Closing the Gap between Bio-Based and Petroleum-Based Plastic through Bioengineering
Abstract
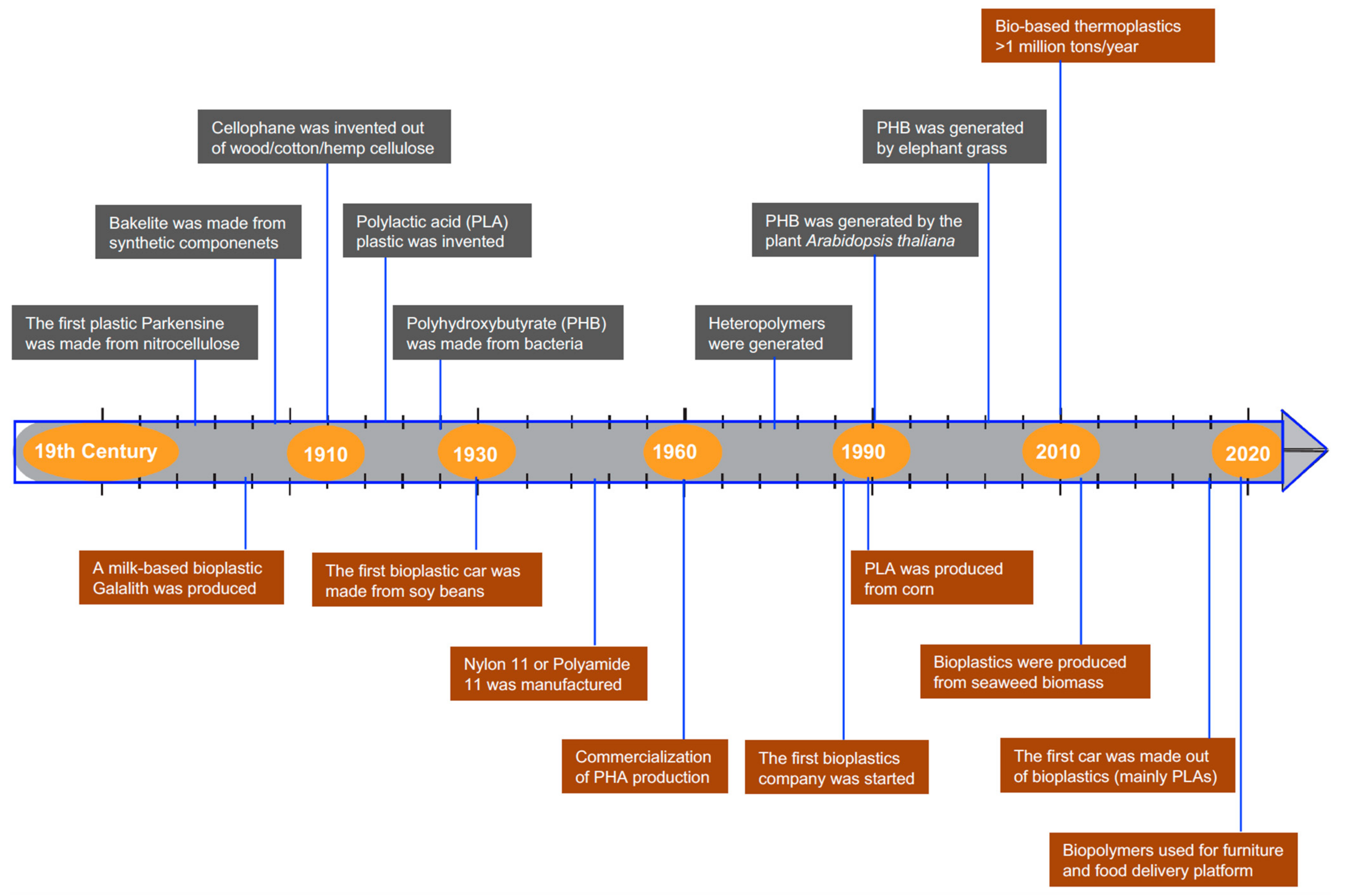
1. Introduction
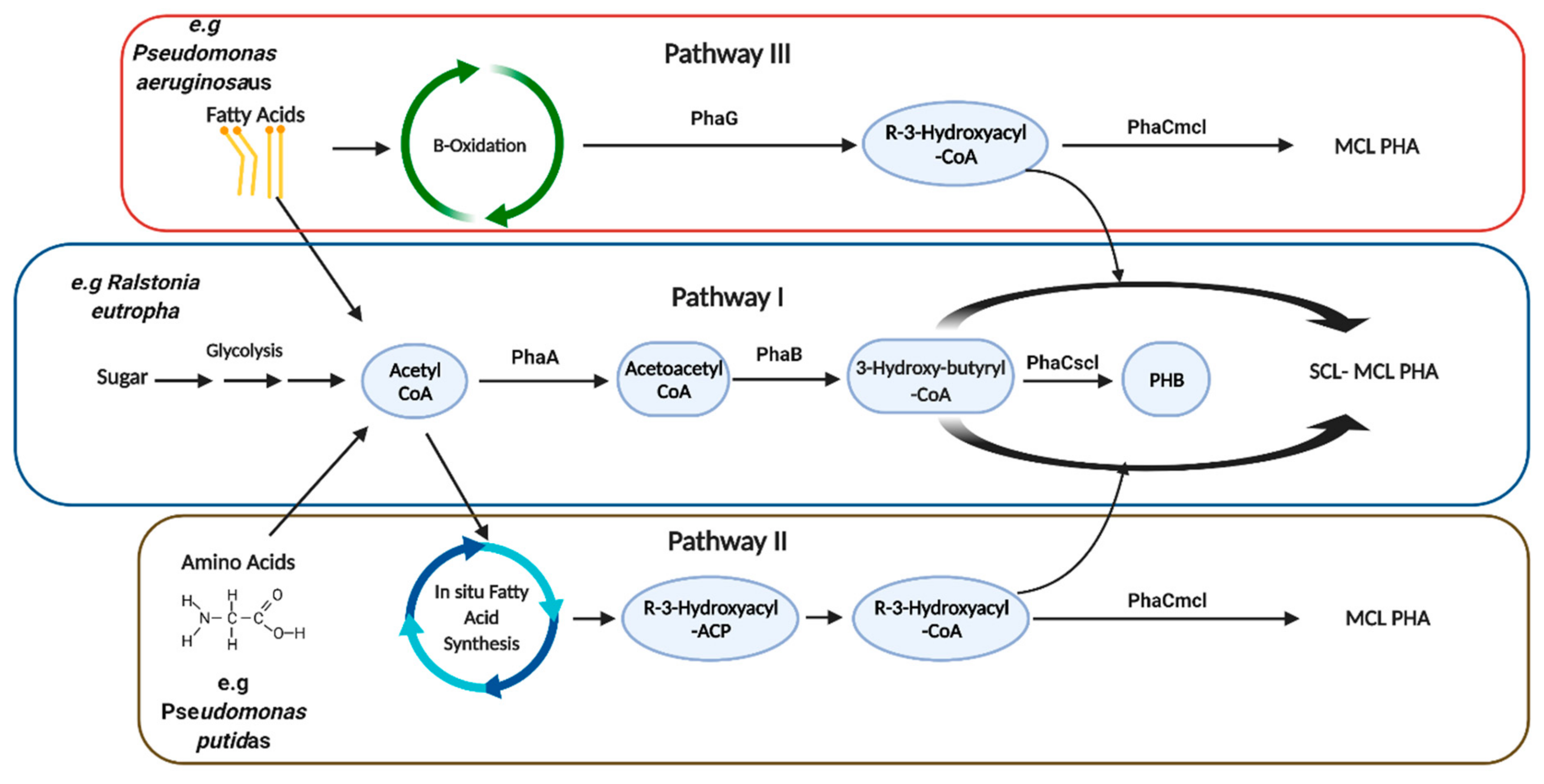
2. Native Polyhydroxyalkanoate Production in Microorganisms
3. Bioplastic Degradation
| Abbreviation | Name | Fossil Fuel | Biomass | Non Biodegradable (NB)/ Biodegradable (B) | References |
|---|---|---|---|---|---|
| PHA | Poly(hydroxyalkanoate) | x | B | [43] | |
| PHB | Poly(hyroxybutyrate) | x | B | [51] | |
| PLA | Poly(lactide) | x | B | [44] | |
| AcC (CTA, TAC) | Acetyl cellulose, cellulose triacetate | x | B | [45,52] | |
| Starch | Starch | x | B | [46] | |
| PBS | Poly(butylene succinate) | x | B | [45,51,53] | |
| PES | Poly(ethylene succinate) | x | B | [45] | |
| PCL | Poly(caprolactone) | x | B | [45,51] | |
| Bio PE | Poly(ethylene) | x | NB | [54] | |
| Bio PP | Poly(propylene) | x | NB | [54] |
4. Bioplastic Degradation: Enzymes and Microorganisms
5. Bioengineering of Polyhydroxyalkanoate Production in Bacteria
6. Polyhydroxyalkanoate Production in Plants
7. Polyhydroxyalkanoate Production in Microalgae
8. Synthetic Approaches and New Advancements
9. Genome Engineering Prospects
10. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2017-2/ (accessed on 8 November 2022).
