Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis
Abstract
1. Introduction
2. Materials and Methods
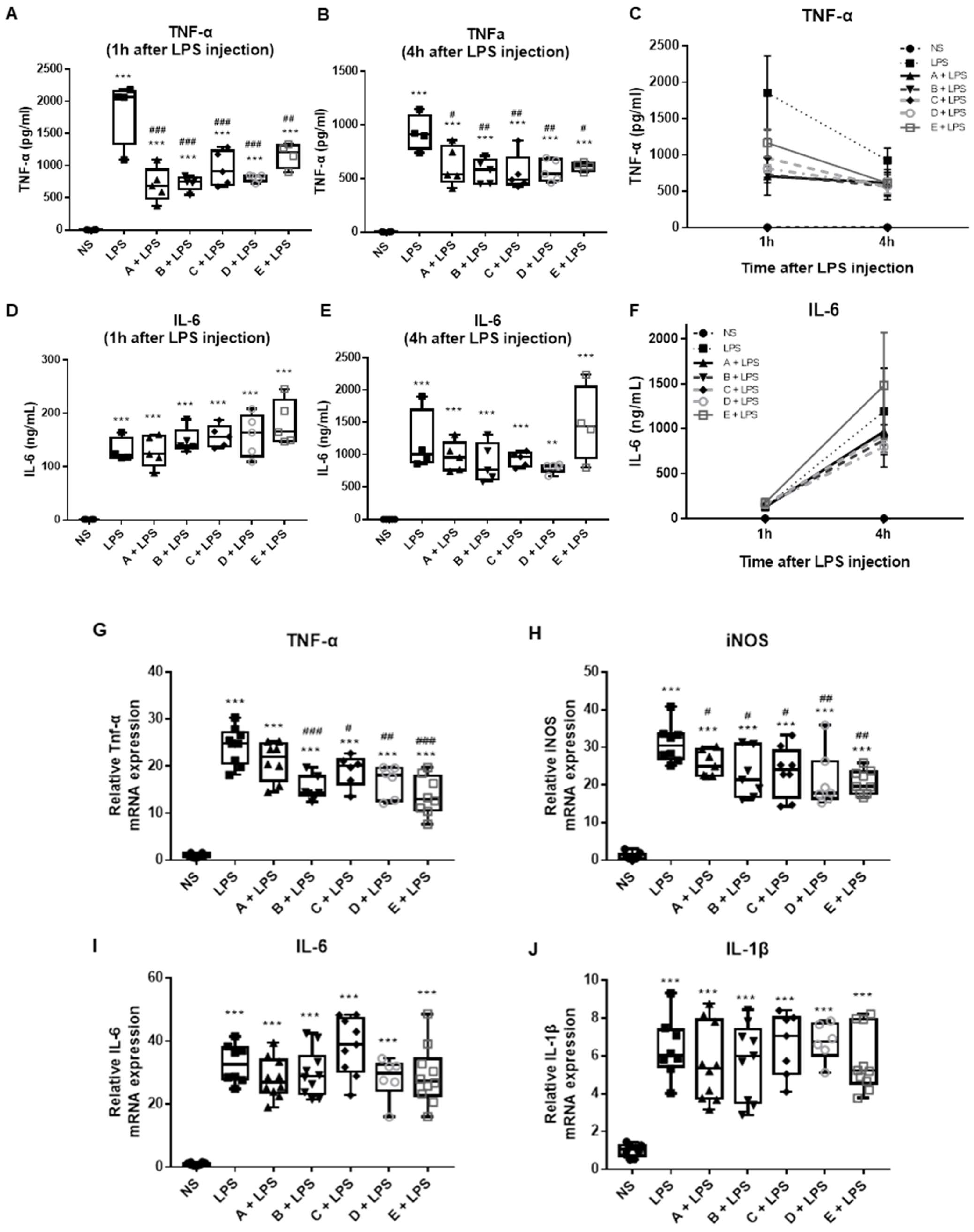
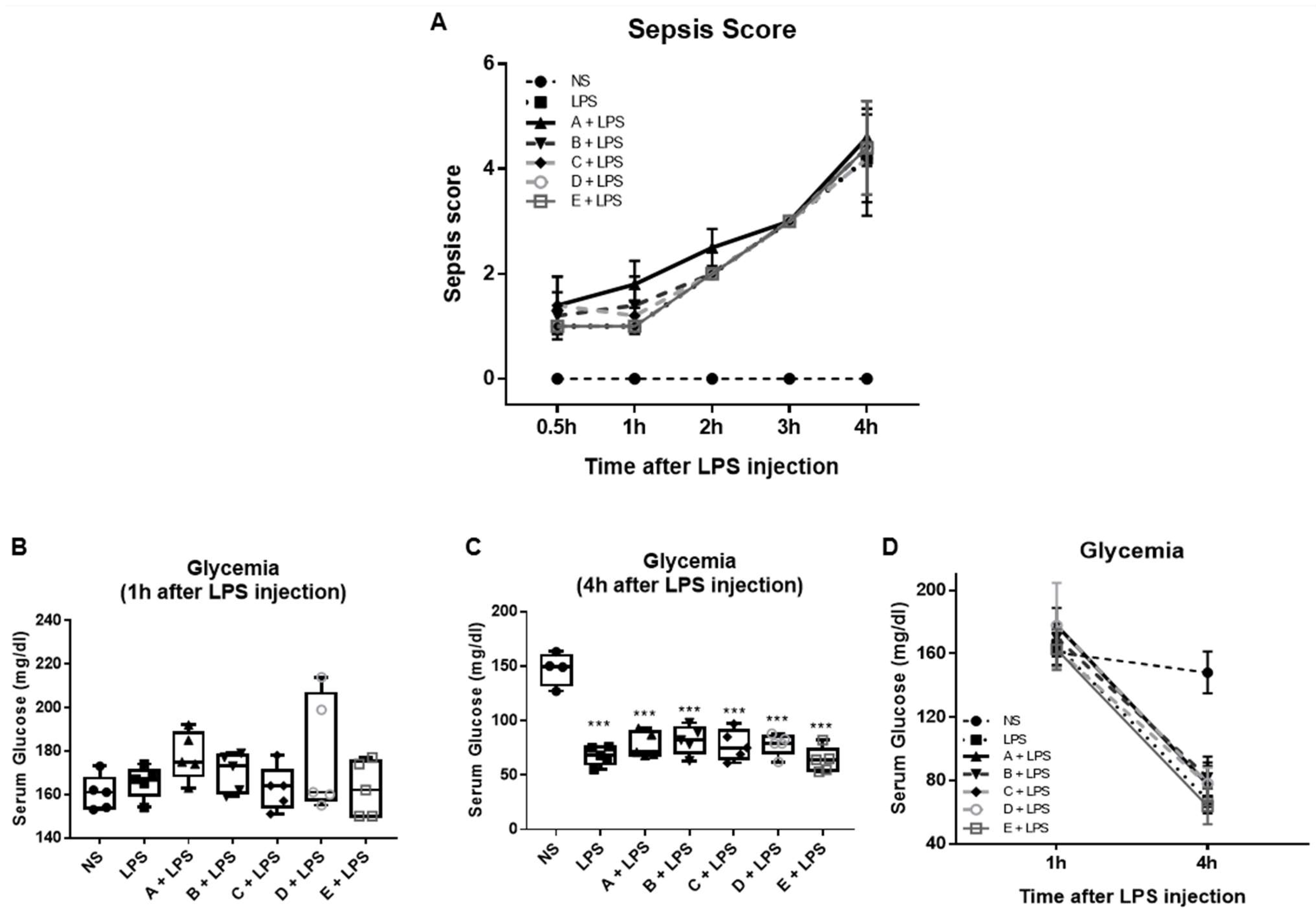
2.1. LPS-Induced Endotoxin Shock
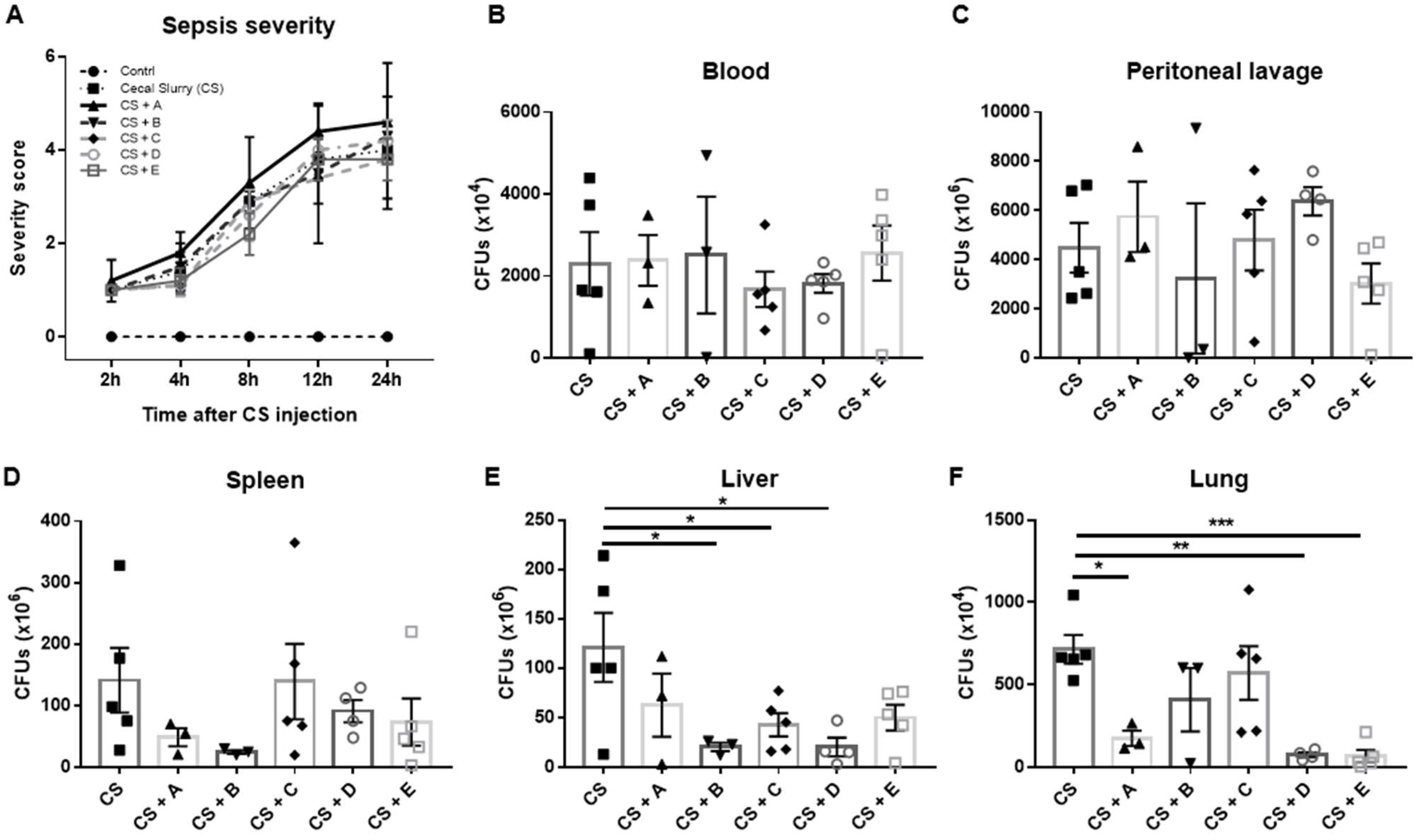
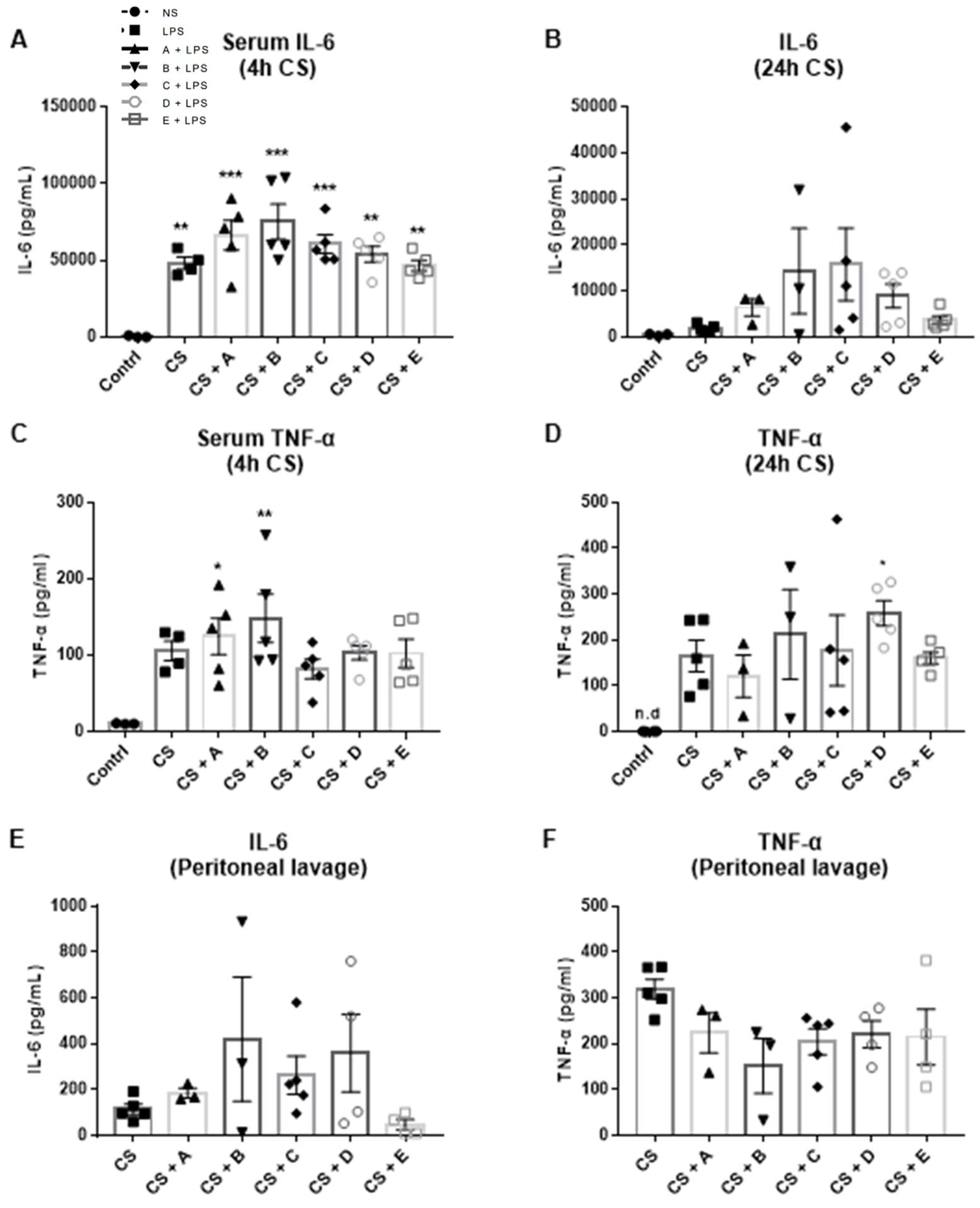
2.2. Cecal-Slurry-Induced Polymicrobial Sepsis
2.3. ELISA
2.4. RNA Isolation and Quantitative PCR
2.5. Statistical Analysis
| Primers for RT-PCR | ||
| TNF-α | Fwd | 5′-CACGCTCTTCTGTCTACTGAACTTCG-3′ |
| Rev | 5′-GGCTGGGTAGAGAATGGATGAACACC-3′ | |
| IL-6 | Fwd | 5′-TTCCATCCAGTTGCCTTCTT-3′ |
| Rev | 5′-CAGAATTGCCATTGCACAAC-3′ | |
| IL-1β | Fwd | 5′-GGATGAGGACATGAGCACCT-3′ |
| Rev | 5′-TCCATTGAGGTGGAGAGCTT-3′ | |
| iNOS | Fwd | 5′-TCCTGGAGGAAGTGGGCCGAAG-3′ |
| Rev | 5′-CCTCCACGGGCCCGGTACTC-3′ | |
| CYP1A1 | Fwd | 5′-TTAACCATGACCGGGAACTGT-3′ |
| Rev | 5′-CACTTTCGCTTGCCCAAACC-3′ | |
| CYP2B10 | Fwd | 5′-GCTCATTCTCCAGCCAGATGTT-3′ |
| Rev | 5′-CTCCATGCGCAGAAGGTAAA-3′ | |
| CYP3A11 | Fwd | 5′-TTCACCCTGCATTCCTTGGC-3′ |
| Rev | 5′-TACGTGGGAGGTGCCTTGTT-3′ | |
| CYP3A25 | Fwd | 5′-ATCTTCGGGGGCTATGATGC-3′ |
| Rev | 5′-AGGTGACAGGTGCCTTATTGG-3′ | |
| Actin | Fwd | 5′-CATTGCTGACAGGATGCAGAAGG-3′ |
| Rev | 5′-TGCTGGAAGGTGGACAGTGAGG-3′ | |
3. Results
3.1. Effect of Greek Honey on Pro-Inflammatory Cytokines in the LPS-Induced Inflammation and Sepsis Model In Vivo
3.2. Evaluation of the Antibacterial Properties of Greek Honey in an In Vivo Model of Polymicrobial Sepsis in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [PubMed]
- El-Kazafy, A.T.; Saad, A.-K.; Reda, T. Comparison of Pollen Spectra and Amount of Mineral Content in Honey Produced by Apis florea F. and Apis mellifera L. J. Kans. Entomol. Soc. 2018, 91, 51–57. [Google Scholar] [CrossRef]
