Clinical and Epidemiological Aspects of Acute Q Fever in Reunion Island over Fourteen Years: A Retrospective Cohort Study
Abstract
:1. Introduction
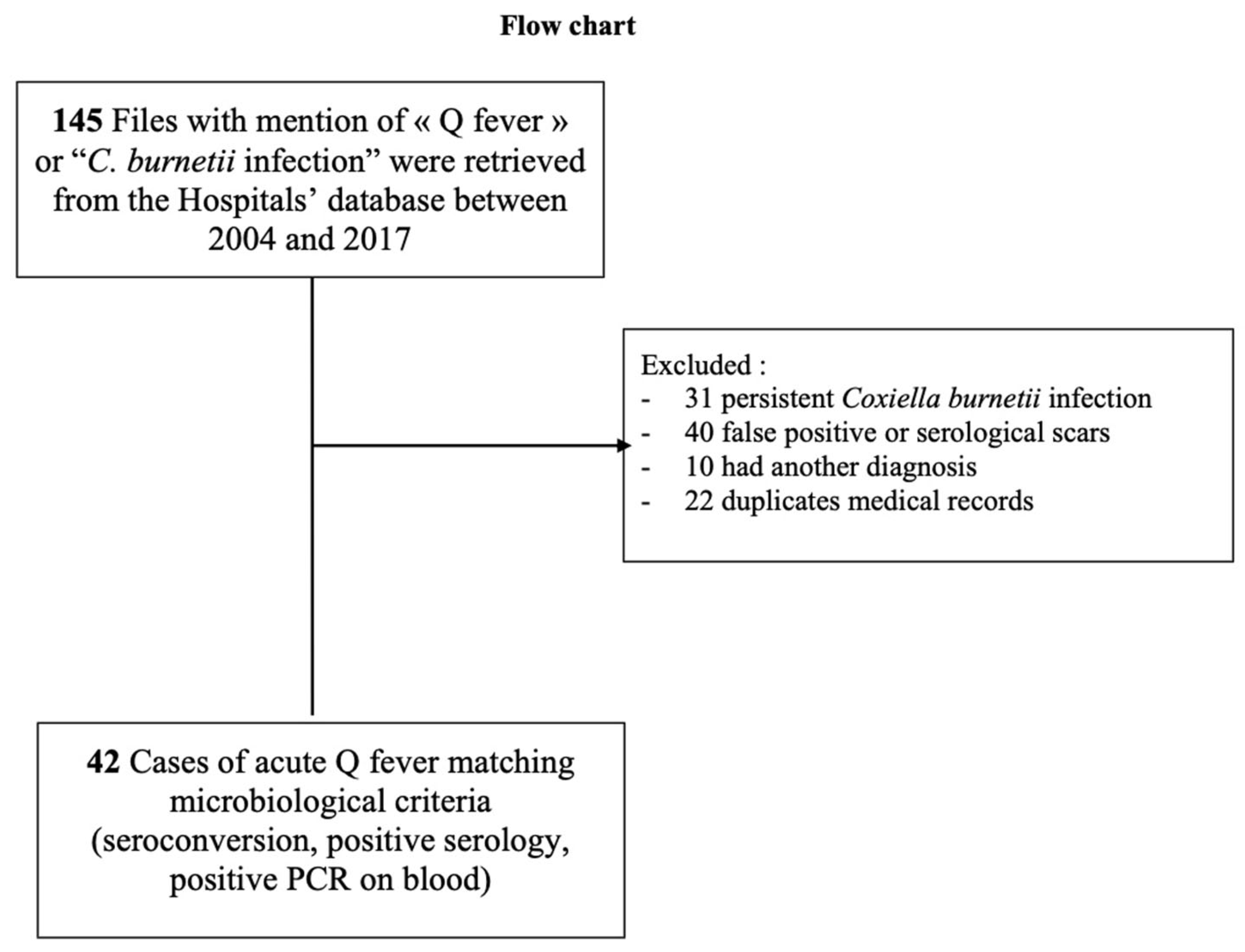
2. Materials and Methods
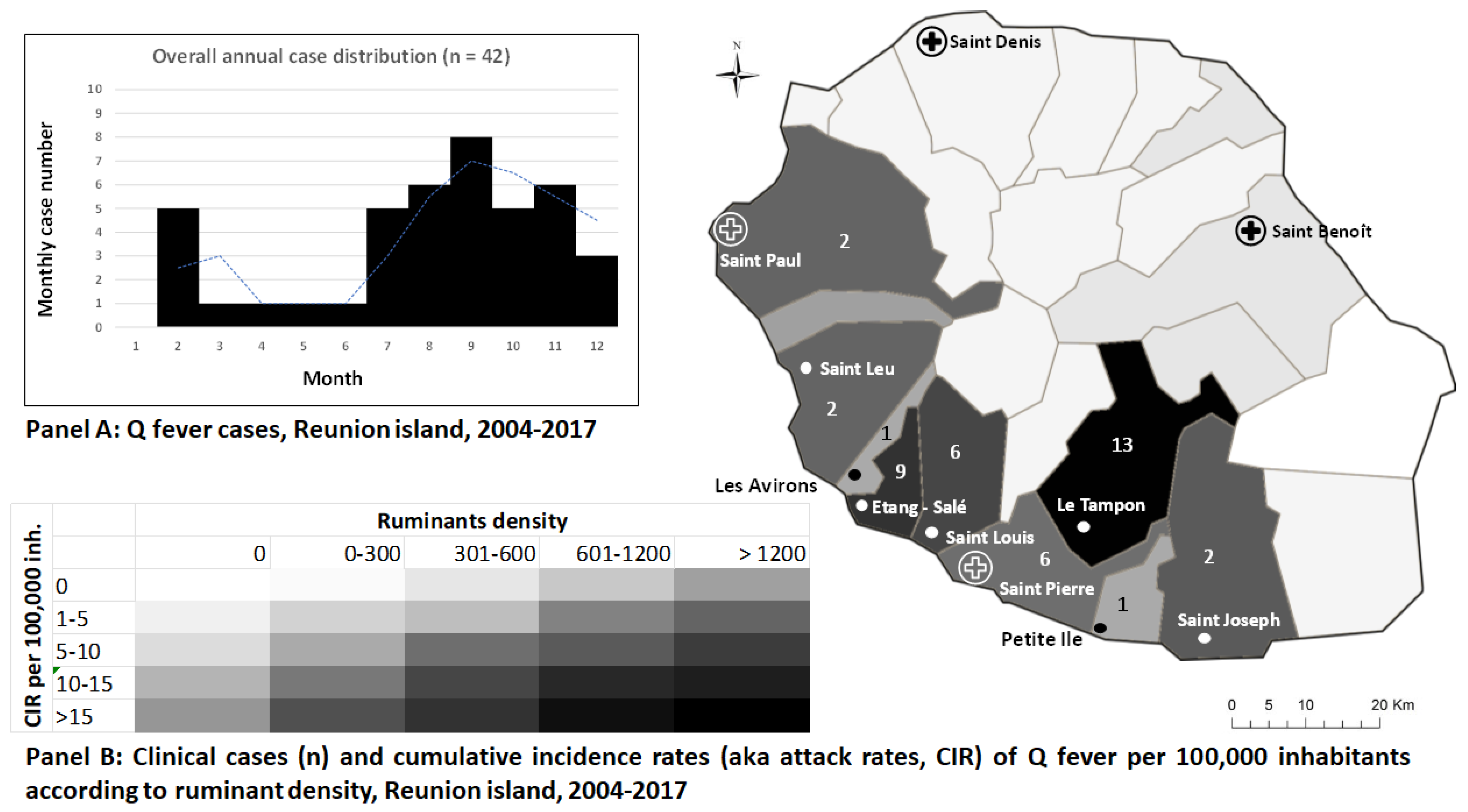
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q Fever to Coxiella burnetii Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, L.; Mahamat, A.; Bonifay, T.; Demar, M.; Abboud, P.; Walter, G.; Drogoul, A.-S.; Berlioz-Arthaud, A.; Nacher, M.; Raoult, D.; et al. Q Fever as a Cause of Community-Acquired Pneumonia in French Guiana. Am. J. Trop. Med. Hyg. 2022, 107, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Pommier de Santi, V.; Briolant, S.; Mahamat, A.; Ilcinkas, C.; Blanchet, D.; de Thoisy, B.; Reynaud, Y.; Hyvert, G.; Marié, J.-L.; Edouard, S.; et al. Q fever epidemic in Cayenne, French Guiana, epidemiologically linked to three-toed sloth. Comp. Immunol. Microbiol. Infect. Dis. 2018, 56, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mahamat, A.; Djossou, F.; Raoult, D. Rainfall and sloth births in May, Q fever in July, Cayenne, French Guiana. Am. J. Trop. Med. Hyg. 2015, 92, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Christen, J.-R.; Edouard, S.; Lamour, T.; Martinez, E.; Rousseau, C.; de Laval, F.; Catzeflis, F.; Djossou, F.; Raoult, D.; de Santi, V.P.; et al. Capybara and Brush Cutter Involvement in Q Fever Outbreak in Remote Area of Amazon Rain Forest, French Guiana, 2014. Emerg. Infect. Dis. 2020, 26, 993–997. [Google Scholar] [CrossRef] [PubMed]
- De França, D.A.; de Mioni, M.S.R.; Fornazari, F.; Duré, A.Í.D.L.; Silva, M.V.F.; Possebon, F.S.; Richini-Pereira, V.B.; Langoni, H.; Megid, J. Seropositivity for Coxiella burnetii in suspected patients with dengue in São Paulo state, Brazil. PLoS Negl. Trop. Dis. 2022, 16, e0010392. [Google Scholar] [CrossRef] [PubMed]
- De França, D.A.; de Mioni, M.S.R.; Fernandes, J.; Lemos, E.R.S.D.; Duré, A.Í.D.L.; Silva, M.V.F.; Langoni, H.; Megid, J. Overview of Q fever in Brazil: An underestimated zoonosis. Rev. Inst. Med. Trop. Sao Paulo 2023, 65, e39. [Google Scholar] [CrossRef] [PubMed]