- EPA National Overview: Facts and Figures on Materials, Wastes and Recycling. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials (accessed on 29 September 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J.G. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. Int 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond\. Ser. B Biol. Sci. 2009, 364, 2097–2113. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.B.O.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Martin, J.; Lusher, A.; Thompson, R.C.; Morley, A. The Deposition and Accumulation of Microplastics in Marine Sediments and Bottom Water from the Irish Continental Shelf. Sci. Rep. 2017, 7, 10772. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- de Sa, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Kontrick, A.V. Microplastics and Human Health: Our Great Future to Think About Now. J. Med. Toxicol. 2018, 14, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- O’Hanlon, N.J.; James, N.A.; Masden, E.A.; Bond, A.L. Seabirds and marine plastic debris in the northeastern Atlantic: A synthesis and recommendations for monitoring and research. Environ. Pollut. 2017, 231, 1291–1301. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.N.; Na, J. Future of microbial polyesters. Microb. Cell Fact. 2013, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Urtuvia, V.; Villegas, P.; Gonzalez, M.; Seeger, M. Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 70, 208–213. [Google Scholar] [CrossRef]
- Varghese, S.; Dhanraj, N.D.; Rebello, S.; Sindhu, R.; Binod, P.; Pandey, A.; Jisha, M.S.; Awasthi, M.K. Leads and hurdles to sustainable microbial bioplastic production. Chemosphere 2022, 305, 135390. [Google Scholar] [CrossRef]
- Dolci, G.; Venturelli, V.; Catenacci, A.; Ciapponi, R.; Malpei, F.; Romano Turri, S.E.; Grosso, M. Evaluation of the anaerobic degradation of food waste collection bags made of paper or bioplastic. J. Environ. Manag. 2022, 305, 114331. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, H.; Masuo, S.; Kaneko, T.; Takaya, N. Fermentation and purification of microbial monomer 4-amminocinnamic acid to produce ultra-high performance bioplastics. Process. Biochem. 2019, 77, 100–105. [Google Scholar] [CrossRef]
- Malik, M.R.; Yang, W.; Patterson, N.; Tang, J.; Wellinghoff, R.L.; Preuss, M.L.; Burkitt, C.; Sharma, N.; Ji, Y.; Jez, J.M.; et al. Production of high levels of poly-3-hydroxybutyrate in plastids of Camelina sativa seeds. Plant Biotechnol. J. 2015, 13, 675–688. [Google Scholar] [CrossRef] [PubMed]
- NESTE Neste and Ikea of Sweden Announce Partnership to Deliver Renewable, Bio-Based Plastics. Available online: https://www.neste.com/neste-and-ikea-sweden-announce-partnership-deliver-renewable-bio-based-plastics (accessed on 29 September 2022).