- Taha, E.-K.A.; Al-Kahtani, S.; Taha, R. Comparison of the physicochemical characteristics of sidr (Ziziphus spp.) honey produced by Apis florea F. and Apis mellifera L. J. Apic. Res. 2021, 60, 470–477. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Molecular mechanism-based therapeutic properties of honey. Biomed. Pharmacother. 2020, 130, 110590. [Google Scholar] [CrossRef]
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Lu, J.; Carter, D.A.; Turnbull, L.; Rosendale, D.; Hedderley, D.; Stephens, J.; Gannabathula, S.; Steinhorn, G.; Schlothauer, R.C.; Whitchurch, C.B.; et al. The Effect of New Zealand Kanuka, Manuka and Clover Honeys on Bacterial Growth Dynamics and Cellular Morphology Varies According to the Species. PLoS ONE 2013, 8, e55898. [Google Scholar] [CrossRef]
- Roberts, A.E.L.; Maddocks, S.E.; Cooper, R.A. Manuka honey reduces the motility of Pseudomonas aeruginosa by suppression of flagella-associated genes. J. Antimicrob. Chemother. 2014, 70, 716–725. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Evaluation of Physiological Effects Induced by Manuka Honey Upon Staphylococcus aureus and Escherichia coli. Microorganisms 2019, 7, 258. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Leticia, M.E.; Teixeira-Santos, R.; Acácio, G.R.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial Action Mechanisms of Honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef]
