Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds
Abstract
1. Introduction
2. Materials and Methods
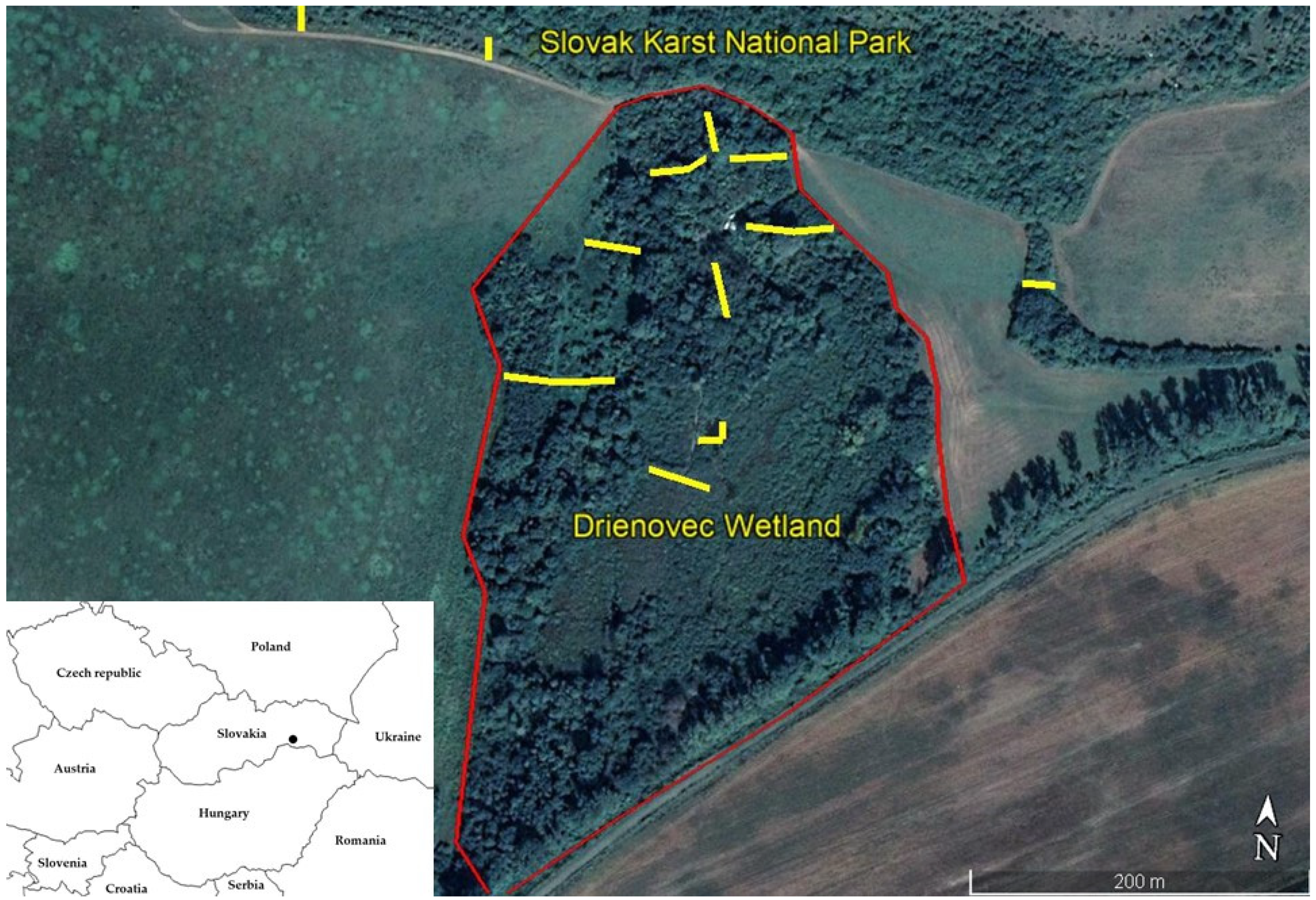
2.1. Description of the Model Area
2.2. Capturing of Birds and Sample Collection
2.3. Tick Diagnostics
2.4. Processing of Blood Samples
2.5. RNA Isolation and RT-PCR for Arbovirus Detection
2.6. μVNT for Arbovirus NAb Screening
2.7. Simultaneous μVNT for Differentiation of Arbovirus Infections
2.8. Quantitative Characteristics of the Captured Bird Population
2.9. Statistical Analysis
3. Results
3.1. Ornithological and Parasitological Findings
3.2. Screening of Arbovirus Infections
3.3. Determination of Arbovirus Infections by Simultaneous μVNT
3.4. Simultaneous Occurrence of Flavivirus and Orbivirus NAb
3.5. Statistical Evaluation of the Link between Tick Infestation, Seropositivity and Arbovirus NAb Titre
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hubalek, Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J. Wildl. Dis. 2004, 40, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.E. The Palaearctic-African Bird Migration Systems; Academic Press: Cambridge, MA, USA, 1972; p. 384. [Google Scholar]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Attoui, H.; Banyai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avsic-Zupanc, T.; Ayllon, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072. [Google Scholar] [CrossRef]
- Mikryukova, T.P.; Moskvitina, N.S.; Kononova, Y.V.; Korobitsyn, I.G.; Kartashov, M.Y.; Tyuten Kov, O.Y.; Protopopova, E.V.; Romanenko, V.N.; Chausov, E.V.; Gashkov, S.I.; et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick Borne Dis. 2014, 5, 145–151. [Google Scholar] [CrossRef]
- Nunn, M.A.; Barton, T.R.; Wanless, S.; Hails, R.S.; Harris, M.P.; Nuttall, P.A. Tick-borne Great Island Virus: (I) Identification of seabird host and evidence for co-feeding and viraemic transmission. Parasitology 2006, 132, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Shope, R.E. Kemerovo virus from a migrating common redstart of Eurasia. Acta Virol. 1971, 15, 112. [Google Scholar]