- Jurasek, L.; Marchessault, R.H. Polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha cells: A computer simulation. Appl. Microbiol. Biotechnol. 2004, 64, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Mravec, F.; Obruca, S.; Krzyzanek, V.; Sedlacek, P.; Hrubanova, K.; Samek, O.; Kucera, D.; Benesova, P.; Nebesarova, J. Accumulation of PHA granules in Cupriavidus necator as seen by confocal fluorescence microscopy. FEMS Microbiol. Lett. 2016, 363, fnw094. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Di Donato, P.; Abbamondi, G.R.; Nicolaus, B. Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011, 2011, 693253. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lenz, R.W. Polyesters from microorganisms. Adv. Biochem. Eng./Biotechnol. 2001, 71, 51–79. [Google Scholar]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Masood, F.; Yasin, T.; Hameed, A. Comparative oxo-biodegradation study of poly-3-hydroxybutyrate-co-3-hydroxyvalerate/polypropylene blend in controlled environments. Int. Biodeterior. Biodegrad. 2014, 87, 1–8. [Google Scholar] [CrossRef]
- Lemoigne, M. Produit de deshydratation et de polymerisation de l’acide β-oxybutyrique. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Peoples, O.P.; Sinskey, A.J. Poly-beta-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identification and characterization of the PHB polymerase gene (phbC). J. Biol. Chem. 1989, 264, 15298–15303. [Google Scholar] [CrossRef]
- Peoples, O.P.; Sinskey, A.J. Poly-beta-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding beta-ketothiolase and acetoacetyl-CoA reductase. J. Biol. Chem. 1989, 264, 15293–15297. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacture of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Pratt, S.; Laycock, B.; Ashworth, P.; Lant, P.A. Public attitudes towards plastics. Resour. Conserv. Recycl. 2019, 147, 227–235. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B 2009, 364, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Pemba, A.G.; Rostagno, M.; Lee, T.A.; Miller, S.A. Cyclic and spirocyclic polyacetal ethers from lignin-based aromatics. Polym. Chem. 2014, 5, 3214–3221. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Accinelli, C.; Sacca, M.L.; Mencarelli, M.; Vicari, A. Deterioration of bioplastic carrier bags in the environment and assessment of a new recycling alternative. Chemosphere 2012, 89, 136–143. [Google Scholar] [CrossRef]
- Kubowicz, S.; Booth, A.M. Biodegradability of Plastics: Challenges and Misconceptions. Environ. Sci. Technol 2017, 51, 12058–12060. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12, 123. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques—A review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Saika, A.; Nomura, K.; Watanabe, T.; Watanabe, T.; Fujimoto, Y.; Enoki, M.; Sato, T.; Kato, C.; Kanehiro, H. Biodegradation of aliphatic polyesters soaked in deep seawaters and isolation of poly(epsilon-caprolactone)-degrading bacteria. Polym. Degrad. Stabil. 2011, 96, 1397–1403. [Google Scholar] [CrossRef]
- McKeen, L. Renewable Resource and Biodegradable Polymers. Pdl. Handb. Ser. 2012, 353–378. [Google Scholar]
- Kim, S.; Park, S.; Lee, K. Fishing Performance of an Octopus minor Net Pot Made of Biodegradable Twines. Turk. J. Fish Aquat Sc 2014, 14, 21–30. [Google Scholar]
- Byun, Y.; Kim, Y.T. Bioplastics for Food Packaging: Chemistry and Physics. In Innovations in Food Packaging, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 353–368. [Google Scholar]