- Wang, R.; Starkey, M.; Hazan, R.; Rahme, L. Honey’s Ability to Counter Bacterial Infections Arises from Both Bactericidal Compounds and QS Inhibition. Front. Microbiol. 2012, 3, 144. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; López-Gálvez, F.; Gil, M.I.; Tomás-Barberán, F.A.; Allende, A. Quorum sensing inhibitory and antimicrobial activities of honeys and the relationship with individual phenolics. Food Chem. 2009, 115, 1337–1344. [Google Scholar] [CrossRef]
- Bouzo, D.; Cokcetin, N.N.; Li, L.; Ballerin, G.; Bottomley, A.L.; Lazenby, J.; Whitchurch, C.B.; Paulsen, I.T.; Hassan, K.A.; Harry, E.J. Characterizing the Mechanism of Action of an Ancient Antimicrobial, Manuka Honey, against Pseudomonas aeruginosa Using Modern Transcriptomics. mSystems 2020, 5, e00106-20. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Jenkins, R.E. Honey: A sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 2013, 8, 1419–1429. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Voidarou, C.C.; Rozos, G.; Vaou, N.; Bardanis, M.; Konstantinidis, T.; Vrioni, G.; Tsakris, A. Antimicrobial Evaluation of Various Honey Types against Carbapenemase-Producing Gram-Negative Clinical Isolates. Antibiotics 2022, 11, 422. [Google Scholar] [CrossRef]
- Arranz, A.; Doxaki, C.; Vergadi, E.; Martinez de la Torre, Y.; Vaporidi, K.; Lagoudaki, E.D.; Ieronymaki, E.; Androulidaki, A.; Venihaki, M.; Margioris, A.N.; et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. USA 2012, 109, 9517–9522. [Google Scholar] [CrossRef]
- Androulidaki, A.; Iliopoulos, D.; Arranz, A.; Doxaki, C.; Schworer, S.; Zacharioudaki, V.; Margioris, A.N.; Tsichlis, P.N.; Tsatsanis, C. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 2009, 31, 220–231. [Google Scholar] [CrossRef]