- Balleydier, E.; Camuset, G.; Socolovschi, C.; Moiton, M.P.; Kuli, B.; Foucher, A.; Poubeau, P.; Borgherini, G.; Wartel, G.; Audin, H.; et al. Murine typhus, Reunion, France, 2011–2013. Emerg. Infect. Dis. 2015, 21, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Ponlot, E.; Oliver, L.; Dommergues, L.; Bellec, L.; Vernier, M. Q fever endocarditis: First reported case in Mayotte. Méd. Mal. Infect. 2018, 48, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Rolain, J.M.; Foucault, C.; Raoult, D. Short report: Q fever and Plasmodium falciparum malaria co-infection in a patient returning from the Comoros archipelago. Am. J. Trop. Med. Hyg. 2005, 73, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, E.; Esnault, O.; Beral, M.; Naze, F.; Michault, A. Emergence of Coxiella burnetii in ruminants on Reunion Island? Prevalence and risk factors. PLoS Negl. Trop. Dis. 2014, 8, e3055. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.; Naze, F.; Camuset, G.; Larrieu, S.; Pascalis, H.; Guernier, V.; Naty, N.; Bertolotti, A.; Manaquin, R.; Mboussou, Y.; et al. Seroprevalence of Coxiella burnetii (Q fever) Exposure in Humans on Reunion Island. Open Forum Infect. Dis. 2019, 6, ofz227. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.; Atiana, L.; Larrieu, S.; De Vos, P.; Somon-Payet, C.; Porcherat, S.; Mboussou, Y.; Naze, F.; Picot, S.; Boukerrou, M.; et al. Q fever seroprevalence in parturient women: The EQRUN cross-sectional study on Reunion Island. BMC Infect. Dis. 2020, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Mboussou, Y.; Jaubert, J.; Larrieu, S.; Atiana, L.; Naze, F.; Folio, C.; Randrianaivo, H.; Bertolotti, A.; Picot, S.; Robillard, P.-Y.; et al. Pregnancy outcomes of Q fever: Prospective follow-up study on Reunion island. BMC Infect. Dis. 2019, 19, 1001. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, L.; Eldin, C.; Thill, P.; de Santi, V.P.; Abboud, P.; Walter, G.; Melzani, A.; Letertre-Gibert, P.; Perez, L.; Demar, M.; et al. Human Q Fever on the Guiana Shield and Brazil: Recent Findings and Remaining Questions. Curr. Trop. Med. Rep. 2021, 8, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Honstettre, A.; Lepidi, H.; Capo, C.; Bayard, F.; Raoult, D.; Mege, J.L. Effect of sex on Coxiella burnetii infection: Protective role of 17beta-estradiol. J. Infect. Dis. 2004, 189, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Soares Magalhães, R.J. Airborne geographical dispersal of Q fever from livestock holdings to human communities: A systematic review and critical appraisal of evidence. BMC Infect. Dis. 2018, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Marrie, T.; Mege, J. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005, 5, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.R.; Milazzo, A.; Marshall, H.; Bi, P. Is a One Health Approach Utilized for Q Fever Control? A Comprehensive Literature Review. Int. J. Environ. Res. Public. Health 2019, 16, 730. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 42) |
|---|---|
| Age, median (Q1–Q3), years | 47.5 (37–59) |
| Age 18 to 45 years 46 to 82 years | 18 (42.9%) 24 (57.1%) |
| Male gender | 29 (69.0%) |
| Area-level exposure to ruminant farm † | 7 (16.7%) |
| Individual-level exposure to ruminants ‡ | 11 (26.2%) |
| Individual exposure to domestic animals # | 12 (28.6%) |
| History of smoking | 17(40.5%) |
| Alcoholism | 7 (16.7%) |
| Obesity | 8 (19.0%) |
| Dyslipidemia | 8 (19.0%) |
| High blood pressure | 7 (16.7%) |
| Obliterative arterial disease of the lower limbs | 5 (11.9%) |
| Stroke | 3 (7.1%) |
| Valve disease | 7 (16.7%) |
| Renal disease | 2 (4.8%) |
| Diabetes mellitus | 11 (26.2%) |
| Heart disease | 5 (11.9%) |
| Immunodeficiency | 4 (9.5%) |
| Bone infection | 0 |
| Fever | 36 (85.7) |
| Median duration of fever (median (Q1–Q3), days | 7 (5–15) |
| >14 days of fever | 8 out 24 (33%) |
| Fatigue | 26 (61.9%) |
| Arthromyalgia | 16 (38.1%) |
| Pulmonary signs | 26 (61.9%) |
| Dyspnea | 7(25%) |
| Cough | 18 (49.2%) |
