A Rationale and Approach to the Development of Specific Treatments for HIV Associated Neurocognitive Impairment
Abstract
1. Introduction
2. Clinical and Neuropathological Features of HIV Associated Cognitive Dysfunction
2.1. Cognitive
2.2. Imaging
2.3. Cerebrospinal Findings
2.4. Viral Persistence in CSF and Viral Escape
2.5. Treatment
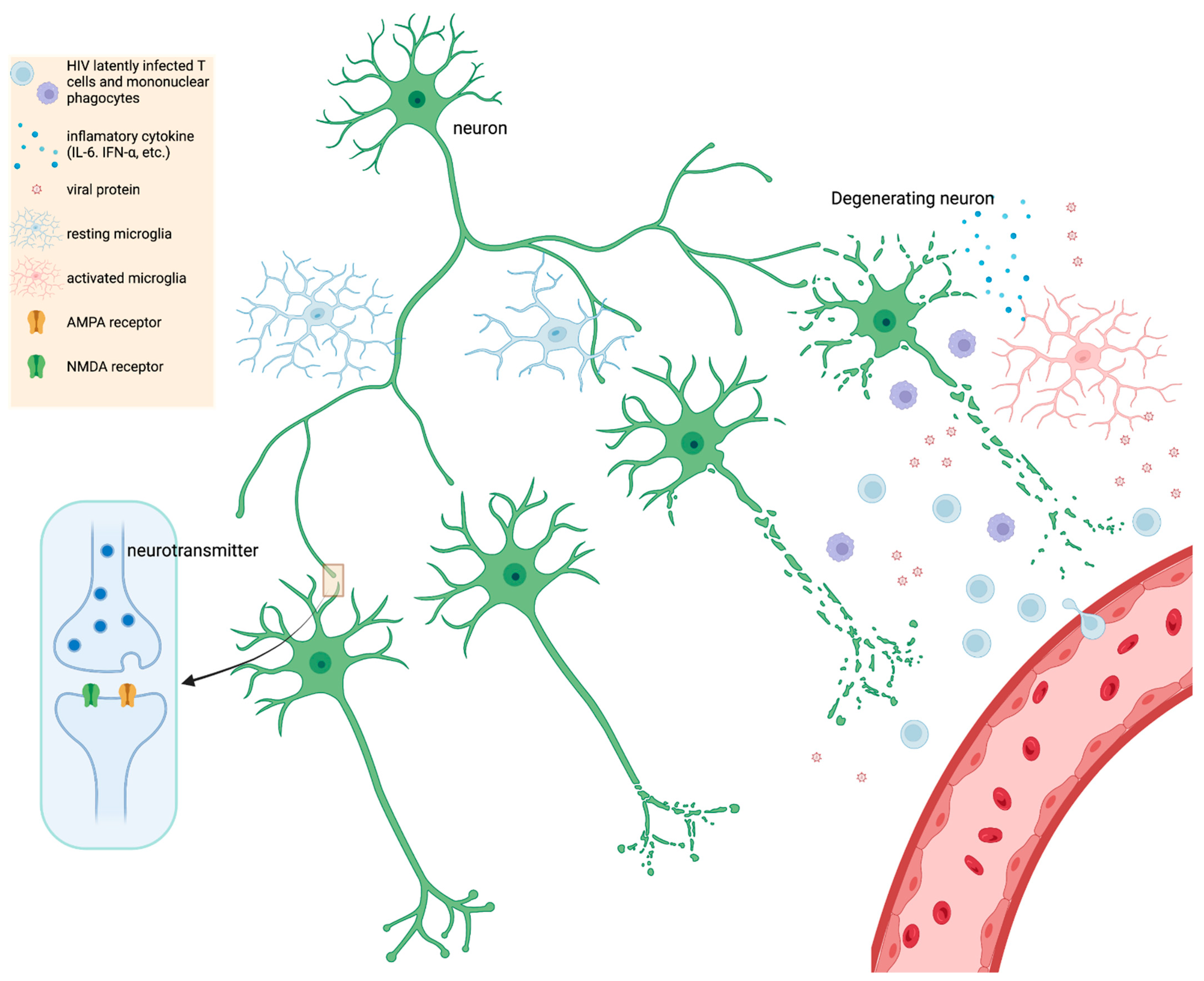
2.6. Pathology
3. Adjunctive Therapies in Animal Models and In Vitro Systems
3.1. Animal Models
3.2. In Vitro Studies
4. Future Treatment Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef]
- Rumbaugh, J.A.; Tyor, W. HIV-associated neurocognitive disorders: Five new things. Neurol. Clin. Pract. 2015, 5, 224–231. [Google Scholar] [CrossRef]
- Guaraldi, G.; Orlando, G.; Zona, S.; Menozzi, M.; Carli, F.; Garlassi, E.; Berti, A.; Rossi, E.; Roverato, A.; Palella, F. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin. Infect. Dis. 2011, 53, 1120–1126. [Google Scholar] [CrossRef]
- Lagathu, C.; Cossarizza, A.; Béréziat, V.; Nasi, M.; Capeau, J.; Pinti, M. Basic science and pathogenesis of ageing with HIV: Potential mechanisms and biomarkers. AIDS 2017, 31 (Suppl. 2), S105–S119. [Google Scholar] [CrossRef]
- Pathai, S.; Bajillan, H.; Landay, A.L.; High, K.P. Is HIV a model of accelerated or accentuated aging? J. Gerontol. A Biol. Sci. Med. Sci 2014, 69, 833–842. [Google Scholar] [CrossRef]
- Lin, K.; Taylor, M.J.; Heaton, R.; Franklin, D.; Jernigan, T.; Fennema-Notestine, C.; McCutchan, A.; Atkinson, J.H.; Ellis, R.J.; McArthur, J.; et al. Effects of traumatic brain injury on cognitive functioning and cerebral metabolites in HIV-infected individuals. J. Clin. Exp. Neuropsychol. 2011, 33, 326–334. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef]
- Bayer, C.; Tyor, W.; Tan, A.; Zola, S.; Marconi, V.; Hu, W.; Penna, S. Estimating the Prevalence and Frequency of HIV-associated Neurocognitive Disorders (HAND) in a Veteran Population. In Proceedings of the 17th Conference of the American Academy of Clinical Neuropsychology, Chicago, IL, USA, 5–8 June 2019. [Google Scholar]
- Bayer, C.; Tyor, W.; Tan, A.; Zola, S.; Hu, W.; Bott, N.; Marconi, V.; Penna, S. Utility of the Neurotrack Visual Paired Comparison (VPC) Task as a Screening Tool for HIV-associated Neurocognitive Disorders (HAND). In Proceedings of the 17th Conference of the American Academy of Clinical Neuropsychology, Chicago, IL, USA, 5–8 June 2019. [Google Scholar]
- Nightingale, S.; Winston, A. Measuring and managing cognitive impairment in HIV. AIDS 2017, 31 (Suppl. 2), S165–S172. [Google Scholar] [CrossRef]
- Robertson, K.R.; Su, Z.; Margolis, D.M.; Krambrink, A.; Havlir, D.V.; Evans, S.; Skiest, D.J. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 2010, 74, 1260–1266. [Google Scholar] [CrossRef]
- Ciavatta, V.T.; Bichler, E.K.; Speigel, I.A.; Elder, C.C.; Teng, S.L.; Tyor, W.R.; García, P.S. In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem. Res. 2017, 42, 3220–3232. [Google Scholar] [CrossRef]
- Valcour, V.; Watters, M.R.; Williams, A.E.; Sacktor, N.; McMurtray, A.; Shikuma, C. Aging exacerbates extrapyramidal motor signs in the era of highly active antiretroviral therapy. J. Neurovirol. 2008, 14, 362–367. [Google Scholar] [CrossRef]
- Kumar, A.M.; Fernandez, J.B.; Singer, E.J.; Commins, D.; Waldrop-Valverde, D.; Ownby, R.L.; Kumar, M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J. Neurovirol. 2009, 15, 257–274. [Google Scholar] [CrossRef]
- Fabbiani, M.; Ciccarelli, N.; Tana, M.; Farina, S.; Baldonero, E.; Di Cristo, V.; Colafigli, M.; Tamburrini, E.; Cauda, R.; Silveri, M.C.; et al. Cardiovascular risk factors and carotid intima-media thickness are associated with lower cognitive performance in HIV-infected patients. HIV Med. 2013, 14, 136–144. [Google Scholar] [CrossRef]
- Wendelken, L.A.; Jahanshad, N.; Rosen, H.J.; Busovaca, E.; Allen, I.; Coppola, G.; Adams, C.; Rankin, K.P.; Milanini, B.; Clifford, K.; et al. ApoE ε4 Is Associated With Cognition, Brain Integrity, and Atrophy in HIV Over Age 60. J. Acquir. Immune Defic. Syndr. 2016, 73, 426–432. [Google Scholar] [CrossRef]
- Valcour, V.; Shikuma, C.; Shiramizu, B.; Watters, M.; Poff, P.; Selnes, O.A.; Grove, J.; Liu, Y.; Abdul-Majid, K.B.; Gartner, S.; et al. Age, apolipoprotein E4, and the risk of HIV dementia: The Hawaii Aging with HIV Cohort. J. Neuroimmunol. 2004, 157, 197–202. [Google Scholar] [CrossRef]
- Cysique, L.A.; Soares, J.R.; Geng, G.; Scarpetta, M.; Moffat, K.; Green, M.; Brew, B.J.; Henry, R.G.; Rae, C. White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. J. Neurovirol. 2017, 23, 539–547. [Google Scholar] [CrossRef]
- Qi, Y.; Li, R.L.; Wang, Y.Y.; Wang, W.; Liu, X.Z.; Liu, J.; Li, X.; Zhang, X.D.; Yu, W.; Liu, J.J.; et al. Characteristics of Brain White Matter Microstructure in HIV Male Patients With Primary Syphilis Co-Infection. Front. Neurol. 2021, 12, 776818. [Google Scholar] [CrossRef]
- Marra, C.M.; Deutsch, R.; Collier, A.C.; Morgello, S.; Letendre, S.; Clifford, D.; Gelman, B.; McArthur, J.; McCutchan, J.A.; Simpson, D.M.; et al. Neurocognitive impairment in HIV-infected individuals with previous syphilis. Int. J. STD AIDS 2013, 24, 351–355. [Google Scholar] [CrossRef]
- Monick, A.J.; Joyce, M.R.; Chugh, N.; Creighton, J.A.; Morgan, O.P.; Strain, E.C.; Marvel, C.L. Characterization of basal ganglia volume changes in the context of HIV and polysubstance use. Sci. Rep. 2022, 12, 4357. [Google Scholar] [CrossRef]
- Smail, R.C.; Brew, B.J. HIV-associated neurocognitive disorder. Handb. Clin. Neurol. 2018, 152, 75–97. [Google Scholar] [CrossRef]
- Chang, L.; Shukla, D.K. Imaging studies of the HIV-infected brain. Handb. Clin. Neurol. 2018, 152, 229–264. [Google Scholar] [CrossRef] [PubMed]