- Starr, M.E.; Steele, A.M.; Saito, M.; Hacker, B.J.; Evers, B.M.; Saito, H. A New Cecal Slurry Preparation Protocol with Improved Long-Term Reproducibility for Animal Models of Sepsis. PLoS ONE 2014, 9, e115705. [Google Scholar] [CrossRef]
- Rincon, J.C.; Efron, P.A.; Moldawer, L.L.; Larson, S.D. Cecal Slurry Injection in Neonatal and Adult Mice. Methods Mol. Biol. 2021, 2321, 27–41. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef]
- Kassim, M.; Achoui, M.; Mansor, M.; Yusoff, K.M. The inhibitory effects of Gelam honey and its extracts on nitric oxide and prostaglandin E(2) in inflammatory tissues. Fitoterapia 2010, 81, 1196–1201. [Google Scholar] [CrossRef]
- Hussein, S.Z.; Mohd Yusoff, K.; Makpol, S.; Mohd Yusof, Y.A. Gelam Honey Attenuates Carrageenan-Induced Rat Paw Inflammation via NF-κB Pathway. PLoS ONE 2013, 8, e72365. [Google Scholar] [CrossRef]
- Kassim, M.; Mansor, M.; Al-Abd, N.; Yusoff, K.M. Gelam honey has a protective effect against lipopolysaccharide (LPS)-induced organ failure. Int. J. Mol. Sci. 2012, 13, 6370–6381. [Google Scholar] [CrossRef]
- Afrin, S.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Manna, P.P.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 1: Enhancement of cellular viability, regulation of cellular apoptosis and improvement of mitochondrial functionality. Food Chem. Toxicol. 2018, 121, 203–213. [Google Scholar] [CrossRef]
- Gasparrini, M.; Afrin, S.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Mahmoud, A.M. Stingless bee honey protects against lipopolysaccharide induced-chronic subclinical systemic inflammation and oxidative stress by modulating Nrf2, NF-κB and p38 MAPK. Nutr. Metab. 2019, 16, 15. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernandez, T.Y.; Alvarez-Suarez, J.M.; Ansary, J.; Quinzi, D.; Amici, A.; Navarro-Hortal, M.D.; Esteban-Muñoz, A.; Quiles, J.L.; Battino, M.; et al. Anti-inflammatory activities of Italian Chestnut and Eucalyptus honeys on murine RAW 264.7 macrophages. J. Funct. Foods 2021, 87, 104752. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Xu, S.; Zhang, J.; Wang, Z.; Mao, X.; Yao, X.; Liu, C. Effect of luteolin on inflammatory responses in RAW264.7 macrophages activated with LPS and IFN-γ. J. Funct. Foods 2017, 32, 123–130. [Google Scholar] [CrossRef]
- Hougee, S.; Sanders, A.; Faber, J.; Graus, Y.M.F.; van den Berg, W.B.; Garssen, J.; Smit, H.F.; Hoijer, M.A. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem. Pharmacol. 2005, 69, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, V.; Gupta, S.; Sharma, N.; Tiwari, H.; Bhardwaj, S.; Dutt, P.; Satti, N.; Nargotra, A.; Bhagat, A.; Ahmed, Z. Kaempferol-3-o-β-d-glucuronate exhibit potential anti-inflammatory effect in LPS stimulated RAW 264.7 cells and mice model. Int. Immunopharmacol. 2018, 57, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Baron, R.M.; Bouadma, L.; Calandra, T.; Daneman, N.; DeWaele, J.; Kollef, M.H.; Lipman, J.; Nair, G.B. Initial antimicrobial management of sepsis. Crit. Care 2021, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Collins, W.; Lowen, N.; Blake, D.J. Caffeic Acid Esters Are Effective Bactericidal Compounds Against Paenibacillus larvae by Altering Intracellular Oxidant and Antioxidant Levels. Biomolecules 2019, 9, 312. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control. 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Al-Waili, N.S.; Al-Waili, F.S.; Akmal, M.; Ali, A.; Salom, K.Y.; Al Ghamdi, A.A. Effects of natural honey on polymicrobial culture of various human pathogens. Arch. Med. Sci. AMS 2014, 10, 246–250. [Google Scholar] [CrossRef]
