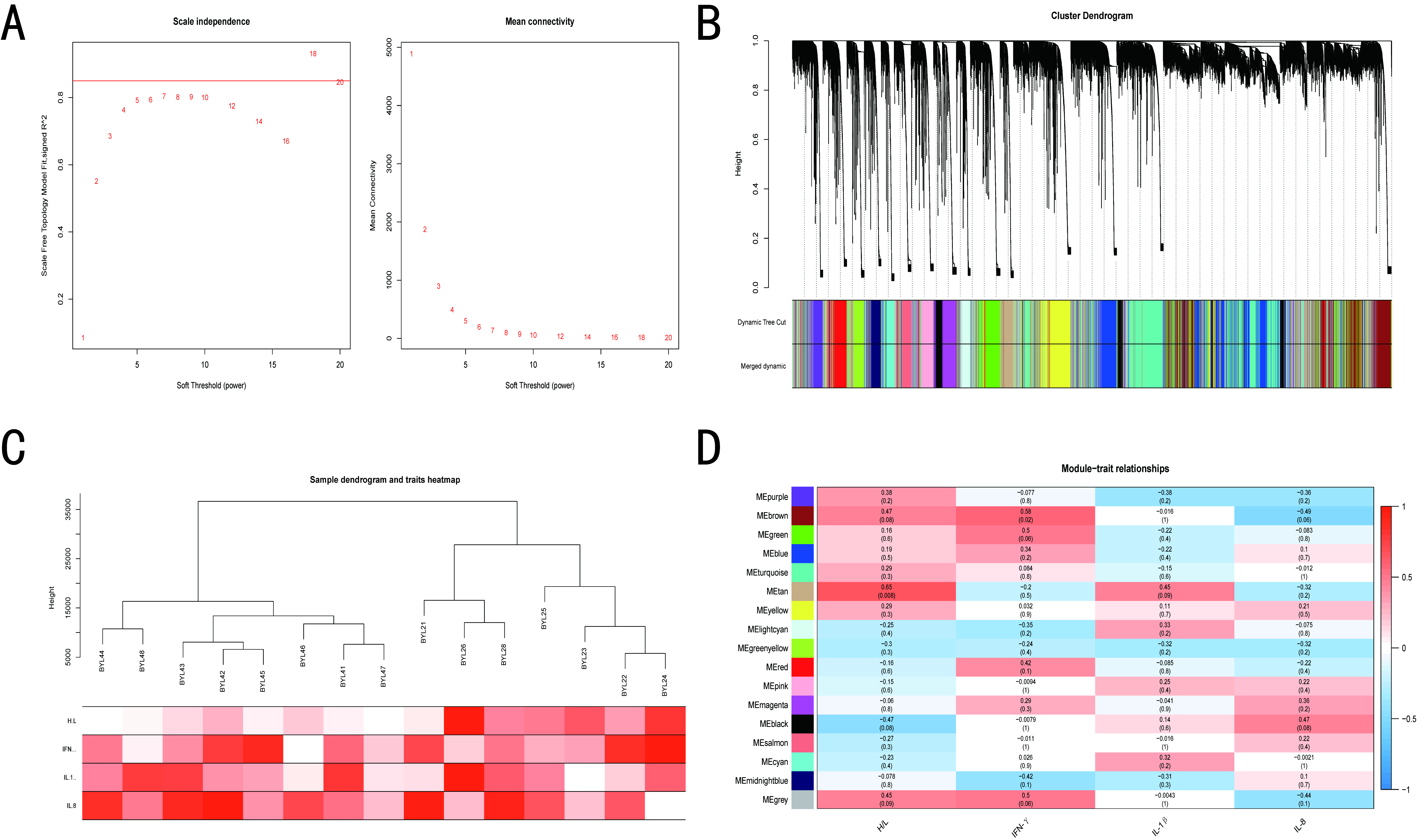
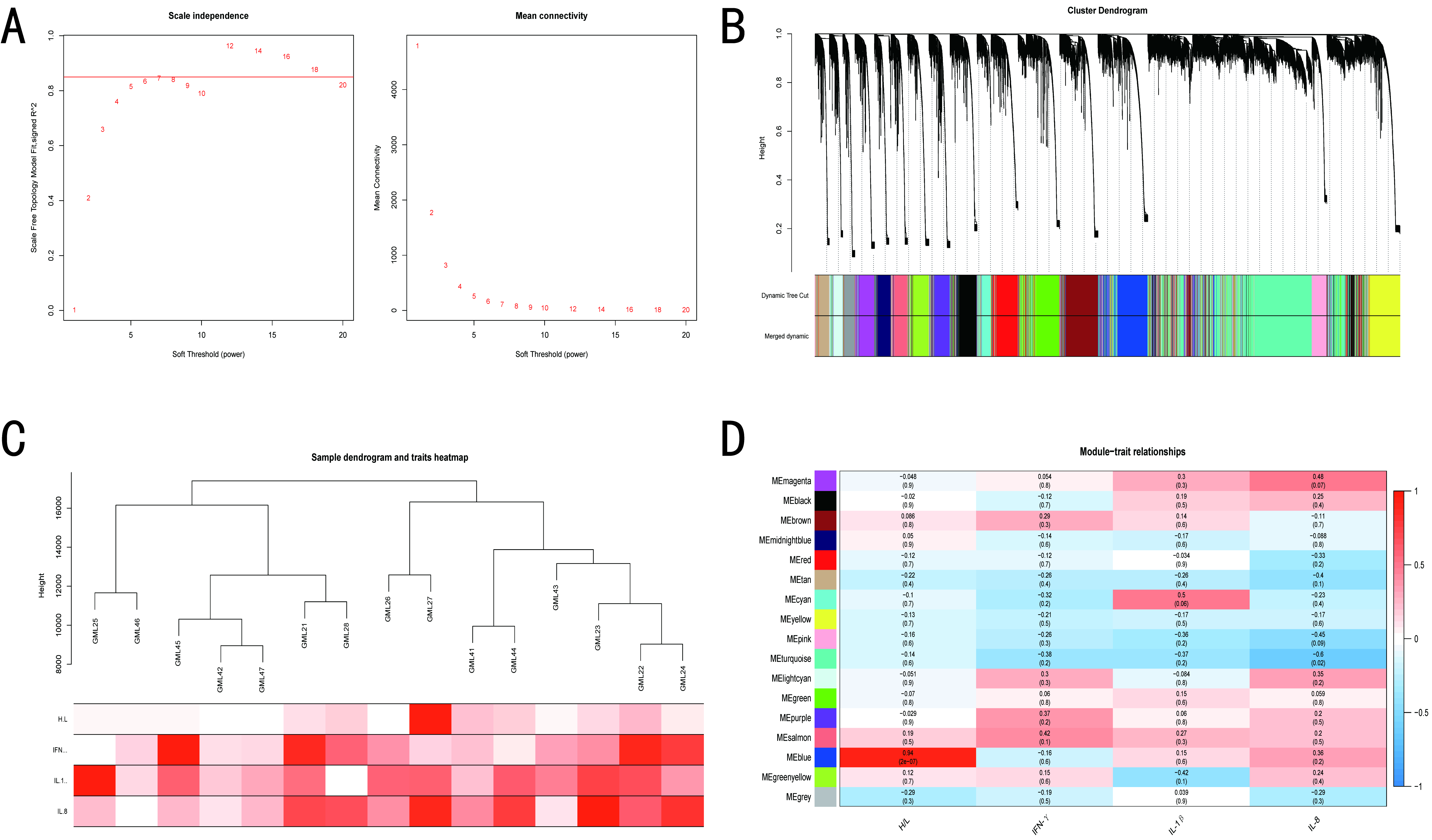
2.7. Weighted Gene Co-Expression Network Analysis (WGCNA)
3. Results
3.1. Changes in Immune Traits before and after ST Infection
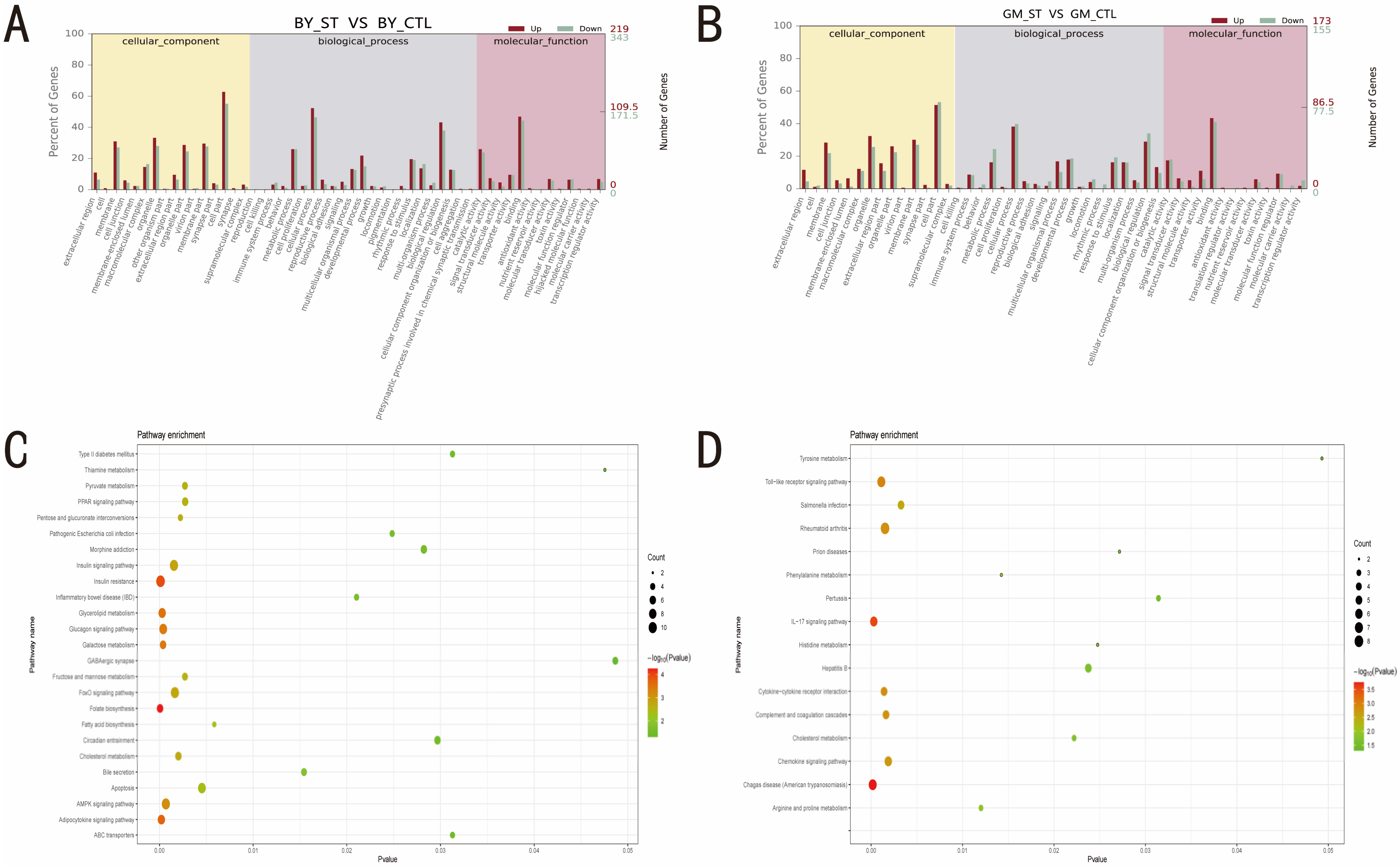
3.2. Identification of DEGs before and after ST Infection
3.3. Identification of the Pathways and Roles of DEGs before and after ST Infection
3.4. WGCNA of BY and GM Chickens
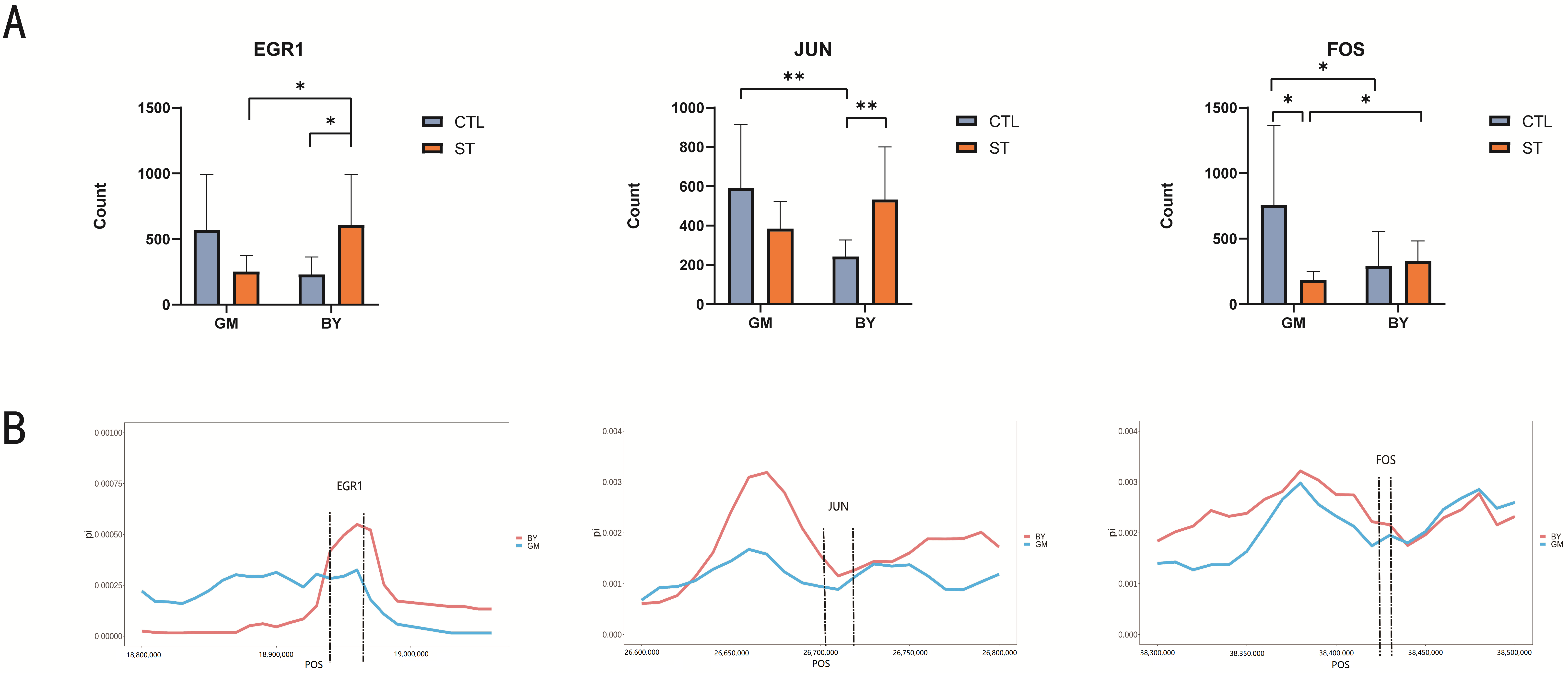
3.5. Screening of Hub Genes Related to ST Infection
3.6. Genome-Wide Selection Signal Analysis to Validate Candidate Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, W.; Li, P.; Wu, G. Amino Acid Nutrition and Metabolism in Chickens. Adv. Exp. Med. Biol. 2021, 1285, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, G.; Meyer, U.; Südekum, K.H. Land Use for Edible Protein of Animal Origin—A Review. Animals 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ohl, M.E.; Miller, S.I. Salmonella: A model for bacterial pathogenesis. Annu. Rev. Med. 2001, 52, 259–274. [Google Scholar] [CrossRef]
- Barrow, P.A.; Jones, M.A.; Smith, A.L.; Wigley, P. The long view: Salmonella—The last forty years. Avian Pathol. 2012, 41, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.D.; Mohakud, N.K.; Panda, R.K.; Sahu, B.R.; Suar, M. Prevalence and multidrug resistance in Salmonella enterica Typhimurium: An overview in South East Asia. World J. Microbiol. Biotechnol. 2021, 37, 185. [Google Scholar] [CrossRef]