- Irollo, E.; Luchetta, J.; Ho, C.; Nash, B.; Meucci, O. Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders. Cell Mol. Life Sci. 2021, 78, 4283–4303. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Behbahani, S.; Shattuck, K.F.; Bronshteyn, M.; Dawson, M.; Diaz, M.; Kumar, P.; Moore, D.J.; Ellis, R.J.; Jiang, X. Low CD4 nadir linked to widespread cortical thinning in adults living with HIV. Neuroimage Clin. 2020, 25, 102155. [Google Scholar] [CrossRef] [PubMed]
- van Zoest, R.A.; Underwood, J.; De Francesco, D.; Sabin, C.A.; Cole, J.H.; Wit, F.W.; Caan, M.W.A.; Kootstra, N.A.; Fuchs, D.; Zetterberg, H.; et al. Structural Brain Abnormalities in Successfully Treated HIV Infection: Associations With Disease and Cerebrospinal Fluid Biomarkers. J. Infect. Dis. 2017, 217, 69–81. [Google Scholar] [CrossRef]
- Chaganti, J.; Brew, B.J. MR spectroscopy in HIV associated neurocognitive disorder in the era of cART: A review. AIDS Res. Ther. 2021, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002, 82, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ernst, T.; Poland, R.E.; Jenden, D.J. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996, 58, 2049–2056. [Google Scholar] [CrossRef]
- Salvan, A.M.; Vion-Dury, J.; Confort-Gouny, S.; Nicoli, F.; Lamoureux, S.; Cozzone, P.J. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: Identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res. Hum. Retrovir. 1997, 13, 1055–1066. [Google Scholar] [CrossRef]
- Harezlak, J.; Buchthal, S.; Taylor, M.; Schifitto, G.; Zhong, J.; Daar, E.; Alger, J.; Singer, E.; Campbell, T.; Yiannoutsos, C.; et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011, 25, 625–633. [Google Scholar] [CrossRef]
- Gongvatana, A.; Harezlak, J.; Buchthal, S.; Daar, E.; Schifitto, G.; Campbell, T.; Taylor, M.; Singer, E.; Algers, J.; Zhong, J.; et al. Progressive cerebral injury in the setting of chronic HIV infection and antiretroviral therapy. J. Neurovirol. 2013, 19, 209–218. [Google Scholar] [CrossRef]
- Young, A.C.; Yiannoutsos, C.T.; Hegde, M.; Lee, E.; Peterson, J.; Walter, R.; Price, R.W.; Meyerhoff, D.J.; Spudich, S. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology 2014, 83, 1592–1600. [Google Scholar] [CrossRef]
- Chang, L.; Ernst, T.; Witt, M.D.; Ames, N.; Gaiefsky, M.; Miller, E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage 2002, 17, 1638–1648. [Google Scholar] [CrossRef]
- Meyerhoff, D.J.; Bloomer, C.; Cardenas, V.; Norman, D.; Weiner, M.W.; Fein, G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology 1999, 52, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ernst, T.; St Hillaire, C.; Conant, K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir. Ther. 2004, 9, 431–440. [Google Scholar] [CrossRef]
- López-Villegas, D.; Lenkinski, R.E.; Frank, I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. USA 1997, 94, 9854–9859. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, J.M.; Wang, Y.; Ma, S.; Yue, C.; Kim, P.K.; Adams, A.V.; Roosa, H.V.; Gage, K.L.; Stathis, M.; Rais, R.; et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J. Neurovirol. 2014, 20, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.H.; Guo, Q.; Cole, J.H.; Boasso, A.; Greathead, L.; Kelleher, P.; Rabiner, E.A.; Kalk, N.; Bishop, C.; Gunn, R.N.; et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016, 86, 1425–1432. [Google Scholar] [CrossRef]
- Rubin, L.H.; Sacktor, N.; Creighton, J.; Du, Y.; Endres, C.J.; Pomper, M.G.; Coughlin, J.M. Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS 2018, 32, 1661–1667. [Google Scholar] [CrossRef]
- Kim, E.J.; Yu, S.W. Translocator protein 18 kDa (TSPO): Old dogma, new mice, new structure, and new questions for neuroprotection. Neural. Regen. Res. 2015, 10, 878–880. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.W.; Yu, S.W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef]
- Garvey, L.J.; Pavese, N.; Politis, M.; Ramlackhansingh, A.; Brooks, D.J.; Taylor-Robinson, S.D.; Winston, A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS 2014, 28, 67–72. [Google Scholar] [CrossRef]
- Boerwinkle, A.; Ances, B.M. Molecular Imaging of Neuroinflammation in HIV. J. Neuroimmune Pharmacol. 2019, 14, 9–15. [Google Scholar] [CrossRef]
- Boerwinkle, A.H.; Strain, J.F.; Burdo, T.; Doyle, J.; Christensen, J.; Su, Y.; Wisch, J.K.; Cooley, S.A.; Vaida, F.; Smith, M.D.; et al. Comparison of [11C]-PBR28 Binding Between Persons Living With HIV and HIV-Uninfected Individuals. J. Acquir. Immune Defic. Syndr. 2020, 85, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Moench, T.R.; Griffin, D.E. Immunocytochemical identification and quantitation of the mononuclear cells in the cerebrospinal fluid, meninges, and brain during acute viral meningoencephalitis. J. Exp. Med. 1984, 159, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Rho, M.B.; Wesselingh, S.; Glass, J.D.; McArthur, J.C.; Choi, S.; Griffin, J.; Tyor, W.R. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav. Immun. 1995, 9, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, R.H.; Liu, Y.; Guo, L.; Wang, X.; Hu, W.H.; Ho, W.Z. HIV infection suppresses TLR3 activation-mediated antiviral immunity in microglia and macrophages. Immunology 2020, 160, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, Y.; Zheng, Y.; Peng, X.; Yang, Z.; Cao, Q.; Xiang, D.; Zhao, H. Differences in cytokine and chemokine profiles in cerebrospinal fluid caused by the etiology of cryptococcal meningitis and tuberculous meningitis in HIV patients. Clin. Exp. Immunol. 2021, 206, 82–90. [Google Scholar] [CrossRef]
- Anderson, A.M.; Lennox, J.L.; Mulligan, M.M.; Loring, D.W.; Zetterberg, H.; Blennow, K.; Kessing, C.; Koneru, R.; Easley, K.; Tyor, W.R. Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-Infected individuals. J. Neurovirol. 2017, 23, 106–112. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal fluid immune markers and HIV-associated neurocognitive impairments: A systematic review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Alammar, L.; Gama, L.; Clements, J.E. Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection. J. Immunol. 2011, 186, 4008–4018. [Google Scholar] [CrossRef]
