Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach
Abstract
1. Introduction
2. Materials and Methods
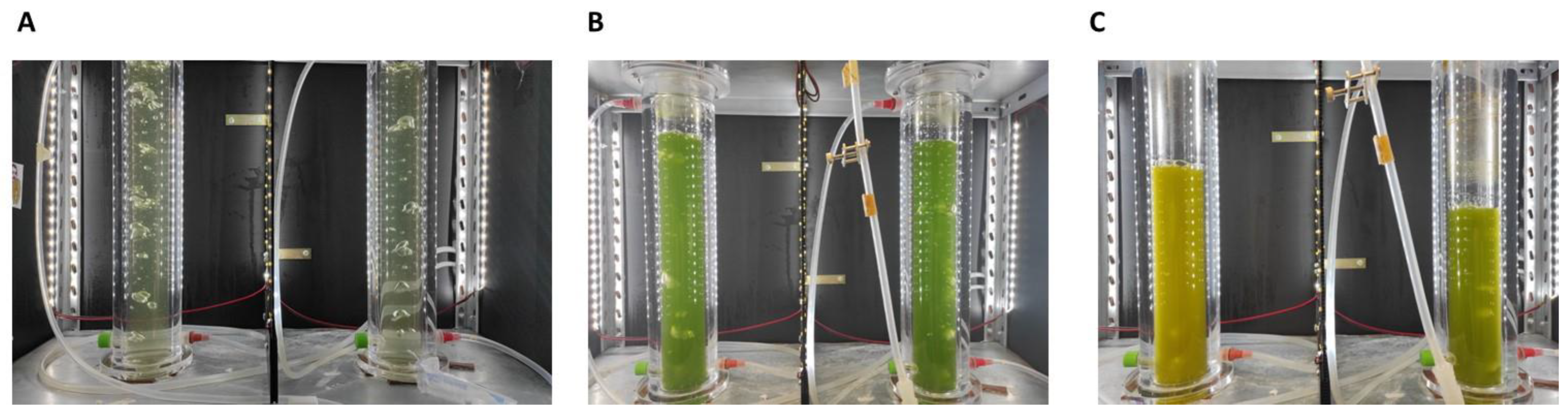
2.1. Microalgae Cultivation
2.2. Yeast Strains, Substrate, and Cultivation Conditions
2.3. Growth and Biomass Determination
2.4. Extraction of Oils from Microalgae
2.5. MELs and Residual Lipids Quantification
2.6. Substrate Quantification
2.7. Statistical Analysis
3. Results and Discussion
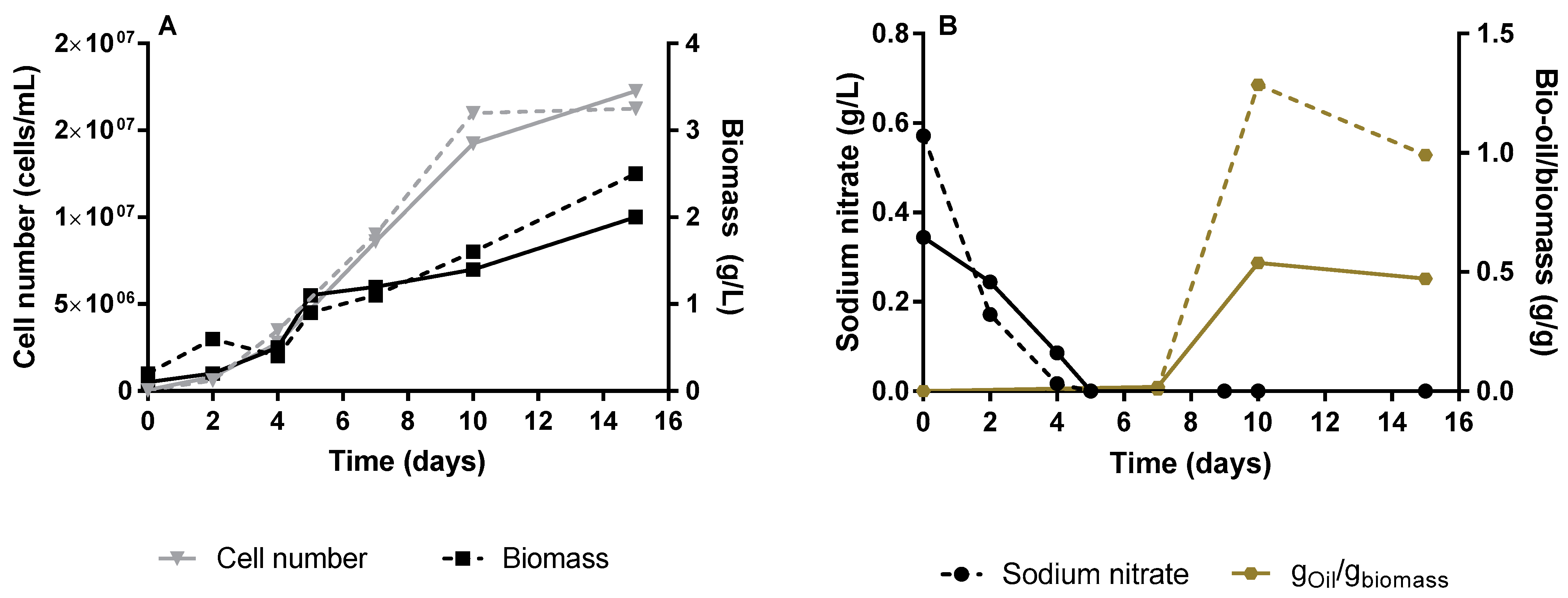
3.1. Neochloris Oleoabundans Growth and Lipids Production
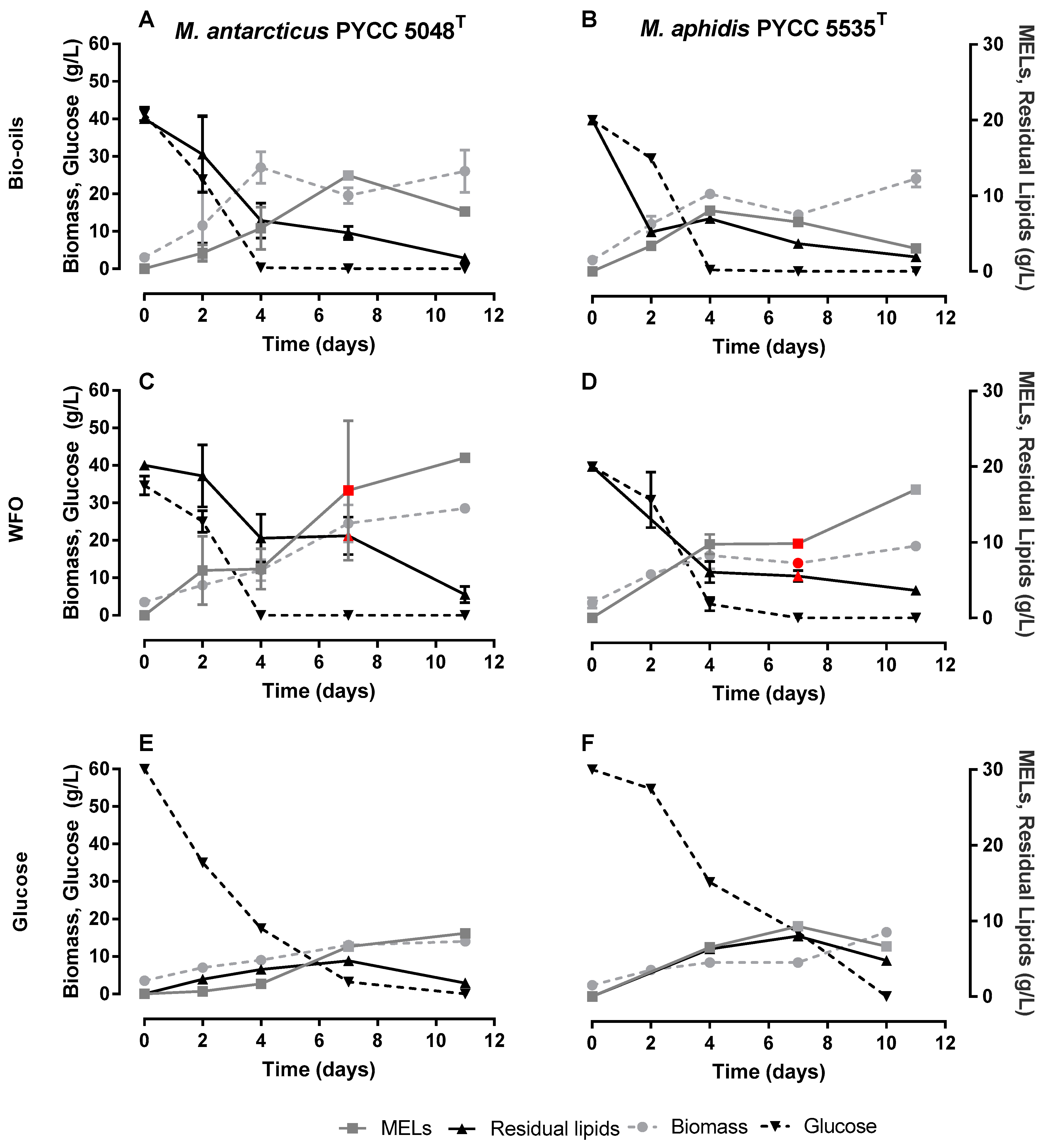
3.2. Production of MELs Using Algae-Derived Bio-Oils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, A. Drought under Global Warming: A Review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Dixit, S.; Danekar, R.; Prasad, E. Surfactants Market Size, Share | Industry Analysis & Forecast, 2027. Available online: https://www.alliedmarketresearch.com/surfactant-market (accessed on 11 January 2021).
- Kronberg, B.; Holmberg, K.; Lindman, B. Types of Surfactants, Their Synthesis, and Applications. Surf. Chem. Surfactants Polym. 2014, 1–47. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential Applications in Medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G. Fate, Behavior and Effects of Surfactants and Their Degradationproducts in the Environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Banat, I.M. Biosurfactants: A Sustainable Replacement for Chemical Surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial Biosurfactants: Current Trends and Applications in Agricultural and Biomedical Industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- MarketWatch. Microbial Biosurfactants Market Share. Available online: https://www.marketwatch.com/press-release/microbial-biosurfactants-market-share-size-2022-global-companies-consumption-drivers-top-leading-countries-trends-growth-factors-forces-analysis-revenue-challenges-and-global-forecast-2028-2022-02-16 (accessed on 24 February 2022).