| Sputum | 10 (23.8%) |
| Abdominal symptoms | 11 (26.2%) |
| Cutaneous rash | 6 (14.3%) |
| Neurological symptoms | 8 (19%) |
| Abnormal chest X ray or CT scan | 21 (50%) |
| Median C-Reactive Protein (median (Q1–Q3), mg/L | 129.7 (47.3–199.8) |
| Leukocytosis (Leukocytes > 10.0 × 109/L) | 27 (64.3%) |
| Leukopenia (Leukocytes < 3.5 × 109/L) | 3 (7%) |
| Thrombocytosis | 2 (4.9%) |
| Thrombocytopenia | 1 (2.4%) |
| Anemia | 15 (35.7%) |
| Median (Q1–Q3) serum creatinin, μmol/L | 81 (68–95) |
| Median ASAT, (Q1–Q3), IU/L | 35 (23.5–57) |
| Median ALAT, (Q1–Q3), IU/L | 38.5 (21.5–67.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubin, A.; Eldin, C.; Zemali, N.; Jaubert, J.; Koumar, Y.; Moiton, M.-P.; Poubeau, P.; Braunberger, E.; Gérardin, P.; Bertolotti, A. Clinical and Epidemiological Aspects of Acute Q Fever in Reunion Island over Fourteen Years: A Retrospective Cohort Study. Microorganisms 2023, 11, 2485. https://doi.org/10.3390/microorganisms11102485
Aubin A, Eldin C, Zemali N, Jaubert J, Koumar Y, Moiton M-P, Poubeau P, Braunberger E, Gérardin P, Bertolotti A. Clinical and Epidemiological Aspects of Acute Q Fever in Reunion Island over Fourteen Years: A Retrospective Cohort Study. Microorganisms. 2023; 11(10):2485. https://doi.org/10.3390/microorganisms11102485
Chicago/Turabian StyleAubin, Alexandra, Carole Eldin, Naël Zemali, Julien Jaubert, Yatrika Koumar, Marie-Pierre Moiton, Patrice Poubeau, Eric Braunberger, Patrick Gérardin, and Antoine Bertolotti. 2023. "Clinical and Epidemiological Aspects of Acute Q Fever in Reunion Island over Fourteen Years: A Retrospective Cohort Study" Microorganisms 11, no. 10: 2485. https://doi.org/10.3390/microorganisms11102485
APA StyleAubin, A., Eldin, C., Zemali, N., Jaubert, J., Koumar, Y., Moiton, M.-P., Poubeau, P., Braunberger, E., Gérardin, P., & Bertolotti, A. (2023). Clinical and Epidemiological Aspects of Acute Q Fever in Reunion Island over Fourteen Years: A Retrospective Cohort Study. Microorganisms, 11(10), 2485. https://doi.org/10.3390/microorganisms11102485