- Hellmuth, J.; Valcour, V.; Spudich, S. CNS reservoirs for HIV: Implications for eradication. J. Virus Erad. 2015, 1, 67–71. [Google Scholar] [CrossRef]
- Mallard, J.; Williams, K. Correction to: An SIV macaque model of SIV and HAND: The need for adjunctive therapies in HIV that target activated monocytes and macrophages. J. Neurovirol. 2018, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Eisele, E.; Siliciano, R.F. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012, 37, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Balcom, E.F.; Roda, W.C.; Cohen, E.A.; Li, M.Y.; Power, C. HIV-1 persistence in the central nervous system: Viral and host determinants during antiretroviral therapy. Curr. Opin. Virol. 2019, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Blankson, J.N.; Persaud, D.; Siliciano, R.F. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002, 53, 557–593. [Google Scholar] [CrossRef]
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the Brain Reservoirs: Toward an HIV Cure. Front. Immunol. 2016, 7, 397. [Google Scholar] [CrossRef]
- Koppensteiner, H.; Brack-Werner, R.; Schindler, M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [Google Scholar] [CrossRef]
- Soulet, D.; Rivest, S. Bone-marrow-derived microglia: Myth or reality? Curr. Opin. Pharmacol. 2008, 8, 508–518. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef]
- Roda, W.C.; Li, M.Y.; Akinwumi, M.S.; Asahchop, E.L.; Gelman, B.B.; Witwer, K.W.; Power, C. Modeling brain lentiviral infections during antiretroviral therapy in AIDS. J. Neurovirol. 2017, 23, 577–586. [Google Scholar] [CrossRef]
- Gelman, B.B.; Lisinicchia, J.G.; Morgello, S.; Masliah, E.; Commins, D.; Achim, C.L.; Fox, H.S.; Kolson, D.L.; Grant, I.; Singer, E.; et al. Neurovirological correlation with HIV-associated neurocognitive disorders and encephalitis in a HAART-era cohort. J. Acquir. Immune Defic. Syndr. 2013, 62, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central nervous system viral invasion and inflammation during acute HIV infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Chaillon, A.; Nakazawa, M.; Vargas, M.; Letendre, S.L.; Strain, M.C.; Ellis, R.J.; Morris, S.; Little, S.J.; Smith, D.M.; et al. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS Pathog. 2017, 13, e1006112. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.; Peterson, J.; Fuchs, D.; Gisslen, M.; Palmer, S.; Price, R.W. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014, 28, 2251–2258. [Google Scholar] [CrossRef]
- Ferretti, F.; Gisslen, M.; Cinque, P.; Price, R.W. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr. HIV/AIDS Rep. 2015, 12, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, S.; Geretti, A.M.; Beloukas, A.; Fisher, M.; Winston, A.; Else, L.; Nelson, M.; Taylor, S.; Ustianowski, A.; Ainsworth, J.; et al. Discordant CSF/plasma HIV-1 RNA in patients with unexplained low-level viraemia. J. Neurovirol. 2016, 22, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Canestri, A.; Lescure, F.X.; Jaureguiberry, S.; Moulignier, A.; Amiel, C.; Marcelin, A.G.; Peytavin, G.; Tubiana, R.; Pialoux, G.; Katlama, C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin. Infect. Dis. 2010, 50, 773–778. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ferretti, F.; Peterson, J.; Lee, E.; Fuchs, D.; Boschini, A.; Gisslén, M.; Angoff, N.; Price, R.W.; Cinque, P.; et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS 2012, 26, 1765–1774. [Google Scholar] [CrossRef]
- Kugathasan, R.; Collier, D.A.; Haddow, L.J.; El Bouzidi, K.; Edwards, S.G.; Cartledge, J.D.; Miller, R.F.; Gupta, R.K. Diffuse White Matter Signal Abnormalities on Magnetic Resonance Imaging Are Associated With Human Immunodeficiency Virus Type 1 Viral Escape in the Central Nervous System Among Patients With Neurological Symptoms. Clin. Infect. Dis. 2017, 64, 1059–1065. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Turrini, F.; de Zan, V.; Caccia, R.; Gerevini, S.; Cinque, P. Symptomatic cerebrospinal fluid escape. AIDS 2019, 33 (Suppl. 2), S159–S169. [Google Scholar] [CrossRef]
- Levy, D.N.; Refaeli, Y.; MacGregor, R.R.; Weiner, D.B. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 1994, 91, 10873–10877. [Google Scholar] [CrossRef] [PubMed]
- Mamik, M.K.; Hui, E.; Branton, W.G.; McKenzie, B.A.; Chisholm, J.; Cohen, E.A.; Power, C. HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: Implications for HIV-1 Associated Neuroinflammation. J. Neuroimmune Pharmacol. 2017, 12, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Evering, T.H.; Kamau, E.; St Bernard, L.; Farmer, C.B.; Kong, X.P.; Markowitz, M. Single genome analysis reveals genetic characteristics of Neuroadaptation across HIV-1 envelope. Retrovirology 2014, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.J.; Johnson, T.P.; Smith, B.R.; Reoma, L.B.; Santamaria, U.A.; Bachani, M.; Demarino, C.; Barclay, R.A.; Snow, J.; Sacktor, N.; et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33 (Suppl. 2), S145–S157. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.J.; Barsby, N.L.; Cohen, E.A.; Holden, J.; Harris, K.; Dickie, P.; Jhamandas, J.; Power, C. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J. Neurosci. 2007, 27, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, H.; Miura, Y.; Ando, Y.; Hoshino, S.; Ishizaka, Y.; Koyanagi, Y. Human immunodeficiency virus type 1 Vpr inhibits axonal outgrowth through induction of mitochondrial dysfunction. J. Virol. 2008, 82, 2528–2542. [Google Scholar] [CrossRef]