- Palacios, G.; Savji, N.; Travassos da Rosa, A.; Guzman, H.; Yu, X.; Desai, A.; Rosen, G.E.; Hutchison, S.; Lipkin, W.I.; Tesh, R. Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): Evidence for seven distinct species. J. Virol. 2013, 87, 3187–3195. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Mackenstedt, U.; Kahl, O.; Petney, T.N. Chapter 3: Transmission/Natural cycle. In The TBE Book, 2nd ed.; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press Pte Ltd: Singapore, 2019; pp. 62–86. [Google Scholar]
- Pfeffer, M.; Schmuck, H.M.; Leschnik, M. Chapter 8: TBE in animals. In The TBE Book, 2nd ed.; Dobler, G., Erber, W., Bröker, M., Schmitt, H.-J., Eds.; Global Health Press Pte Ltd: Singapore, 2019; pp. 144–160. [Google Scholar]
- Kunze, U. Report of the 21st Annual Meeting of the International Scientific Working Group on Tick-Borne Encephalitis (ISW-TBE): TBE—record year 2018. Ticks Tick Borne Dis. 2020, 11, 101287. [Google Scholar] [CrossRef]
- Van Tongeren, H.A.E. Experimental infection of coots (Fulica atra) with Russian spring summer encephalitis virus. In Biology of Viruses of the Tick-Borne Encephalitis Complex: Proceedings; Libíková, H., Ed.; House of the Czechoslovak Academy of Sciences, Praha: Smolenice, Slovakia, 1962; pp. 383–386. [Google Scholar]
- Van Tongeren, H.A.E. Viraemia and antibody response of the mallard (Anas platyrhynchos) to infection with tick-borne encephalitis virus. J. Comp. Pathol. 1983, 93, 521–530. [Google Scholar] [CrossRef]
- Csank, T.; Bhide, K.; Bencurova, E.; Dolinska, S.; Drzewniokova, P.; Major, P.; Korytar, L.; Bockova, E.; Bhide, M.; Pistl, J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch. Virol. 2016, 161, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z.; Rudolf, I. Tick-borne viruses in Europe. Parasitol. Res. 2012, 111, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Kraminskiy, V.A.; Kraminskaya, N.N.; Brom, I.P.; Zhivolyapina, R.R.; Zonov, G.B.; Perevoznikov, V.A.; Sotnikova, A.N.; Soldatov, G.M. Transovarial (transembryonal) transmission of TBE virus in migratory birds. In Transcontinental Connections of Migratory Birds and Their Role in the Distribution of Arboviruses; Cherepanov, A.I.E.A., Ed.; Nauka: Novosibirsk, Russia, 1972; pp. 274–276. (In Russian) [Google Scholar]