- Rau, U.; Nguyen, L.A.; Roeper, H.; Koch, H.; Lang, S. Fed-Batch Bioreactor Production of Mannosylerythritol Lipids Secreted by Pseudozyma Aphidis. Appl. Microbiol. Biotechnol. 2005, 68, 607–613. [Google Scholar] [CrossRef]
- Lang, S.; Wullbrandt, D. Rhamnose Lipids—Biosynthesis, Microbial Production and Application Potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [Google Scholar] [CrossRef]
- Konoshi, M.; Fukuoka, T.; Morita, T.; Imura, T.; Kitamoto, D. Production of New Types of Sophorolipids by Candida Batistae. J. Oleo Sci. 2008, 57, 359–369. [Google Scholar] [CrossRef]
- Imura, T.; Ohta, N.; Inoue, K.; Yagi, N.; Negishi, H.; Yanagishita, H.; Kitamoto, D. Naturally Engineered Glycolipid Biosurfactants Leading to Distinctive Self-Assembled Structures. Chemistry 2006, 12, 2434–2440. [Google Scholar] [CrossRef]
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functions and Potential Applications of Glycolipid Biosurfactants—From Energy-Saving Materials to Gene Delivery Carriers. J. Biosci. Bioeng. 2002, 94, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of Mannosylerythritol Lipids and Their Application in Cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Yoshida, S.; Nakamura, J.; Koitabashi, M.; Sakai, H.; Abe, M.; Kitamoto, D.; Kitamoto, H. Application of Yeast Glycolipid Biosurfactant, Mannosylerythritol Lipid, as Agrospreaders. J. Oleo Sci. 2015, 64, 689–695. [Google Scholar] [CrossRef]
- Shu, Q.; Wei, T.; Lu, H.; Niu, Y.; Chen, Q. Mannosylerythritol Lipids: Dual Inhibitory Modes against Staphylococcus Aureus through Membrane-Mediated Apoptosis and Biofilm Disruption. Appl. Microbiol. Biotechnol. 2020, 104, 5053–5064. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as Green Energy Reserve: Technological Outlook on Biofuel Production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wu, J.; Wang, W.; Chen, Q. Production and Characterization of a New Glycolipid, Mannosylerythritol Lipid, from Waste Cooking Oil Biotransformation by Pseudozyma aphidis ZJUDM34. Food Sci. Nutr. 2019, 7, 937–948. [Google Scholar] [CrossRef]