- Zou, W.; Kim, B.O.; Zhou, B.Y.; Liu, Y.; Messing, A.; He, J.J. Protection against human immunodeficiency virus type 1 Tat neurotoxicity by Ginkgo biloba extract EGb 761 involving glial fibrillary acidic protein. Am. J. Pathol. 2007, 171, 1923–1935. [Google Scholar] [CrossRef]
- Kim, B.O.; Liu, Y.; Ruan, Y.; Xu, Z.C.; Schantz, L.; He, J.J. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am. J. Pathol. 2003, 162, 1693–1707. [Google Scholar] [CrossRef]
- Bachis, A.; Wenzel, E.; Boelk, A.; Becker, J.; Mocchetti, I. The neurotrophin receptor p75 mediates gp120-induced loss of synaptic spines in aging mice. Neurobiol. Aging 2016, 46, 160–168. [Google Scholar] [CrossRef]
- Toggas, S.M.; Masliah, E.; Rockenstein, E.M.; Rall, G.F.; Abraham, C.R.; Mucke, L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 1994, 367, 188–193. [Google Scholar] [CrossRef]
- Thaney, V.E.; O’Neill, A.M.; Hoefer, M.M.; Maung, R.; Sanchez, A.B.; Kaul, M. IFNβ Protects Neurons from Damage in a Murine Model of HIV-1 Associated Brain Injury. Sci. Rep. 2017, 7, 46514. [Google Scholar] [CrossRef] [PubMed]
- Ditiatkovski, M.; Mukhamedova, N.; Dragoljevic, D.; Hoang, A.; Low, H.; Pushkarsky, T.; Fu, Y.; Carmichael, I.; Hill, A.F.; Murphy, A.J.; et al. Modification of lipid rafts by extracellular vesicles carrying HIV-1 protein Nef induces redistribution of amyloid precursor protein and Tau, causing neuronal dysfunction. J. Biol. Chem. 2020, 295, 13377–13392. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.; Tyor, W. Aging, comorbidities, and the importance of finding biomarkers for HIV-associated neurocognitive disorders. J. Neurovirol. 2019, 25, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.M.; Jang, J.H.; Easley, K.A.; Fuchs, D.; Gisslen, M.; Zetterberg, H.; Blennow, K.; Ellis, R.J.; Franklin, D.; Heaton, R.K.; et al. Cognitive and Neuronal Link With Inflammation: A Longitudinal Study in People With and Without HIV Infection. J. Acquir. Immune Defic. Syndr. 2020, 85, 617–625. [Google Scholar] [CrossRef]
- Robertson, K.R.; Robertson, W.T.; Ford, S.; Watson, D.; Fiscus, S.; Harp, A.G.; Hall, C.D. Highly active antiretroviral therapy improves neurocognitive functioning. J. Acquir. Immune Defic. Syndr. 2004, 36, 562–566. [Google Scholar] [CrossRef]
- Schmitt, F.A.; Bigley, J.W.; McKinnis, R.; Logue, P.E.; Evans, R.W.; Drucker, J.L. Neuropsychological outcome of zidovudine (AZT) treatment of patients with AIDS and AIDS-related complex. N. Engl. J. Med. 1988, 319, 1573–1578. [Google Scholar] [CrossRef]
- Letendre, S.; Marquie-Beck, J.; Capparelli, E.; Best, B.; Clifford, D.; Collier, A.C.; Gelman, B.B.; McArthur, J.C.; McCutchan, J.A.; Morgello, S.; et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol. 2008, 65, 65–70. [Google Scholar] [CrossRef]
- Letendre, S.L.; Ellis, R.J.; Ances, B.M.; McCutchan, J.A. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010, 18, 45–55. [Google Scholar]
- Asahchop, E.L.; Meziane, O.; Mamik, M.K.; Chan, W.F.; Branton, W.G.; Resch, L.; Gill, M.J.; Haddad, E.; Guimond, J.V.; Wainberg, M.A.; et al. Reduced antiretroviral drug efficacy and concentration in HIV-infected microglia contributes to viral persistence in brain. Retrovirology 2017, 14, 47. [Google Scholar] [CrossRef]
- Gates, T.M.; Cysique, L.A.; Siefried, K.J.; Chaganti, J.; Moffat, K.J.; Brew, B.J. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 2016, 30, 591–600. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; D’Antoni, M.L.; Ananworanich, J.; Byron, M.M.; Chalermchai, T.; Sithinamsuwan, P.; Tipsuk, S.; Ho, E.; Slike, B.M.; Schuetz, A.; et al. Loss of CCR2 expressing non-classical monocytes are associated with cognitive impairment in antiretroviral therapy-naïve HIV-infected Thais. J. Neuroimmunol. 2015, 288, 25–33. [Google Scholar] [CrossRef]
- D’Antoni, M.L.; Paul, R.H.; Mitchell, B.I.; Kohorn, L.; Fischer, L.; Lefebvre, E.; Seyedkazemi, S.; Nakamoto, B.K.; Walker, M.; Kallianpur, K.J.; et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J. Acquir. Immune Defic. Syndr. 2018, 79, 108–116. [Google Scholar] [CrossRef]
- Lin, S.P.; Calcagno, A.; Letendre, S.L.; Ma, Q. Clinical Treatment Options and Randomized Clinical Trials for Neurocognitive Complications of HIV Infection: Combination Antiretroviral Therapy, Central Nervous System Penetration Effectiveness, and Adjuvants. Curr. Top Behav. Neurosci. 2021, 50, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Letendre, S.L.; Roa, J.; Chen, H.; McKhann, A.; Marra, C.M.; Daar, E.S.; Hunt, P.W.; Campbell, T.; Swaminathan, S.; Ha, B.; et al. ACTG A5324: A Randomized Trial of ART Intensification for Cognitive Impairment in PWH. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 12–16 February 2022. [Google Scholar]
- Decloedt, E.H.; Freeman, C.; Howells, F.; Casson-Crook, M.; Lesosky, M.; Koutsilieri, E.; Lovestone, S.; Maartens, G.; Joska, J.A. Moderate to severe HIV-associated neurocognitive impairment: A randomized placebo-controlled trial of lithium. Medicine 2016, 95, e5401. [Google Scholar] [CrossRef] [PubMed]
- Schifitto, G.; Yiannoutsos, C.T.; Ernst, T.; Navia, B.A.; Nath, A.; Sacktor, N.; Anderson, C.; Marra, C.M.; Clifford, D.B. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology 2009, 73, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Schifitto, G.; Zhang, J.; Evans, S.R.; Sacktor, N.; Simpson, D.; Millar, L.L.; Hung, V.L.; Miller, E.N.; Smith, E.; Ellis, R.J.; et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 2007, 69, 1314–1321. [Google Scholar] [CrossRef]
- Sacktor, N.; Skolasky, R.L.; Moxley, R.; Wang, S.; Mielke, M.M.; Munro, C.; Steiner, J.; Nath, A.; Haughey, N.; McArthur, J. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: Results from a double-blind, placebo-controlled trial. J. Neurovirol. 2018, 24, 16–27. [Google Scholar] [CrossRef]