- Grešíková, M.; Nosek, J.; Kožuch, O.; Ernek, E.; Lichard, M. Study on ecology of Tribeč virus. Acta Virol. 1965, 9, 83–88. [Google Scholar]
- Libíková, H.; Rehacek, J.; Ernek, E.; Gresikova, M.; Somogyio, J.; Kozuch, O. Cytopathic viruses isolated from Ixodes ricinus ticks in Czechoslovakia. Acta Virol. 1964, 8, 96–106. [Google Scholar] [PubMed]
- Csank, T.; Korytár, Ľ.; Pošiváková, T.; Bakonyi, T.; Pistl, J.; Csanády, A. Surveillance on antibodies against West Nile virus, Usutu virus, tick-borne encephalitis virus and Tribeč virus in wild birds in Drienovská wetland, Slovakia. Biologia 2019, 74, 813–820. [Google Scholar] [CrossRef]
- Chumakov, M.P. Report on the isolation from Ixodes persulcatus ticks and from patients in western Siberia of a virus differing from the agent of tick-borne encephalitis. Acta Virol. 1963, 7, 82–83. [Google Scholar] [PubMed]
- Franková, V.; Marhoul, Z.; Duniewicz, M.; Pruklová, A. Meningoencephalitis associated with orbivirus infection. Sb. Lek. 1982, 84, 181–186. [Google Scholar] [PubMed]
- Kerlik, J.; Pántiková Valachová, M.; Csank, T.; Avdičová, M. Výskyt západonílskej horúčky v Európe. In Proceedings of the VI. Ročník Vedeckého Kongresu Zoonózy, Alimentárne Nákazy a Nákazy z Vody—Spoločná Ochrana Zdravia Ľudí a Zvierat a XXIII, Červenkove Dni Preventívnej Medicíny, Banská Bystrica, Slovakia, 15–17 October 2018. (In Slovak). [Google Scholar]
- Olekšák, M.; Pjenčák, P.; Fulín, M.; Matis, Š. Bird nesting community of the Drienovec bird Ringing Station—CES pro-gramme. Tichodroma 2007, 19, 41–47. [Google Scholar]
- Csank, T.; Drzewnioková, P.; Korytár, L.; Major, P.; Gyuranecz, M.; Pistl, J.; Bakonyi, T. A Serosurvey of Flavivirus Infection in Horses and Birds in Slovakia. Vector-Borne Zoonotic Dis. 2018, 18, 206–213. [Google Scholar] [CrossRef]
- Reports from Bird Ringing Station Drienovec. Available online: https://brsdrienovec.webnode.sk/publikovanie/ (accessed on 10 October 2022).
- Guidelines for Constant Effort Ringing. Available online: https://euring.org/files/documents/research/euro_ces_guidelines210904.pdf (accessed on 10 October 2022).