- Nascimento, M.F.; Barreiros, R.; Cristina, A.; Frederico, O.; Ferreira, C.; Faria, N.T. Moesziomyces spp. Cultivation Using Cheese Whey: New Yeast Extract-Free Media, β-Galactosidase Biosynthesis and Mannosylerythritol Lipids Production. Biomass Convers. Biorefin. 2022, 1–14. [Google Scholar] [CrossRef]
- Bhuiya, M.M.K.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Azad, A.K. Prospects of 2nd Generation Biodiesel as a Sustainable Fuel—Part: 1 Selection of Feedstocks, Oil Extraction Techniques and Conversion Technologies. Renew. Sustain. Energy Rev. 2016, 55, 1109–1128. [Google Scholar] [CrossRef]
- Gouveia, L.; Evangelista, A.; Lopes, T.; Reis, A. Neochloris Oleabundans UTEX # 1185: A Suitable Renewable Lipid Source for Biofuel Production. J. Ind. Microbiol. Biotechnol. 2009, 36, 821–826. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus Obliquus Microalga-Based Biorefinery—From Brewery Effluent to Bioactive Compounds, Biofuels and Biofertilizers—Aiming at a Circular Bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of Lipids from Microalgae by Ultrasound Application: Prospection of the Optimal Extraction Method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Welz, W.; Sattler, W.; Leis, H.J.; Malle, E. Rapid Analysis of Non-Esterified Fatty Acids as Methyl Esters from Different Biological Specimens by Gas Chromatography after One-Step Esterification. J. Chromatogr. B Biomed. Sci. Appl. 1990, 526, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.T.; Santos, M.V.; Fernandes, P.; Fonseca, L.L.; Fonseca, C.; Ferreira, F.C. Production of Glycolipid Biosurfactants, Mannosylerythritol Lipids, from Pentoses and d-Glucose/d-Xylose Mixtures by Pseudozyma Yeast Strains. Process Biochem. 2014, 49, 1790–1799. [Google Scholar] [CrossRef][Green Version]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the Production of Lipid and Carotenoids: A Review with Focus on Stress Regulation and Adaptation. Biotechnol. Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of Nitrogen Sources on Cell Growth and Lipid Accumulation of Green Alga Neochloris Oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Janelas, J.; Tropecêlo, A.I.; Oliveira, A.C. Microalga Nannochloropsis sp. Biomass for Biodiesel Production: Conventional (Cell Disruption) and in Situ Transesterification. J. Mar. Biol. Oceanogr. 2016, 5, 1–7. [Google Scholar] [CrossRef]