- Steiner, J.P.; Bachani, M.; Wolfson-Stofko, B.; Lee, M.H.; Wang, T.; Li, G.; Li, W.; Strayer, D.; Haughey, N.J.; Nath, A. Interaction of paroxetine with mitochondrial proteins mediates neuroprotection. Neurotherapeutics 2015, 12, 200–216. [Google Scholar] [CrossRef]
- Meulendyke, K.A.; Queen, S.E.; Engle, E.L.; Shirk, E.N.; Liu, J.; Steiner, J.P.; Nath, A.; Tarwater, P.M.; Graham, D.R.; Mankowski, J.L.; et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J. Neurovirol. 2014, 20, 591–602. [Google Scholar] [CrossRef]
- Ashraf, T.; Jiang, W.; Hoque, M.T.; Henderson, J.; Wu, C.; Bendayan, R. Role of anti-inflammatory compounds in human immunodeficiency virus-1 glycoprotein120-mediated brain inflammation. J. Neuroinflammation 2014, 11, 91. [Google Scholar] [CrossRef]
- Yadav, A.; Betts, M.R.; Collman, R.G. Statin modulation of monocyte phenotype and function: Implications for HIV-1-associated neurocognitive disorders. J. Neurovirol. 2016, 22, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, V.V.; Mielke, M.M.; Sacktor, N.; McArthur, J.C.; Grant, I.; Letendre, S.; Chang, L.; Wojna, V.; Pardo, C.; Calabresi, P.; et al. A lipid storage-like disorder contributes to cognitive decline in HIV-infected subjects. Neurology 2013, 81, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Probasco, J.C.; Spudich, S.S.; Critchfield, J.; Lee, E.; Lollo, N.; Deeks, S.G.; Price, R.W. Failure of atorvastatin to modulate CSF HIV-1 infection: Results of a pilot study. Neurology 2008, 71, 521–524. [Google Scholar] [CrossRef]
- Saylor, D.; Molsberry, S.A.; Seaberg, E.C.; Cheng, Y.; Levine, A.; Martin, E.; Munro, C.; Palella, F.; Becker, J.; Sacktor, N. Statin Use and Cognitive Performance in the Multicenter Aids Cohort Study. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021. [Google Scholar]
- Sacktor, N.; Miyahara, S.; Evans, S.; Schifitto, G.; Cohen, B.; Haughey, N.; Drewes, J.L.; Graham, D.; Zink, M.C.; Anderson, C.; et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J. Neurovirol. 2014, 20, 620–626. [Google Scholar] [CrossRef]
- Nakasujja, N.; Miyahara, S.; Evans, S.; Lee, A.; Musisi, S.; Katabira, E.; Robertson, K.; Ronald, A.; Clifford, D.B.; Sacktor, N. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 2013, 80, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Schifitto, G.; Navia, B.A.; Yiannoutsos, C.T.; Marra, C.M.; Chang, L.; Ernst, T.; Jarvik, J.G.; Miller, E.N.; Singer, E.J.; Ellis, R.J.; et al. Memantine and HIV-associated cognitive impairment: A neuropsychological and proton magnetic resonance spectroscopy study. AIDS 2007, 21, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Marconi, V.C.; Moser, C.; Gavegnano, C.; Deeks, S.G.; Lederman, M.M.; Overton, E.T.; Tsibris, A.; Hunt, P.W.; Kantor, A.; Sekaly, R.P.; et al. Randomized Trial of Ruxolitinib in Antiretroviral-Treated Adults With Human Immunodeficiency Virus. Clin. Infect. Dis. 2022, 74, 95–104. [Google Scholar] [CrossRef]
- Bastard, J.P.; Soulié, C.; Fellahi, S.; Haïm-Boukobza, S.; Simon, A.; Katlama, C.; Calvez, V.; Marcelin, A.G.; Capeau, J. Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir. Ther. 2012, 17, 915–919. [Google Scholar] [CrossRef]
- Lichtfuss, G.F.; Cheng, W.J.; Farsakoglu, Y.; Paukovics, G.; Rajasuriar, R.; Velayudham, P.; Kramski, M.; Hearps, A.C.; Cameron, P.U.; Lewin, S.R.; et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J. Immunol. 2012, 189, 1491–1499. [Google Scholar] [CrossRef]
- Kamat, A.; Misra, V.; Cassol, E.; Ancuta, P.; Yan, Z.; Li, C.; Morgello, S.; Gabuzda, D. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS ONE 2012, 7, e30881. [Google Scholar] [CrossRef]
- Lederman, M.M.; Funderburg, N.T.; Sekaly, R.P.; Klatt, N.R.; Hunt, P.W. Residual immune dysregulation syndrome in treated HIV infection. Adv. Immunol. 2013, 119, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel mechanisms to inhibit HIV reservoir seeding using Jak inhibitors. PLoS Pathog. 2017, 13, e1006740. [Google Scholar] [CrossRef] [PubMed]
- Vier, J.; Groth, M.; Sochalska, M.; Kirschnek, S. The anti-apoptotic Bcl-2 family protein A1/Bfl-1 regulates neutrophil survival and homeostasis and is controlled via PI3K and JAK/STAT signaling. Cell Death Dis. 2016, 7, e2103. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Chipuk, J.E. Getting away with murder: How does the BCL-2 family of proteins kill with immunity? Ann. N. Y. Acad. Sci. 2013, 1285, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Chetoui, N.; Boisvert, M.; Gendron, S.; Aoudjit, F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology 2010, 130, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Haile, W.B.; Hurwitz, S.; Tao, S.; Jiang, Y.; Schinazi, R.F.; Tyor, W.R. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J. Neuroinflammation 2019, 16, 182. [Google Scholar] [CrossRef]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Titanji, B.K.; Farley, M.M.; Mehta, A.; Connor-Schuler, R.; Moanna, A.; Cribbs, S.K.; O’Shea, J.; DeSilva, K.; Chan, B.; Edwards, A.; et al. Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 72, 1247–1250. [Google Scholar] [CrossRef]
- Bronte, V.; Ugel, S.; Tinazzi, E.; Vella, A.; De Sanctis, F.; Canè, S.; Batani, V.; Trovato, R.; Fiore, A.; Petrova, V.; et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J. Clin. Investig. 2020, 130, 6409–6416. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Gelman, B.B. Neuropathology of HAND With Suppressive Antiretroviral Therapy: Encephalitis and Neurodegeneration Reconsidered. Curr. HIV/AIDS Rep. 2015, 12, 272–279. [Google Scholar] [CrossRef]
- Kolson, D.L.; Sabnekar, P.; Baybis, M.; Crino, P.B. Gene expression in TUNEL-positive neurons in human immunodeficiency virus-infected brain. J. Neurovirol. 2004, 10 (Suppl. 1), 102–107. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Ge, N.; Morey, M.; DeTeresa, R.; Terry, R.D.; Wiley, C.A. Cortical dendritic pathology in human immunodeficiency virus encephalitis. Lab Investig. 1992, 66, 285–291. [Google Scholar] [PubMed]