- Hoysak, D.J.; Weatherhead, P.J. Sampling Blood from Birds: A Technique and an Assessment of Its Effect. Condor 1991, 93, 746–752. [Google Scholar] [CrossRef]
- Peňazziová, K.; Korytár, Ľ.; Pistl, J.; Ondrejková, A.; Csank, T. Detekcia arbovírusov z kliešťov u vtákov odchytených v Drienovskej mokradi. In Seminár Doktorandov Venovaný Pamiatke Akademika Boďu. Vedecké práce Doktorandov 2020: Zborník zo Seminára Doktorandov Venovaného Pamiatke Akademika Boďu; Slovenská Akadémia Vied, Centrum Biovied: Košice, Slovakia, 2020; pp. 77–79. [Google Scholar]
- Kuno, G. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 1998, 72, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Drzewniokova, P.; Csank, T.; Vargová, B.; Majláthová, V.; Pistl, J. Molecular detection of Tick-borne encephalitis virus and Tribeč virus from ticks in Eastern Slovakia. In Proceedings of the Czechoslovak Virology Conference, České Budějovice, Czech Republic, 16–17 February 2017; Biologické Centrum AV ČR: Branišovská, Czech Republic, 2017; p. 87. [Google Scholar]
- Drzewnioková, P.; Barzon, L.; Franchin, E.; Lavezzo, E.; Bakonyi, T.; Pistl, J.; Csank, T. The complete genome sequence analysis of West Nile virus strains isolated in Slovakia (central Europe). Arch. Virol. 2019, 164, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Zając, Z.; Kulisz, J.; Kunc-Kozioł, R.; Woźniak, A.; Filipiuk, M.; Rudolf, R.; Bartosik, K.; Cabezas-Cruz, A. Tick Infestation in Migratory Birds of the Vistula River Valley, Poland. Int. J. Environ. Res. Public Health 2022, 19, 13781. [Google Scholar] [CrossRef]
- Margolis, L.; Esch, G.W.; Holmes, J.C.; Kuris, A.M.; Schad, G.A. The Use of Ecological Terms in Parasitology (Report of an Ad Hoc Committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Taragel’ova, V.; Koci, J.; Hanincova, K.; Kurtenbach, K.; Derdakova, M.; Ogden, N.H.; Literak, I.; Kocianova, E.; Labuda, M. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl. Environ. Microbiol. 2008, 74, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Spitalska, E.; Literak, I.; Sparagano, O.A.; Golovchenko, M.; Kocianova, E. Ticks (Ixodidae) from passerine birds in the Carpathian region. Wien Klin. Wochenschr. 2006, 118, 759–764. [Google Scholar] [CrossRef]
- Berthova, L.; Slobodnik, V.; Slobodnik, R.; Oleksak, M.; Sekeyova, Z.; Svitalkova, Z.; Kazimirova, M.; Spitalska, E. The natural infection of birds and ticks feeding on birds with Rickettsia spp. and Coxiella burnetii in Slovakia. Exp. Appl. Acarol. 2016, 68, 299–314. [Google Scholar] [CrossRef]
- Snow, D.; Perrins, C.M.; Gillmor, R. The Birds of the Western Palearctic (Concise); Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Ferianc, O. Vtáky Slovenska 2; Veda: Bratislava, Slovakia, 1979; p. 472. [Google Scholar]
- Davison, F.; Magor, K.E.; Kaspers, B. Structure and evolution of avian immunoglobulins. In Avian Immunology; Davison, F., Kaspers, B., Schat, K.A., Eds.; Academic Press: Amsterdam, The Netherlands, 2008; pp. 107–127. [Google Scholar]
- Kuno, G. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: Its occurrence and impacts. Rev. Med. Virol. 2001, 11, 165–190. [Google Scholar] [CrossRef]