- Thiam, M.; Barreto Sanchez, A.L.; Zhang, J.; Zheng, M.; Wen, J.; Zhao, G.; Wang, Q. Association of Heterophil/Lymphocyte Ratio with Intestinal Barrier Function and Immune Response to Salmonella enteritidis Infection in Chicken. Animals 2021, 11, 3498. [Google Scholar] [CrossRef]
- Threlfall, E.J. Antimicrobial drug resistance in Salmonella: Problems and perspectives in food- and water-borne infections. FEMS Microbiol. Rev. 2002, 26, 141–148. [Google Scholar] [CrossRef]
- Barbour, E.K.; el-Khatib, N.U.; al Haddad, I.C.; Iytani, D.A.; Eid, A.M.; Hamadeh, S.K.; Safieh-Garabedian, B. Macrophage recruitment and activation: A model for comparing resistance to Salmonella enteritidis in different broiler breeds. Rev. Sci. Tech. Int. Off. Epizoot. 2000, 19, 831–840. [Google Scholar] [CrossRef]
- Reed, L.J. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Fidan, E.; Nazlıgül, A.; Kenan, M.; Ünübol Aypak, S.; Sevil-Kilimci, F.; Karaarslan, S.; Kaya, M. Effect of photoperiod length and light intensity on some welfare criteria, carcass, and meat quality characteristics in broilers. Rev. Bras. Zootec. 2017, 46, 202–210. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Liang, W.; Sun, F.; Zhao, Y.; Shan, L.; Lou, H. Identification of Susceptibility Modules and Genes for Cardiovascular Disease in Diabetic Patients Using WGCNA Analysis. J. Diabetes Res. 2020, 2020, 4178639. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Cheng, H.H.; Kaiser, P.; Lamont, S.J. Integrated genomic approaches to enhance genetic resistance in chickens. Annu. Rev. Anim. Biosci. 2013, 1, 239–260. [Google Scholar] [CrossRef]