- Masliah, E.; Heaton, R.K.; Marcotte, T.D.; Ellis, R.J.; Wiley, C.A.; Mallory, M.; Achim, C.L.; McCutchan, J.A.; Nelson, J.A.; Atkinson, J.H.; et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 1997, 42, 963–972. [Google Scholar] [CrossRef]
- Adle-Biassette, H.; Chretien, F.; Wingertsmann, L.; Hery, C.; Ereau, T.; Scaravilli, F.; Tardieu, M.; Gray, F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 1999, 25, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Langford, D.; Masliah, E. HIV and antiretroviral therapy in the brain: Neuronal injury and repair. Nat. Rev. Neurosci. 2007, 8, 33–44. [Google Scholar] [CrossRef]
- Weis, S.; Haug, H.; Budka, H. Neuronal damage in the cerebral cortex of AIDS brains: A morphometric study. Acta Neuropathol. 1993, 85, 185–189. [Google Scholar] [CrossRef]
- Carroll, A.; Brew, B. HIV-associated neurocognitive disorders: Recent advances in pathogenesis, biomarkers, and treatment. F1000Res 2017, 6, 312. [Google Scholar] [CrossRef]
- Manji, H.; Jäger, H.R.; Winston, A. HIV, dementia and antiretroviral drugs: 30 years of an epidemic. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1126–1137. [Google Scholar] [CrossRef]
- Spudich, S.; Gisslen, M.; Hagberg, L.; Lee, E.; Liegler, T.; Brew, B.; Fuchs, D.; Tambussi, G.; Cinque, P.; Hecht, F.M.; et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J. Infect. Dis. 2011, 204, 753–760. [Google Scholar] [CrossRef]
- Green, M.V.; Raybuck, J.D.; Zhang, X.; Wu, M.M.; Thayer, S.A. Scaling Synapses in the Presence of HIV. Neurochem. Res. 2019, 44, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation 2021. [Google Scholar] [CrossRef]
- Koneru, R.; Bimonte-Nelson, H.; Ciavatta, V.; Haile, W.; Elmore, K.; Ward, J.; Maroun, L.; Tyor, W.R. Reversing interferon-alpha neurotoxicity in a HIV-associated neurocognitive disorder mouse model. AIDS 2018, 32, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia regulation of synaptic plasticity and learning and memory. Neural. Regen. Res. 2022, 17, 705–716. [Google Scholar] [CrossRef]
- Hudson, L.; Liu, J.; Nath, A.; Jones, M.; Raghavan, R.; Narayan, O.; Male, D.; Everall, I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000, 6, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.V.; Bell, J.E.; Nath, A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS 2000, 14, 2709–2713. [Google Scholar] [CrossRef]
- Wheeler, E.D.; Achim, C.L.; Ayyavoo, V. Immunodetection of human immunodeficiency virus type 1 (HIV-1) Vpr in brain tissue of HIV-1 encephalitic patients. J. Neurovirol. 2006, 12, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ranki, A.; Nyberg, M.; Ovod, V.; Haltia, M.; Elovaara, I.; Raininko, R.; Haapasalo, H.; Krohn, K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 1995, 9, 1001–1008. [Google Scholar] [CrossRef]
- Pushkarsky, T.; Ward, A.; Ivanov, A.; Lin, X.; Sviridov, D.; Nekhai, S.; Bukrinsky, M.I. Abundance of Nef and p-Tau217 in Brains of Individuals Diagnosed with HIV-Associated Neurocognitive Disorders Correlate with Disease Severance. Mol. Neurobiol. 2022, 59, 1088–1097. [Google Scholar] [CrossRef]
- Donoso, M.; D’Amico, D.; Valdebenito, S.; Hernandez, C.A.; Prideaux, B.; Eugenin, E.A. Identification, Quantification, and Characterization of HIV-1 Reservoirs in the Human Brain. Cells 2022, 11, 2379. [Google Scholar] [CrossRef]
- Everall, I.P.; Heaton, R.K.; Marcotte, T.D.; Ellis, R.J.; McCutchan, J.A.; Atkinson, J.H.; Grant, I.; Mallory, M.; Masliah, E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, E.; Morrison, D.; Sullivan, P.; Morgello, S.; Fischer, T. Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr. HIV Res. 2014, 12, 97–110. [Google Scholar] [CrossRef]
- Ginsberg, S.D.; Alldred, M.J.; Gunnam, S.M.; Schiroli, C.; Lee, S.H.; Morgello, S.; Fischer, T. Expression profiling suggests microglial impairment in human immunodeficiency virus neuropathogenesis. Ann. Neurol. 2018, 83, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Tyor, W.R.; Power, C.; Gendelman, H.E.; Markham, R.B. A model of human immunodeficiency virus encephalitis in scid mice. Proc. Natl. Acad. Sci. USA 1993, 90, 8658–8662. [Google Scholar] [CrossRef] [PubMed]
- Tyor, W.R.; Bimonte-Nelson, H. A mouse model of HIV-associated neurocognitive disorders: A brain-behavior approach to discover disease mechanisms and novel treatments. J. Neurovirol. 2018, 24, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.E.; Dasgupta, S.; Middaugh, L.D.; Terry, E.C.; Gorry, P.R.; Wesselingh, S.L.; Tyor, W.R. Highly active antiretroviral therapy and human immunodeficiency virus encephalitis. Ann. Neurol. 2005, 57, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Cook-Easterwood, J.; Middaugh, L.D.; Griffin, W.C., 3rd; Khan, I.; Tyor, W.R. Highly active antiretroviral therapy of cognitive dysfunction and neuronal abnormalities in SCID mice with HIV encephalitis. Exp. Neurol. 2007, 205, 506–512. [Google Scholar] [CrossRef]
- Koneru, R.; Olive, M.F.; Tyor, W.R. Combined antiretroviral therapy reduces brain viral load and pathological features of HIV encephalitis in a mouse model. J. Neurovirol. 2014, 20, 9–17. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Tyor, W.; Scanlan, A.; Koneru, R.; Haile, W.; Reece, M.; Gavegnano, C. Treatment of HIV Associated Neurocognitive Disorder Mice with Curcumin Improves Cognitive Deficits. In Proceedings of the 73rd American Academy of Neurology Annual Meeting, Virtual, 17–22 April 2021. [Google Scholar]
- Haile, W.B.; Gavegnano, C.; Tao, S.; Jiang, Y.; Schinazi, R.F.; Tyor, W.R. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol. Dis. 2016, 92, 137–143. [Google Scholar] [CrossRef]