- Phukon, P.; Saikia, J.P.; Konwar, B.K. Bio-plastic (P-3HB-co-3HV) from Bacillus circulans (MTCC 8167) and its biodegradation. Colloids Surf. B Biointerfaces 2012, 92, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Calabia, B.P.; Tokiwa, Y. Microbial degradation of poly(D-3-hydroxybutyrate) by a new thermophilic Streptomyces isolate. Biotechnol. Lett. 2004, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P. Degradation of microbial polyesters. Biotechnol. Lett. 2004, 26, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hidalgo, J.; Hormigo, D.; Arroyo, M.; de la Mata, I. Novel Extracellular PHB Depolymerase from Streptomyces ascomycinicus: PHB Copolymers Degradation in Acidic Conditions. PLoS ONE 2013, 8, e71699. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Jarerat, A. Biodegradation of poly(L-lactide). Biotechnol. Lett. 2004, 26, 771–777. [Google Scholar] [CrossRef]
- Nakajima-Kambe, T.; Toyoshima, K.; Saito, C.; Takaguchi, H.; Akutsu-Shigeno, Y.; Sato, M.; Miyama, K.; Nomura, N.; Uchiyama, H. Rapid monomerization of poly(butylene succinate)-co-(butylene adipate) by Leptothrix sp. J. Biosci. Bioeng. 2009, 108, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Penkhrue, W.; Khanongnuch, C.; Masaki, K.; Pathom-aree, W.; Punyodom, W.; Lumyong, S. Isolation and screening of biopolymer-degrading microorganisms from northern Thailand. World J. Microb. Biot. 2015, 31, 1431–1442. [Google Scholar] [CrossRef]
- Chen, G.Q.; Jiang, X.R.; Guo, Y. Synthetic biology of microbes synthesizing polyhydroxyalkanoates (PHA). Synth. Syst. Biotechnol. 2016, 1, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Sangkharak, K.; Prasertsan, P. Screening and identification of polyhydroxyalkanoates producing bacteria and biochemical characterization of their possible application. J. Gen. Appl. Microbiol. 2012, 58, 173–182. [Google Scholar] [CrossRef]
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.-Y.A.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.M.N.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Mezzolla, V.; D’Urso, O.F.; Poltronieri, P. Role of PhaC Type I and Type II Enzymes during PHA Biosynthesis. Polymers 2018, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Rehm, B.H. Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: Identification of catalytic residues. Biochem. J. 2003, 374, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Health Sci. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.I.; Sikorska, W.; Musiol, M.; Zieba, M.; Marek, A.A.; Keddie, D.; et al. The Microbial Production of Polyhydroxyalkanoates from Waste Polystyrene Fragments Attained Using Oxidative Degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Jari, M.; Khatami, S.R.; Galehdari, H.; Shafiei, M. Cloning and Expression of Poly 3-Hydroxybutyrate Operon Into Escherichia coli. Jundishapur. J. Microb. 2015, 8, e16318. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, K.; Motoda, Y.; Numata, K.; Tsuge, T. Phasin Proteins Activate Aeromonas caviae Polyhydroxyalkanoate (PHA) Synthase but Not Ralstonia eutropha PHA Synthase. Appl. Environ. Microb. 2014, 80, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Z.; Han, J.; Chen, G.Q. Metabolic engineering of Aeromonas hydrophila for the enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl. Microbiol. Biot. 2006, 69, 537–542. [Google Scholar] [CrossRef]
- Adkins, J.; Pugh, S.; McKenna, R.; Nielsen, D.R. Engineering microbial chemical factories to produce renewable “biomonomers”. Front. Microbiol. 2012, 3, 313. [Google Scholar] [CrossRef]