- Al-Murrani, W.K.; Kassab, A.; al-Sam, H.Z.; al-Athari, A.M. Heterophil/lymphocyte ratio as a selection criterion for heat resistance in domestic fowls. Br. Poult. Sci. 1997, 38, 159–163. [Google Scholar] [CrossRef]
- Al-Murrani, W.K.; Al-Rawi, A.J.; Al-Hadithi, M.F.; Al-Tikriti, B. Association between heterophil/lymphocyte ratio, a marker of ‘resistance’ to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 2006, 47, 443–448. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, B.; Wen, J.; Li, Q.; Zhao, G. Genome-Wide Association Study and Pathway Analysis for Heterophil/Lymphocyte (H/L) Ratio in Chicken. Genes 2020, 11, 1005. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Q.; Liu, R.; Zheng, M.; Wen, J.; Zhao, G. Genome-Wide Association Study of H/L Traits in Chicken. Animals 2019, 9, 260. [Google Scholar] [CrossRef]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. JASN 2016, 27, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Hyland, K.A.; Brown, D.R.; Murtaugh, M.P. Salmonella enterica serovar Choleraesuis infection of the porcine jejunal Peyer’s patch rapidly induces IL-1beta and IL-8 expression. Vet. Immunol. Immunopathol. 2006, 109, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.S.; Wang, H.; Ding, J.; Madkour, M.; Wang, Q.; Zhang, Q.; Zhang, N.; Li, Q.; Zhao, G.; Wen, J. Transcriptomic Analysis of the Spleen of Different Chicken Breeds Revealed the Differential Resistance of Salmonella Typhimurium. Genes 2022, 13, 811. [Google Scholar] [CrossRef] [PubMed]
- Zoabi, Y.; Shomron, N. Processing and Analysis of RNA-seq Data from Public Resources. Methods Mol. Biol. 2021, 2243, 81–94. [Google Scholar] [CrossRef]