- Reisen, W.; Lothrop, H.; Chiles, R.; Madon, M.; Cossen, C.; Woods, L.; Husted, S.; Kramer, V.; Edman, J. West Nile virus in California. Emerg. Infect. Dis. 2004, 10, 1369–1378. [Google Scholar] [CrossRef]
- Reisen, W.K.; Kramer, L.D.; Chiles, R.E.; Green, E.G.; Martinez, V.M. Encephalitis virus persistence in California birds: Preliminary studies with house finches. J. Med. Entomol. 2001, 38, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.W.; Moss, R.; Pow, I.; Buxton, D. The response of three grouse species (Tetrao urogallus, Lagopus mutus, Lagopus lagopus) to louping-ill virus. J. Comp. Pathol. 1980, 90, 257–263. [Google Scholar] [CrossRef] [PubMed]
- McKee, E.M.; Walker, E.D.; Anderson, T.K.; Kitron, U.D.; Brawn, J.D.; Krebs, B.L.; Newman, C.; Ruiz, M.O.; Levine, R.S.; Carrington, M.E.; et al. West Nile Virus Antibody Decay Rate in Free-Ranging Birds. J. Wildl. Dis. 2015, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Ernek, E.; Kozuch, O.; Lichard, M.; Nosek, J. The role of birds in the circulation of tick-borne encephalitis virus in the Tribeč region. Acta Virol. 1968, 12, 468–470. [Google Scholar]
- Lommano, E.; Dvorak, C.; Vallotton, L.; Jenni, L.; Gern, L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick Borne Dis. 2014, 5, 871–882. [Google Scholar] [CrossRef]
- Saikku, P. Blackbird, Turdus merula L., and tick-borne encephalitis (TBE) virus. Acta Virol. 1973, 17, 442. [Google Scholar]
- Waldenstrom, J.; Lundkvist, A.; Falk, K.I.; Garpmo, U.; Bergstrom, S.; Lindegren, G.; Sjostedt, A.; Mejlon, H.; Fransson, T.; Haemig, P.D.; et al. Migrating birds and tickborne encephalitis virus. Emerg. Infect. Dis. 2007, 13, 1215–1218. [Google Scholar] [CrossRef]
- Gaumann, R.; Muhlemann, K.; Strasser, M.; Beuret, C.M. High-throughput procedure for tick surveys of tick-borne encephalitis virus and its application in a national surveillance study in Switzerland. Appl. Environ. Microbiol. 2010, 76, 4241–4249. [Google Scholar] [CrossRef]
- Pettersson, J.H.O.; Golovljova, I.; Vene, S.; Jaenson, T.G.T. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasites Vectors 2014, 7, 102. [Google Scholar] [CrossRef]
- Stefanoff, P.; Pfeffer, M.; Hellenbrand, W.; Rogalska, J.; Rühe, F.; Makówka, A.; Michalik, J.; Wodecka, B.; Rymaszewska, A.; Kiewra, D.; et al. Virus detection in questing ticks is not a sensitive indicator for risk assessment of tick-borne encephalitis in humans. Zoonoses Public Health 2013, 60, 215–226. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.-E. Migratory birds as disseminators of ticks and the tick-borne pathogens Borrelia bacteria and tick-borne encephalitis (TBE) virus: A seasonal study at Ottenby Bird Observatory in South-eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef] [PubMed]
- Libíková, H.; Buckley, S.M. Serological characterization of Eurasian Kemerovo group viruses. II. Cross plaque neutralization tests. Acta Virol. 1971, 15, 79–86. [Google Scholar] [PubMed]