- Zhou, Y.; Dominguez, J.M.; Cao, N.J.; Du, J.X.; Tsao, G.T. Optimization of L-lactic acid production from glucose by Rhizopus oryzae ATCC 52311. Appl. Biochem. Biotech. 1999, 77–79, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, S.; Clomburg, J.M.; Gonzalez, R. Escherichia coli Strains Engineered for Homofermentative Production of D-Lactic Acid from Glycerol. Appl. Environ. Microb. 2010, 76, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.K.; Kim, T.Y.; Park, S.J.; Lee, S.Y. Metabolic Engineering of Escherichia coli for the Production of Polylactic Acid and Its Copolymers. Biotechnol. Bioeng. 2010, 105, 161–171. [Google Scholar] [CrossRef]
- Yang, T.H.; Kim, T.W.; Kang, H.O.; Lee, S.H.; Lee, E.J.; Lim, S.C.; Oh, S.O.; Song, A.J.; Park, S.J.; Lee, S.Y. Biosynthesis of Polylactic Acid and Its Copolymers Using Evolved Propionate CoA Transferase and PHA Synthase. Biotechnol. Bioeng. 2010, 105, 150–160. [Google Scholar] [CrossRef]
- Chaudhry, W.; Jamil, N.; Ali, I.; Ayaz, M.; Hasnain, S. Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann. Microbiol. 2010, 61, 623–629. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, Y.B.; Rhee, Y.H. Evaluation of various carbon substrates for the biosynthesis of polyhydroxyalkanoates bearing functional groups by Pseudomonas putida. Int. J. Biol. Macromol. 2000, 28, 23–29. [Google Scholar] [CrossRef]
- Somleva, M.N.; Peoples, O.P.; Snell, K.D. PHA Bioplastics, Biochemicals, and Energy from Crops. Plant Biotechnol. J. 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Poirier, Y.; Somerville, C.; Schechtman, L.A.; Satkowski, M.M.; Noda, I. Synthesis of high-molecular-weight poly([R]-(-)-3-hydroxybutyrate) in transgenic Arabidopsis thaliana plant cells. Int. J. Biol. Macromol. 1995, 17, 7–12. [Google Scholar] [CrossRef]
- Poirier, Y.; Dennis, D.E.; Klomparens, K.; Somerville, C. Polyhydroxybutyrate, a biodegradable thermoplastic, produced in transgenic plants. Science 1992, 256, 520–523. [Google Scholar] [CrossRef]
- Nawrath, C.; Poirier, Y.; Somerville, C. Targeting of the polyhydroxybutyrate biosynthetic pathway to the plastids of Arabidopsis thaliana results in high levels of polymer accumulation. Proc. Natl. Acad. Sci. USA 1994, 91, 12760–12764. [Google Scholar] [CrossRef] [PubMed]
- Bohmert, K.; Balbo, I.; Kopka, J.; Mittendorf, V.; Nawrath, C.; Poirier, Y.; Tischendorf, G.; Trethewey, R.N.; Willmitzer, L. Transgenic Arabidopsis plants can accumulate polyhydroxybutyrate to up to 4% of their fresh weight. Planta 2000, 211, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Yoshida, K.; Akiyoshi, M.; Manji, S. Hybrid fiber production: A wood and plastic combination in transgenic rice and Tamarix made by accumulating poly-3-hydroxybutyrate. Plant Biotechnol. J. 2006, 3, 99–109. [Google Scholar] [CrossRef][Green Version]
- Lössl, A.; Eibl, C.; Harloff, H.-J.; Jung, C.; Koop, H.U. Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L.): Significant contents of polyhydroxybutyrate are associated with growth reduction. Plant Cell Rep. 2003, 21, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bohmert-Tatarev, K.; McAvoy, S.; Daughtry, S.; Peoples, O.P.; Snell, K.D. High Levels of Bioplastic Are Produced in Fertile Transplastomic Tobacco Plants Engineered with a Synthetic Operon for the Production of Polyhydroxybutyrate. Plant Physiol 2011, 155, 1690. [Google Scholar] [CrossRef] [PubMed]
- Somleva, M.N.; Snell, K.D.; Beaulieu, J.J.; Peoples, O.P.; Garrison, B.R.; Patterson, N.A. Production of polyhydroxybutyrate in switchgrass, a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnol. J. 2008, 6, 663–678. [Google Scholar] [CrossRef]