- Schroeder, L.; Herwartz, C.; Jordanovski, D.; Steger, G. ZNF395 Is an Activator of a Subset of IFN-Stimulated Genes. Mediat. Inflamm. 2017, 2017, 1248201. [Google Scholar] [CrossRef]
- Jacobsen, M.; Repsilber, D.; Gutschmidt, A.; Neher, A.; Feldmann, K.; Mollenkopf, H.J.; Ziegler, A.; Kaufmann, S.H. Ras-associated small GTPase 33A, a novel T cell factor, is down-regulated in patients with tuberculosis. J. Infect. Dis. 2005, 192, 1211–1218. [Google Scholar] [CrossRef]
- Klees, S.; Schlüter, J.S.; Schellhorn, J.; Bertram, H.; Kurzweg, A.C.; Ramzan, F.; Schmitt, A.O.; Gültas, M. Comparative Investigation of Gene Regulatory Processes Underlying Avian Influenza Viruses in Chicken and Duck. Biology 2022, 11, 219. [Google Scholar] [CrossRef]
- Hao, F.; Tan, M.; Xu, X.; Cui, M.Z. Histamine induces Egr-1 expression in human aortic endothelial cells via the H1 receptor-mediated protein kinase Cdelta-dependent ERK activation pathway. J. Biol. Chem. 2008, 283, 26928–26936. [Google Scholar] [CrossRef]
- Yan, S.F.; Fujita, T.; Lu, J.; Okada, K.; Shan Zou, Y.; Mackman, N.; Pinsky, D.J.; Stern, D.M. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat. Med. 2000, 6, 1355–1361. [Google Scholar] [CrossRef]
- Guo, R.; Zhao, M.; Liu, H.; Su, R.; Mao, Q.; Gong, L.; Cao, X.; Hao, Y. Uncovering the pharmacological mechanisms of Xijiao Dihuang decoction combined with Yinqiao powder in treating influenza viral pneumonia by an integrative pharmacology strategy. Biomed. Pharmacother. 2021, 141, 111676. [Google Scholar] [CrossRef]