- Libíková, H.; Casals, J. Serological characterization of Eurasian Kemerovo group viruses. I. Cross complement fixation tests. Acta Virol. 1971, 15, 65–78. [Google Scholar]
- Libikova, H.; Mayer, V.; Kozuch, O.; Rehacek, J.; Ernek, E.; Albrecht, P. Isolation from Ixodes persulcatus ticks of cytopathic agents (Kemerovo virus) differing from tick-borne encephalitis virus and some of their properties. Acta Virol. 1964, 8, 289–301. [Google Scholar]
- Fulín, M.; Gálffyová, M.; Kiss, E.; Krišovský, P.; Olekšák, M. Personal Communication; Drienovec Bird Ringing Station, Slovak Karst National Park: Brzotín, Slovakia, 2022. [Google Scholar]
- Cepák, J. Hawfinch. In Atlas Migrace Ptáku České a Slovenské Republiky; Cepák, J., Klvaňa, P., Škopek, J., Schropfer, L., Jelínek, L., Hořák, D., Formánek, J., Zárybnický, J., Eds.; Aventinum: Prague, Czech Republic, 2008; pp. 552–559. [Google Scholar]
- Dedkov, V.G.; Markelov, M.L.; Gridneva, K.A.; Bekova, M.V.; Gmyl, A.P.; Kozlovskaya, L.I.; Karganova, G.G.; Romanova, L.; Pogodina, V.V.; Yakimenko, V.V.; et al. Prevalence of Kemerovo virus in ixodid ticks from the Russian Federation. Ticks Tick Borne Dis. 2014, 5, 651–655. [Google Scholar] [CrossRef]
- Kozlova, T.V.; Khomyakova, T.I.; Dedkov, V.G.; Safonova, M.V.; Karan, L.S.; Grigoryeva, Y.E.; Kozlov, V.V.; Lopatin, A.A.; Ivanova, S.M.; Khomyakov, Y.N. The identification of new pathogens for focal infections in Ixodes ticks on the territory of Tula region. EIDj 2018, 23, 172–177. [Google Scholar] [CrossRef]
- Safonova, M.V.; Gmyl, A.P.; Lukashev, A.N.; Speranskaya, A.S.; Neverov, A.D.; Fedonin, G.G.; Pimkina, E.V.; Matsvay, A.D.; Khafizov, K.F.; Karganova, G.G.; et al. Genetic diversity of Kemerovo virus and phylogenetic relationships within the Great Island virus genetic group. Ticks Tick Borne Dis. 2020, 11, 101333. [Google Scholar] [CrossRef]
- Tkachev, S.; Panov, V.; Dobler, G.; Tikunova, N. First detection of Kemerovo virus in Ixodes pavlovskyi and Ixodes persulcatus ticks collected in Novosibirsk region, Russia. Ticks Tick Borne Dis. 2014, 5, 494–496. [Google Scholar] [CrossRef]
- ECDC. Tick Maps. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 10 October 2022).
- Migné, C.V.; Hönig, V.; Bonnet, S.I.; Palus, M.; Rakotobe, S.; Galon, C.; Heckmann, A.; Vyletova, E.; Devillers, E.; Attoui, H.; et al. Evaluation of two artificial infection methods of live ticks as tools for studying interactions between tick-borne viruses and their tick vectors. Sci. Rep. 2022, 12, 491. [Google Scholar] [CrossRef]
- Hubalek, Z.; Calisher, C.H.; Mittermayer, T. A new subtype (“Brezova”) of Tribec orbivirus (Kemerovo group) isolated from Ixodes ricinus males in Czechoslovakia. Acta Virol. 1987, 31, 91–92. [Google Scholar] [PubMed]
- Libíková, H.; Heinz, F.; Ujházyová, D.; Stünzner, D. Orbiviruses of the Kemerovo complex and neurological diseases. Med. Microbiol. Immunol. 1978, 166, 255–263. [Google Scholar] [CrossRef] [PubMed]
| Bird Species | No. of Caught Birds | IED (%) | MS | No. of Tick Infested Birds | No. of Collected Ticks | Tick Species | PTI (%) | MITB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I. ricinus | I. frontalis | H. concinna | Ixodes spp. | ||||||||||||