- Yuzbasioglu, M.F.; Kurutas, E.B.; Bulbuloglu, E.; Goksu, M.; Atli, Y.; Bakan, V.; Kale, I.T. Administration of honey to prevent peritoneal adhesions in a rat peritonitis model. Int. J. Surg. 2009, 7, 54–57. [Google Scholar] [CrossRef]
- Gencay, C.; Kilicoglu, S.S.; Kismet, K.; Kilicoglu, B.; Erel, S.; Muratoglu, S.; Sunay, A.E.; Erdemli, E.; Akkus, M.A. Effect of honey on bacterial translocation and intestinal morphology in obstructive jaundice. World J. Gastroenterol. 2008, 14, 3410–3415. [Google Scholar] [CrossRef]
- Oguz, S.; Salt, O.; Ibis, A.C.; Gurcan, S.; Albayrak, D.; Yalta, T.; Sagiroglu, T.; Erenoglu, C. Combined Effectiveness of Honey and Immunonutrition on Bacterial Translocation Secondary to Obstructive Jaundice in Rats: Experimental Study. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3374–3381. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef]
- Bilsel, Y.; Bugra, D.; Yamaner, S.; Bulut, T.; Cevikbas, U.; Turkoglu, U. Could honey have a place in colitis therapy? Effects of honey, prednisolone, and disulfiram on inflammation, nitric oxide, and free radical formation. Dig. Surg. 2002, 19, 306–311. [Google Scholar] [CrossRef]
- Nooh, H.Z.; Nour-Eldien, N.M. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochem. 2016, 118, 588–595. [Google Scholar] [CrossRef]