- Curran, T.; Morgan, J.I. Fos: An immediate-early transcription factor in neurons. J. Neurobiol. 1995, 26, 403–412. [Google Scholar] [CrossRef]
- Van Dam, H.; Castellazzi, M. Distinct roles of Jun: Fos and Jun: ATF dimers in oncogenesis. Oncogene 2001, 20, 2453–2464. [Google Scholar] [CrossRef]
- He, Y.; Fu, W.; Cao, K.; He, Q.; Ding, X.; Chen, J.; Zhu, L.; Chen, T.; Ding, L.; Yang, Y.; et al. IFN-κ suppresses the replication of influenza A viruses through the IFNAR-MAPK-Fos-CHD6 axis. Sci. Signal. 2020, 13, eaaz3381. [Google Scholar] [CrossRef]
- Matsumoto, K.; Saitoh, K.; Koike, C.; Narita, T.; Yasugi, S.; Iba, H. Differential expression of fos and jun family members in the developing chicken gastrointestinal tract. Oncogene 1998, 16, 1611–1616. [Google Scholar] [CrossRef]
- Li, X.; Nie, C.; Zhang, Z.; Wang, Q.; Shao, P.; Zhao, Q.; Chen, Y.; Wang, D.; Li, Y.; Jiao, W.; et al. Evaluation of genetic resistance to Salmonella pullorum in three chicken lines. Poult. Sci. 2018, 97, 764–769. [Google Scholar] [CrossRef]
- Schokker, D.; Peters, T.H.; Hoekman, A.J.; Rebel, J.M.; Smits, M.A. Differences in the early response of hatchlings of different chicken breeding lines to Salmonella enterica serovar Enteritidis infection. Poult. Sci. 2012, 91, 346–353. [Google Scholar] [CrossRef]
- Coble, D.J.; Redmond, S.B.; Hale, B.; Lamont, S.J. Distinct lines of chickens express different splenic cytokine profiles in response to Salmonella Enteritidis challenge. Poult. Sci. 2011, 90, 1659–1663. [Google Scholar] [CrossRef]