| La | N | La | N | La | N | La | N | ||||||||
| Hawfinch | 14 | 3.6 | S | 3 | 7 | 5 | 2 | - | - | - | - | - | - | 21.4 | 2.3 |
| European robin | 93 | 23.7 | S | 23 | 48 | 23 | 23 | - | 1 | 1 | - | - | - | 24.7 | 2.1 |
| Common chaffinch | 11 | 2.8 | S | 3 | 5 | 1 | 4 | - | - | - | - | - | - | 27.3 | 1.7 |
| Common nightingale | 10 | 2.5 | L | 2 | 4 | - | 4 | - | - | - | - | - | - | 20.0 | 2.0 |
| Great tit | 72 | 18.3 | S | 18 | 18 | 1 | 14 | - | - | - | - | - | 3 | 25.0 | 1.0 |
| Dunnock | 5 | 1.3 | S | 2 | 4 | 1 | 3 | - | - | - | - | - | - | 40.0 | 2.0 |
| Eurasian blackcap | 93 | 23.7 | S/L | 3 | 4 | 3 | 1 | - | - | - | - | - | - | 3.2 | 1.3 |
| Common blackbird | 31 | 7.9 | S | 27 | 93 | 16 | 74 | - | - | 1 | - | 2 | - | 87.1 | 3.4 |
| Song thrush | 13 | 3.3 | S | 7 | 11 | 2 | 9 | - | - | - | - | - | - | 53.8 | 1.6 |
| Dependent Var. | Factors | p-Values | Effect Size (eta2) | Group Means (No. of Ticks) | Significant Pairwise Comparisons | |||
|---|---|---|---|---|---|---|---|---|
| Prevalence of ticks | Migration status | p ≤ 0.0000 * | 0.0962 moderate | S | S/L | L | p ≤ 0.0000 *; S vs. S/L | |
| 0.8 | 0.04 | 0.4 | ||||||
| Prevalence of larvae | Migration status | p ≤ 0.0177 * | 0.0179 small | S | S/L | L | p ≤ 0.0082 *; S vs. S/L | |
| 0.22 | 0.03 | 0 | ||||||
| Prevalence of nymphs | Migration status | p ≤ 0.0000 * | 0.0713 moderate | S | S/L | L | p ≤ 0.0000 *; S vs. S/L | |
| 0.56 | 0.01 | 0.4 | ||||||
| Prevalence of ticks | Species (S1–S9) | p ≤ 0.0000 * | 0.295 large | S1 | S8 | p ≤ 0.0000 *; S1 vs. S8 | ||
| 0.5 | 3 | |||||||
| Prevalence of ticks | Sex | p ≤ 0.226 | - | - | - | |||
| Prevalence of ticks | Age | p ≤ 0.306 | - | - | - | |||
| Prevalence of ticks | Feeding behaviour | p ≤ 0.0000 * | 0.236 large | Ground | Shrubs | - | ||
| 2.36 | 0.3 | |||||||
| Prevalence of larvae | Feeding behaviour | p ≤ 0.0000 * | 0.049 small | Ground | Shrubs | - | ||
| 0.48 | 0.18 | |||||||
| Prevalence of nymphs | Feeding behaviour | p ≤ 0.0000 * | 0.210 large | Ground | Shrubs | - | ||
| 1.89 | 0.19 | |||||||
| Dependent Variables | Independent Variables (Factors) | p-Values |
|---|---|---|
| Tick infestation | Sex | p ≤ 0.4725 |
| Tick infestation | Age | p ≤ 0.3834 |
| Tick infestation | Feeding behaviour | p ≤ 0.0000 * |
| Tick infestation | Migration status | p ≤ 0.0000 * |
| Species | Sample | Infestation | Estimated Age | TBEV NAb | WNV NAb | USUV NAb |
|---|---|---|---|---|---|---|
| Common blackbird | 86.B/19 | + | +1K | 1:10–1:40 | 1:10–1:40 | 1:160 |
| Common blackbird | 118.B/19 | + | +1K | 1:10–1:40 | 1:10–1:40 | - |
| Common blackbird | 216.B/19 | + | +1K | 1:10–1:40 | - | - |
| Common blackbird | 245.B/20 | + | 1K | 1:10–1:40 | - | - |
| Eurasian blackcap | 278.B/19 | - | +1K | 1:10 | - | - |
| Common blackbird | 509.B/19 | - | 1K | 1:10 | - | - |
| Eurasian blackcap | 223.B/20 | - | +1K | 1:10 | - | - |
| European robin | 225.B/20 | - | +1K | 1:10 | - | - |
| Species | Sample | Infestation | Estimated Age | TRBV NAb | KEMV NAb |
|---|---|---|---|---|---|
| Common chaffinch | 60.B/19 | + | +1K | 1:640 | - |