- Potash, M.J.; Chao, W.; Bentsman, G.; Paris, N.; Saini, M.; Nitkiewicz, J.; Belem, P.; Sharer, L.; Brooks, A.I.; Volsky, D.J. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc. Natl. Acad. Sci. USA 2005, 102, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Hadas, E.; Chao, W.; He, H.; Saini, M.; Daley, E.; Saifuddin, M.; Bentsman, G.; Ganz, E.; Volsky, D.J.; Potash, M.J. Transmission of chimeric HIV by mating in conventional mice: Prevention by pre-exposure antiretroviral therapy and reduced susceptibility during estrus. Dis. Model. Mech. 2013, 6, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.J.; Borjabad, A.; Hadas, E.; Kelschenbach, J.; Kim, B.H.; Chao, W.; Arancio, O.; Suh, J.; Polsky, B.; McMillan, J.; et al. EcoHIV infection of mice establishes latent viral reservoirs in T cells and active viral reservoirs in macrophages that are sufficient for induction of neurocognitive impairment. PLoS Pathog. 2018, 14, e1007061. [Google Scholar] [CrossRef]
- Kim, B.H.; Kelschenbach, J.; Borjabad, A.; Hadas, E.; He, H.; Potash, M.J.; Nedelcovych, M.T.; Rais, R.; Haughey, N.J.; McArthur, J.C.; et al. Intranasal insulin therapy reverses hippocampal dendritic injury and cognitive impairment in a model of HIV-associated neurocognitive disorders in EcoHIV-infected mice. AIDS 2019, 33, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Nedelcovych, M.T.; Kim, B.H.; Zhu, X.; Lovell, L.E.; Manning, A.A.; Kelschenbach, J.; Hadas, E.; Chao, W.; Prchalová, E.; Dash, R.P.; et al. Glutamine Antagonist JHU083 Normalizes Aberrant Glutamate Production and Cognitive Deficits in the EcoHIV Murine Model of HIV-Associated Neurocognitive Disorders. J. Neuroimmune Pharmacol. 2019, 14, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Saikali, M.F.; Currier, S.; Volsky, D.J.; Cummins, C.L.; Bendayan, R. Selective peroxisome proliferator-activated receptor-gamma modulator, INT131 exhibits anti-inflammatory effects in an EcoHIV mouse model. FASEB J. 2020, 34, 1996–2010. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications--a review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Polak, P.E.; Kalinin, S.; Dello Russo, C.; Gavrilyuk, V.; Sharp, A.; Peters, J.M.; Richardson, J.; Willson, T.M.; Weinberg, G.; Feinstein, D.L. Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2005, 168, 65–75. [Google Scholar] [CrossRef]
- Barbiero, J.K.; Santiago, R.; Tonin, F.S.; Boschen, S.; da Silva, L.M.; Werner, M.F.; da Cunha, C.; Lima, M.M.; Vital, M.A. PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 35–44. [Google Scholar] [CrossRef]
- Sauer, S. Ligands for the Nuclear Peroxisome Proliferator-Activated Receptor Gamma. Trends Pharmacol. Sci. 2015, 36, 688–704. [Google Scholar] [CrossRef]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. Sci. Rep. 2019, 9, 9428. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Hoque, M.T.; Choi, U.Y.; Bendayan, R. Peroxisome proliferator-activated receptor-gamma: Potential molecular therapeutic target for HIV-1-associated brain inflammation. J. Neuroinflammation 2017, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Wahl, A.; Baker, C.; Spagnuolo, R.A.; Foster, J.; Zakharova, O.; Wietgrefe, S.; Caro-Vegas, C.; Madden, V.; Sharpe, G.; et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016, 126, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Liao, B.; Nixon, C.C.; Cleary, R.A.; Thayer, W.O.; Birath, S.L.; Swanson, M.D.; Sheridan, P.; Zakharova, O.; Prince, F.; et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J. Clin. Investig. 2018, 128, 2862–2876. [Google Scholar] [CrossRef] [PubMed]
- Denton, P.W.; Olesen, R.; Choudhary, S.K.; Archin, N.M.; Wahl, A.; Swanson, M.D.; Chateau, M.; Nochi, T.; Krisko, J.F.; Spagnuolo, R.A.; et al. Generation of HIV latency in humanized BLT mice. J. Virol. 2012, 86, 630–634. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.C.; Johnson, T.P. Chronic inflammation mediates brain injury in HIV infection: Relevance for cure strategies. Curr. Opin. Neurol. 2020, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Rissiek, B.; Stabernack, J.; Cordes, M.; Duan, Y.; Behr, S.; Menzel, S.; Magnus, T.; Koch-Nolte, F. Astrocytes and Microglia Are Resistant to NAD(+)-Mediated Cell Death Along the ARTC2/P2X7 Axis. Front. Mol. Neurosci. 2019, 12, 330. [Google Scholar] [CrossRef]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef]
- Mediouni, S.; Jablonski, J.; Paris, J.J.; Clementz, M.A.; Thenin-Houssier, S.; McLaughlin, J.P.; Valente, S.T. Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice. Curr HIV Res. 2015, 13, 64–79. [Google Scholar] [CrossRef]
- Fields, J.A.; Overk, C.; Adame, A.; Florio, J.; Mante, M.; Pineda, A.; Desplats, P.; Rockenstein, E.; Achim, C.; Masliah, E. Neuroprotective effects of the immunomodulatory drug FK506 in a model of HIV1-gp120 neurotoxicity. J. Neuroinflammation 2016, 13, 120. [Google Scholar] [CrossRef]
- Churchill, M.J.; Gorry, P.R.; Cowley, D.; Lal, L.; Sonza, S.; Purcell, D.F.; Thompson, K.A.; Gabuzda, D.; McArthur, J.C.; Pardo, C.A.; et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J. Neurovirol. 2006, 12, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.C.; Yao, Q.; Stohlman, S.A. Microglia exhibit clonal variability in eliciting cytotoxic T lymphocyte responses independent of class I expression. Cell Immunol. 1999, 198, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Lokensgard, J.R. Glial Cell Expression of PD-L1. Int. J. Mol. Sci. 2019, 20, 1677. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Hu, S.; Prasad, S.; Sheng, W.S.; Lokensgard, J.R. Programmed death ligand-1 induction restrains the cytotoxic T lymphocyte response against microglia. Glia 2021, 69, 858–871. [Google Scholar] [CrossRef] [PubMed]
- PD-1 Inhibition to Determine CNS Reservoir of HIV-Infection. Available online: https://ClinicalTrials.gov/show/NCT03239899 (accessed on 27 October 2022).