- Shapiro, H.; Lev, S.; Cohen, J.; Singer, P. Polyphenols in the prevention and treatment of sepsis syndromes: Rationale and pre-clinical evidence. Nutrition 2009, 25, 981–997. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Johnson, B.J.; Hickey, T.E.; Pons, T.; Ligler, F.S. Binding and neutralization of lipopolysaccharides by plant proanthocyanidins. J. Nat. Prod. 2007, 70, 1718–1724. [Google Scholar] [CrossRef]
- Hertel, W.; Peschel, G.; Ozegowski, J.-H.; Müller, P.-J. Inhibitory Effects of Triterpenes and Flavonoids on the Enzymatic Activity of Hyaluronic Acid-Splitting Enzymes. Arch. Pharm. 2006, 339, 313–318. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulou, E.; Ieronymaki, E.; Dimitroulia, E.; Constantinidis, T.C.; Vrioni, G.; Tsatsanis, C.; Tsakris, A. Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms 2022, 10, 2374. https://doi.org/10.3390/microorganisms10122374
Stavropoulou E, Ieronymaki E, Dimitroulia E, Constantinidis TC, Vrioni G, Tsatsanis C, Tsakris A. Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms. 2022; 10(12):2374. https://doi.org/10.3390/microorganisms10122374
Chicago/Turabian StyleStavropoulou, Elisavet, Eleftheria Ieronymaki, Evangelia Dimitroulia, Theodoros C. Constantinidis, Georgia Vrioni, Christos Tsatsanis, and Athanasios Tsakris. 2022. "Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis" Microorganisms 10, no. 12: 2374. https://doi.org/10.3390/microorganisms10122374
APA StyleStavropoulou, E., Ieronymaki, E., Dimitroulia, E., Constantinidis, T. C., Vrioni, G., Tsatsanis, C., & Tsakris, A. (2022). Anti-Inflammatory and Antibacterial Effects and Mode of Action of Greek Arbutus, Chestnut, and Fir Honey in Mouse Models of Inflammation and Sepsis. Microorganisms, 10(12), 2374. https://doi.org/10.3390/microorganisms10122374