- Institute for Bioplastics and Biocomposites. Biopolymers, Facts and Statistics; Institute for Bioplastics and Biocomposites: Hannover, Germany, 2018. [Google Scholar]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production-Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef]
- Troschl, C.; Meixner, K.; Fritz, I.; Lechner, K.; Romero, A.; Kovalcik, A.; Sedlacek, P.; Drosg, B. Pilot-scale production of poly-β-hydroxybutyrate with the cyanobacterium Synechocytis sp. CCALA192 in a non-sterile tubular photobioreactor. Algal. Res. 2018, 34, 116–125. [Google Scholar] [CrossRef]
- Katayama, N.; Iijima, H.; Osanai, T. Production of Bioplastic Compounds by Genetically Manipulated and Metabolic Engineered Cyanobacteria. Adv. Exp. Med. Biol. 2018, 1080, 155–169. [Google Scholar]
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef]
- Huang, W.; Daboussi, F. Genetic and metabolic engineering in diatoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160411. [Google Scholar] [CrossRef]
- Poulsen, N.; Kroger, N. A new molecular tool for transgenic diatoms: Control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J. 2005, 272, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Hempel, F.; Bozarth, A.S.; Lindenkamp, N.; Klingl, A.; Zauner, S.; Linne, U.; Steinbuchel, A.; Maier, U.G. Microalgae as bioreactors for bioplastic production. Microb. Cell Fact. 2011, 10, 81. [Google Scholar] [CrossRef]
- Chaogang, W.; Zhangli, H.; Anping, L.; Baohui, J. Biosynthesis of poly-3-hydroxybutyrate (phb) in the transgenic green alga chlamydomonas reinhardtii1. J. Phycol. 2010, 46, 396–402. [Google Scholar] [CrossRef]
- Chen, G.Q.; Hajnal, I.; Wu, H.; Lv, L.; Ye, J. Engineering Biosynthesis Mechanisms for Diversifying Polyhydroxyalkanoates. Trends Biotechnol. 2015, 33, 565–574. [Google Scholar] [CrossRef]
- Meng, D.C.; Shen, R.; Yao, H.; Chen, J.C.; Wu, Q.; Chen, G.Q. Engineering the diversity of polyesters. Curr. Opin. Biotechnol. 2014, 29, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Hajnal, I. The ‘PHAome’. Trends Biotechnol. 2015, 33, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Lv, L.; Chen, G.Q. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi. Microb. Cell Fact. 2017, 16, 48. [Google Scholar] [CrossRef]
- Wang, H.H.; Isaacs, F.J.; Carr, P.A.; Sun, Z.Z.; Xu, G.; Forest, C.R.; Church, G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature 2009, 460, 894–898. [Google Scholar] [CrossRef]
- Wang, H.H.; Kim, H.; Cong, L.; Jeong, J.; Bang, D.; Church, G.M. Genome-scale promoter engineering by coselection MAGE. Nat. Methods 2012, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, J.E.; Conley, A.J.; Penttila, M.; Jantti, J.; Wang, H.H.; Church, G.M. Yeast Oligo-Mediated Genome Engineering (YOGE). Acs Synth. Biol. 2013, 2, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Mitchell, L.A.; Stracquadanio, G.; Yang, K.; Dymond, J.S.; DiCarlo, J.E.; Lee, D.; Huang, C.L.V.; Chandrasegaran, S.; Cai, Y.Z.; et al. Design of a synthetic yeast genome. Science 2017, 355, 1040–1044. [Google Scholar] [CrossRef]
- Annaluru, N.; Muller, H.; Mitchell, L.A.; Ramalingam, S.; Stracquadanio, G.; Richardson, S.M.; Dymond, J.S.; Kuang, Z.; Scheifele, L.Z.; Cooper, E.M.; et al. Total Synthesis of a Functional Designer Eukaryotic Chromosome. Science 2014, 344, 55–58. [Google Scholar] [CrossRef]