| Common blackbird | 86.B/19 | + | +1K | 1:2560 | - |
| Common blackbird | 216.B/19 | + | +1K | 1:2560 | 1:40 |
| Song thrush | 224.B/19 | + | +1K | 1:640 | - |
| Hawfinch | 226.B/19 | + | +1K | 1:2560 | - |
| Hawfinch | 230.B/19 | + | +1K | 1:160–1:640 | - |
| Common blackbird | 236.B/19 | + | +1K | 1:2560–1:10,240 | - |
| Common blackbird | 242.B/19 | + | +1K | 1:10,240 | 1:10–1:40 |
| Dunnock | 266.B/19 | + | +1K | 1:640 | - |
| Common blackbird | 286.B/19 | + | +1K | 1:640 | 1:40 |
| Song thrush | 513.B/19 | + | +1K | 1:10–1:40 | - |
| Common blackbird | 517.B/19 | + | 1K | 1:10 | - |
| Common blackbird | 541.B/19 | + | 1K | 1:640 | 1:10 |
| Common blackbird | 549.B/19 | + | 1K | 1:640 | 1:10–1:40 |
| Common blackbird | 579.B/19 | + | 1K | 1:160 | - |
| Hawfinch | 5.B/20 | + | +1K | 1:10 | 1:10 |
| Common blackbird | 107.B/20 | + | 1K | 1:10 | - |
| Song thrush | 215.B/20 | + | 1K | 1:2560–1:10,240 | - |
| Song thrush | 235.B/20 | + | 1K | 1:10 | - |
| Redwing | 258.B/19 | - | +1K | 1:10 | - |
| Eurasian jay | 425.B/19 | - | +1K | 1:640–1:2560 | 1:10 |
| Hawfinch | 507.B/19 | - | +1K | 1:40 | - |
| Common blackbird | 529.B/19 | - | 1K | 1:40–1:160 | - |
| Hawfinch | 29.B/20 | - | +1K | 1:10–1:40 | - |
| Eurasian jay | 49.B/20 | - | +1K | - | 1:10 |
| European robin | 275.B/20 | - | 1K | 1:10 | - |
| European robin | 279.B/20 | - | +1K | 1:40–1:160 | 1:10 * |
| Dependent Variables | Independent Variables (Factors) | p-Values |
|---|---|---|
| Tick infestation | Seroprevalence TRBV | p ≤ 0.0016 * |
| Tick infestation | Seroprevalence KEMV | p ≤ 0.2186 |
| Tick infestation | Seroprevalence TBEV | p ≤ 0.3895 |
| Tick infestation | TRBV antibody titre ≥1:160 | p ≤ 0.0261 * |
| Tick infestation | KEMV antibody titre ≥1:10 | p ≤ 0.4286 |
| Tick infestation | TBEV antibody titre ≥1:10 | p ≤ 0.0119 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peňazziová, K.; Korytár, Ľ.; Cingeľová Maruščáková, I.; Schusterová, P.; Loziak, A.; Pivka, S.; Ondrejková, A.; Pistl, J.; Csank, T. Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds. Microorganisms 2022, 10, 2397. https://doi.org/10.3390/microorganisms10122397
Peňazziová K, Korytár Ľ, Cingeľová Maruščáková I, Schusterová P, Loziak A, Pivka S, Ondrejková A, Pistl J, Csank T. Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds. Microorganisms. 2022; 10(12):2397. https://doi.org/10.3390/microorganisms10122397
Chicago/Turabian StylePeňazziová, Katarína, Ľuboš Korytár, Ivana Cingeľová Maruščáková, Petra Schusterová, Alexander Loziak, Soňa Pivka, Anna Ondrejková, Juraj Pistl, and Tomáš Csank. 2022. "Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds" Microorganisms 10, no. 12: 2397. https://doi.org/10.3390/microorganisms10122397
APA StylePeňazziová, K., Korytár, Ľ., Cingeľová Maruščáková, I., Schusterová, P., Loziak, A., Pivka, S., Ondrejková, A., Pistl, J., & Csank, T. (2022). Serologic Investigation on Tick-Borne Encephalitis Virus, Kemerovo Virus and Tribeč Virus Infections in Wild Birds. Microorganisms, 10(12), 2397. https://doi.org/10.3390/microorganisms10122397