- Robles Arévalo, A. Substrates for the Sustainable Production of Mannosylerythritol Lipids: Biological Oils vs. Nanofiltrated Lignocellulosic Hydrolysates. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2015. [Google Scholar]
- Faria, N.T.; Nascimento, M.F.; Ferreira, F.A.; Esteves, T.; Santos, M.V.; Ferreira, F.C. Efficient Production of Mannosylerythritol Lipids by Moesziomyces Spp.: Substrates of Opposite Polarities for Optimal Biosurfactant Production and Downstream Processing. Appl. Biochem. Biotechnol. 2022. submittedi. [Google Scholar]
- Ueda, H.; Mitsuhara, I.; Tabata, J.; Kugimiya, S.; Watanabe, T.; Suzuki, K.; Yoshida, S.; Kitamoto, H. Extracellular Esterases of Phylloplane Yeast Pseudozyma Antarctica Induce Defect on Cuticle Layer Structure and Water-Holding Ability of Plant Leaves. Appl. Microbiol. Biotechnol. 2015, 99, 6405–6415. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, D.; Yanagishita, H.; Haraya, K.; Kitamoto, H.K. Contribution of a Chain-Shortening Pathway to the Biosynthesis of the Fatty Acids of Mannosylerythritol Lipid (Biosurfactant) in the Yeast Candida Antarctica: Effect of β-Oxidation Inhibitors on Biosurfactant Synthesis. Biotechnol. Lett. 1998, 20, 813–818. [Google Scholar] [CrossRef]
- Akkermans, V.; Verstraete, R.; Braem, C.; D’Aes, J.; Dries, J. Mannosylerythritol Lipid Production from Oleaginous Yeast Cell Lysate by Moesziomyces Aphidis. Ind. Biotechnol. 2020, 16, 222–232. [Google Scholar] [CrossRef]


| Fatty Acid Chain (%) | Algae-Derived Bio-Oils | WFO |
|---|---|---|
| C14:0 | - | - |
| C16:0 | 12.66 | 0.13 |
| C16:1 | 8.88 | 0 |
| C18:0 | 59.84 | 95.43 |
| C18:1 | 18.62 | 4.45 |
| Parameters | M. antarcticus PYCC 5048T | M. aphidis PYCC 5535T | ||||
|---|---|---|---|---|---|---|
| Algae-Derived Bio-Oils | WFO | D-Glucose | Algae-Derived Bio-Oils | WFO | D-Glucose | |
| Rs (g/L/h) | 0.43 ± 0.02 | 0.36 ± 0.02 | 0.31 | 0.41 ± 0 | 0.38 ± 0.01 | 0.36 |