- Huang, H.; Liu, A.; Wu, H.; Ansari, A.R.; Wang, J.; Huang, X.; Zhao, X.; Peng, K.; Zhong, J.; Liu, H. Transcriptome analysis indicated that Salmonella lipopolysaccharide-induced thymocyte death and thymic atrophy were related to TLR4-FOS/JUN pathway in chicks. BMC Genom. 2016, 17, 322. [Google Scholar] [CrossRef]


| Gene ID | Gene Description | Gene Name |
|---|---|---|
| ENSGALG00000000217 | PTPRF-interacting protein alpha 4 | PPFIA4 |
| ENSGALG00000007426 | mohawk homeobox | MKX |
| ENSGALG00000008562 | dystrotelin | DYTN |
| ENSGALG00000009624 | fibrous sheath-interacting protein 1 | FSIP1 |
| ENSGALG00000010379 | synuclein alpha | SNCA |
| ENSGALG00000010572 | thyroid-stimulating hormone receptor | TSHR |
| ENSGALG00000014861 | NIM1 serine/threonine protein kinase | NIM1K |
| Gene ID | Gene Description | Gene Name |
|---|---|---|
| ENSGALG00000000168 | adenosine A1 receptor | ADORA1 |
| ENSGALG00000001866 | melanin-concentrating hormone receptor 2 | MCHR2 |
| ENSGALG00000008865 | aminolevulinate dehydratase | ALAD |
| ENSGALG00000009774 | E74-like ETS transcription factor 2 | ELF2 |
| ENSGALG00000012619 | PR/SET domain 4 | PRDM4 |
| ENSGALG00000012816 | solute carrier family 22 member 23 | SLC22A23 |
| ENSGALG00000014751 | ADAM metallopeptidase with thrombospondin type 1 motif 6 | ADAMTS6 |
| ENSGALG00000030879 | androgen receptor | AR |
| ENSGALG00000034140 | zinc finger protein 395 | ZNF395 |
| ENSGALG00000034348 | ATPase family, AAA domain containing 2 | ATAD2 |
| ENSGALG00000037361 | Krueppel-like factor 8 | KLF8 |
| ENSGALG00000039363 | thioredoxin reductase 3 | TXNRD3 |