- Dymond, J.S.; Richardson, S.M.; Coombes, C.E.; Babatz, T.; Muller, H.; Annaluru, N.; Blake, W.J.; Schwerzmann, J.W.; Dai, J.; Lindstrom, D.L.; et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 2011, 477, 471–476. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, R.-Y.; Mitchell, L.A.; Ma, L.; Liu, R.; Zhao, M.; Jia, B.; Xu, H.; Li, Y.-X.; Yang, Z.-M.; et al. In vitro DNA SCRaMbLE. Nat. Commun. 2018, 9, 1935. [Google Scholar] [CrossRef]
- Jia, B.; Wu, Y.; Li, B.Z.; Mitchell, L.A.; Liu, H.; Pan, S.; Wang, J.; Zhang, H.R.; Jia, N.; Li, B.; et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast. Nat. Commun. 2018, 9, 1933. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Chapter 6—Bioactive Compounds From Microalgae: Current Development and Prospects. In Studies in Natural Products Chemistry; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Karemore, A.; Nayak, M.; Sen, R. Recent Inventions and Trends in Algal Biofuels Research. Recent Pat. Biotechnol. 2016, 10, 30–42. [Google Scholar] [CrossRef]
- Jijakli, K.; Abdrabu, R.; Khraiwesh, B.; Nelson, D.R.; Koussa, J.; Salehi-Ashtiani, K. Molecular Genetic Techniques for Algal Bioengineering. In Biomass and Biofuels from Microalgae: Advances in Engineering and Biology; Moheimani, N.R., McHenry, M.P., de Boer, K., Bahri, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 155–171. [Google Scholar]
- Munoz-Bonilla, A.; Echeverria, C.; Sonseca, A.; Arrieta, M.P.; Fernandez-Garcia, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.B.; Chen, J.C.; Wu, Q.; Chen, G.Q. Open and continuous fermentation: Products, conditions and bioprocess economy. Biotechnol. J. 2014, 9, 1503–1511. [Google Scholar] [CrossRef]
- Li, Z.J.; Shi, Z.Y.; Jian, J.; Guo, Y.Y.; Wu, Q.; Chen, G.Q. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab. Eng. 2010, 12, 352–359. [Google Scholar] [CrossRef]
- Tripathi, L.; Wu, L.P.; Dechuan, M.; Chen, J.; Wu, Q.; Chen, G.Q. Pseudomonas putida KT2442 as a platform for the biosynthesis of polyhydroxyalkanoates with adjustable monomer contents and compositions. Bioresour. Technol. 2013, 142, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, Z.; Jiang, X.; Chen, J.; Chen, G.Q. Enhanced production of polyhydroxybutyrate by multiple dividing E. coli. Microb. Cell Fact. 2016, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Jiang, X.-R. Engineering bacteria for enhanced polyhydroxyalkanoates (PHA) biosynthesis. Synth. Syst. Biotechnol. 2017, 2, 192–197. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khairy, D.; Fu, W.; Alzahmi, A.S.; Twizere, J.-C.; Amin, S.A.; Salehi-Ashtiani, K.; Mystikou, A. Closing the Gap between Bio-Based and Petroleum-Based Plastic through Bioengineering. Microorganisms 2022, 10, 2320. https://doi.org/10.3390/microorganisms10122320
Al-Khairy D, Fu W, Alzahmi AS, Twizere J-C, Amin SA, Salehi-Ashtiani K, Mystikou A. Closing the Gap between Bio-Based and Petroleum-Based Plastic through Bioengineering. Microorganisms. 2022; 10(12):2320. https://doi.org/10.3390/microorganisms10122320
Chicago/Turabian StyleAl-Khairy, Dina, Weiqi Fu, Amnah Salem Alzahmi, Jean-Claude Twizere, Shady A. Amin, Kourosh Salehi-Ashtiani, and Alexandra Mystikou. 2022. "Closing the Gap between Bio-Based and Petroleum-Based Plastic through Bioengineering" Microorganisms 10, no. 12: 2320. https://doi.org/10.3390/microorganisms10122320
APA StyleAl-Khairy, D., Fu, W., Alzahmi, A. S., Twizere, J.-C., Amin, S. A., Salehi-Ashtiani, K., & Mystikou, A. (2022). Closing the Gap between Bio-Based and Petroleum-Based Plastic through Bioengineering. Microorganisms, 10(12), 2320. https://doi.org/10.3390/microorganisms10122320