| Biomassmax (g/L) | 27.0 ± 3.0 (Day 4) | 28.5 ± 0.5 (Day 11) | 17 | 24.5 ± 1.5 (Day 11) | 28.5 ± 0.5 (Day 11) | 14 |
| MELmax (g/L) | 12.47 ± 0.28 (Day 7) | 21.94 ± 1.31 (Day 11) | 8.09 (Day 11) | 5.72 ± 2.32 (Day 4) | 16.98 ± 0.39 (Day 11) | 6.64 (Day 11) |
| Y MEL/Substrate (g/g) | 0.21 ± 0.01 | 0.37 ± 0.02 | 0.13 | 0.1 ± 0.04 | 0.28 ± 0.39 | 0.11 |
| Productivitymax (g/L/day) | 1.78 ± 0.04 | 1.99 ± 0.12 | 0.73 | 1.43 ± 0.58 | 1.54 ± 0.01 | 0.60 |
| MEL purity (g/g) | 0.84 ± 0.01 | 0.88 ± 0.03 | 0.84 | 0.61 ± 0.06 | 0.88 ± 0.04 | 0.58 |
| Bio-Oil Producing Strain | Product Recovery | MELs Producing Strain | MELs Max (g/L) | Yield (gMELs/gsubstrate) | Productivity (g/L/h) | Ref |
|---|---|---|---|---|---|---|
| Cutaneotrichosporon oleoginosus | Use of cell lysates | M. aphidis | 2.3 | 0.19 * | 0.5 * | Akkermans et al. [34] |
| Neochloris oleoabundans | Use or organic solvents | M. aphidis | 5.72 ± 2.32 | 0.1 ± 0.04 | 1.43 ± 0.58 | This study |
| M. antarcticus | 12.47 ± 0.28 | 0.21 ± 0.01 | 1.78 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.F.; Coelho, T.; Reis, A.; Gouveia, L.; Faria, N.T.; Ferreira, F.C. Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach. Microorganisms 2022, 10, 2390. https://doi.org/10.3390/microorganisms10122390
Nascimento MF, Coelho T, Reis A, Gouveia L, Faria NT, Ferreira FC. Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach. Microorganisms. 2022; 10(12):2390. https://doi.org/10.3390/microorganisms10122390
Chicago/Turabian StyleNascimento, Miguel Figueiredo, Tiago Coelho, Alberto Reis, Luísa Gouveia, Nuno Torres Faria, and Frederico Castelo Ferreira. 2022. "Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach" Microorganisms 10, no. 12: 2390. https://doi.org/10.3390/microorganisms10122390
APA StyleNascimento, M. F., Coelho, T., Reis, A., Gouveia, L., Faria, N. T., & Ferreira, F. C. (2022). Production of Mannosylerythritol Lipids Using Oils from Oleaginous Microalgae: Two Sequential Microorganism Culture Approach. Microorganisms, 10(12), 2390. https://doi.org/10.3390/microorganisms10122390