| ENSGALG00000042895 | BCAR1, Cas family scaffolding protein | BCAR1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, H.; Barreto Sánchez, A.L.; Thiam, M.; Zhang, J.; Li, Q.; Zheng, M.; Wen, J.; Li, H.; Zhao, G.; et al. Comparative Analysis of the Liver Transcriptome of Beijing You Chickens and Guang Ming Broilers under Salmonella enterica Serovar Typhimurium Infection. Microorganisms 2022, 10, 2440. https://doi.org/10.3390/microorganisms10122440
Wang Z, Wang H, Barreto Sánchez AL, Thiam M, Zhang J, Li Q, Zheng M, Wen J, Li H, Zhao G, et al. Comparative Analysis of the Liver Transcriptome of Beijing You Chickens and Guang Ming Broilers under Salmonella enterica Serovar Typhimurium Infection. Microorganisms. 2022; 10(12):2440. https://doi.org/10.3390/microorganisms10122440
Chicago/Turabian StyleWang, Zixuan, Hailong Wang, Astrid Lissette Barreto Sánchez, Mamadou Thiam, Jin Zhang, Qinghe Li, Maiqing Zheng, Jie Wen, Hegang Li, Guiping Zhao, and et al. 2022. "Comparative Analysis of the Liver Transcriptome of Beijing You Chickens and Guang Ming Broilers under Salmonella enterica Serovar Typhimurium Infection" Microorganisms 10, no. 12: 2440. https://doi.org/10.3390/microorganisms10122440
APA StyleWang, Z., Wang, H., Barreto Sánchez, A. L., Thiam, M., Zhang, J., Li, Q., Zheng, M., Wen, J., Li, H., Zhao, G., & Wang, Q. (2022). Comparative Analysis of the Liver Transcriptome of Beijing You Chickens and Guang Ming Broilers under Salmonella enterica Serovar Typhimurium Infection. Microorganisms, 10(12), 2440. https://doi.org/10.3390/microorganisms10122440