- Dow, S.W.; Poss, M.L.; Hoover, E.A. Feline immunodeficiency virus: A neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. (1988) 1990, 3, 658–668. [Google Scholar]
- Dua, N.; Reubel, G.; Moore, P.F.; Higgins, J.; Pedersen, N.C. An experimental study of primary feline immunodeficiency virus infection in cats and a historical comparison to acute simian and human immunodeficiency virus diseases. Vet. Immunol. Immunopathol. 1994, 43, 337–355. [Google Scholar] [CrossRef]
- Dow, S.W.; Dreitz, M.J.; Hoover, E.A. Feline immunodeficiency virus neurotropism: Evidence that astrocytes and microglia are the primary target cells. Vet. Immunol. Immunopathol. 1992, 35, 23–35. [Google Scholar] [CrossRef]
- Hein, A.; Martin, J.P.; Koehren, F.; Bingen, A.; Dörries, R. In vivo infection of ramified microglia from adult cat central nervous system by feline immunodeficiency virus. Virology 2000, 268, 420–429. [Google Scholar] [CrossRef]
- Maingat, F.; Vivithanaporn, P.; Zhu, Y.; Taylor, A.; Baker, G.; Pearson, K.; Power, C. Neurobehavioral performance in feline immunodeficiency virus infection: Integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J. Neurosci. 2009, 29, 8429–8437. [Google Scholar] [CrossRef]
- Huitron-Resendiz, S.; De Rozières, S.; Sanchez-Alavez, M.; Bühler, B.; Lin, Y.C.; Lerner, D.L.; Henriksen, N.W.; Burudi, M.; Fox, H.S.; Torbett, B.E.; et al. Resolution and prevention of feline immunodeficiency virus-induced neurological deficits by treatment with the protease inhibitor TL-3. J. Virol. 2004, 78, 4525–4532. [Google Scholar] [CrossRef] [PubMed]
- Gómez, N.V.; Fontanals, A.; Castillo, V.; Gisbert, M.A.; Suraniti, A.; Mira, G.; Pisano, P.B. Evaluation of different antiretroviral drug protocols on naturally infected feline immunodeficiency virus (FIV) cats in the late phase of the asymptomatic stage of infection. Viruses 2012, 4, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Vergote, D.; Pardo, C.; Noorbakhsh, F.; McArthur, J.C.; Hollenberg, M.D.; Overall, C.M.; Power, C. CXCR3 activation by lentivirus infection suppresses neuronal autophagy: Neuroprotective effects of antiretroviral therapy. FASEB J. 2009, 23, 2928–2941. [Google Scholar] [CrossRef] [PubMed]
- Freiherr, J.; Hallschmid, M.; Frey, W.H., 2nd; Brünner, Y.F.; Chapman, C.D.; Hölscher, C.; Craft, S.; De Felice, F.G.; Benedict, C. Intranasal insulin as a treatment for Alzheimer’s disease: A review of basic research and clinical evidence. CNS Drugs 2013, 27, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Mamik, M.K.; Asahchop, E.L.; Chan, W.F.; Zhu, Y.; Branton, W.G.; McKenzie, B.A.; Cohen, E.A.; Power, C. Insulin Treatment Prevents Neuroinflammation and Neuronal Injury with Restored Neurobehavioral Function in Models of HIV/AIDS Neurodegeneration. J. Neurosci. 2016, 36, 10683–10695. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.C.; Suryanarayana, K.; Mankowski, J.L.; Shen, A.; Piatak, M., Jr.; Spelman, J.P.; Carter, D.L.; Adams, R.J.; Lifson, J.D.; Clements, J.E. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 1999, 73, 10480–10488. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, J.L.; Clements, J.E.; Zink, M.C. Searching for clues: Tracking the pathogenesis of human immunodeficiency virus central nervous system disease by use of an accelerated, consistent simian immunodeficiency virus macaque model. J. Infect. Dis. 2002, 186 (Suppl. 2), S199–S208. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Kuroda, M.J.; Santra, S.; Sasseville, V.G.; Simon, M.A.; Lifton, M.A.; Racz, P.; Tenner-Racz, K.; Dalesandro, M.; Scallon, B.J.; et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999, 283, 857–860. [Google Scholar] [CrossRef]
- Dinoso, J.B.; Rabi, S.A.; Blankson, J.N.; Gama, L.; Mankowski, J.L.; Siliciano, R.F.; Zink, M.C.; Clements, J.E. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J. Virol. 2009, 83, 9247–9257. [Google Scholar] [CrossRef]
- Whitney, J.B.; Hill, A.L.; Sanisetty, S.; Penaloza-MacMaster, P.; Liu, J.; Shetty, M.; Parenteau, L.; Cabral, C.; Shields, J.; Blackmore, S.; et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014, 512, 74–77. [Google Scholar] [CrossRef]
- Gama, L.; Abreu, C.M.; Shirk, E.N.; Price, S.L.; Li, M.; Laird, G.M.; Pate, K.A.; Wietgrefe, S.W.; O’Connor, S.L.; Pianowski, L.; et al. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 2017, 31, 5–14. [Google Scholar] [CrossRef]
- Beck, S.E.; Queen, S.E.; Metcalf Pate, K.A.; Mangus, L.M.; Abreu, C.M.; Gama, L.; Witwer, K.W.; Adams, R.J.; Zink, M.C.; Clements, J.E.; et al. An SIV/macaque model targeted to study HIV-associated neurocognitive disorders. J. Neurovirol. 2018, 24, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, J.L.; Queen, S.E.; Clements, J.E.; Zink, M.C. Cerebrospinal fluid markers that predict SIV CNS disease. J. Neuroimmunol. 2004, 157, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Pendyala, G.; Siuzdak, G.; Fox, H.S. Metabolomic analysis of the cerebrospinal fluid reveals changes in phospholipase expression in the CNS of SIV-infected macaques. J. Clin. Investig. 2008, 118, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Mothapo, K.M.; Ten Oever, J.; Koopmans, P.; Stelma, F.F.; Burm, S.; Bajramovic, J.; Verbeek, M.M.; Rikkert, M.G.O.; Netea, M.G.; Koopman, G.; et al. Soluble TLR2 and 4 concentrations in cerebrospinal fluid in HIV/SIV-related neuropathological conditions. J. Neurovirol. 2017, 23, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Avalos, C.R.; Price, S.L.; Forsyth, E.R.; Pin, J.N.; Shirk, E.N.; Bullock, B.T.; Queen, S.E.; Li, M.; Gellerup, D.; O’Connor, S.L.; et al. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J. Virol. 2016, 90, 5643–5656. [Google Scholar] [CrossRef]
- Zink, M.C.; Uhrlaub, J.; DeWitt, J.; Voelker, T.; Bullock, B.; Mankowski, J.; Tarwater, P.; Clements, J.; Barber, S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 2005, 293, 2003–2011. [Google Scholar] [CrossRef]
- Ratai, E.M.; Bombardier, J.P.; Joo, C.G.; Annamalai, L.; Burdo, T.H.; Campbell, J.; Fell, R.; Hakimelahi, R.; He, J.; Autissier, P.; et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS ONE 2010, 5, e10523. [Google Scholar] [CrossRef]
- Campbell, J.H.; Burdo, T.H.; Autissier, P.; Bombardier, J.P.; Westmoreland, S.V.; Soulas, C.; González, R.G.; Ratai, E.M.; Williams, K.C. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS ONE 2011, 6, e18688. [Google Scholar] [CrossRef]
- Veenstra, M.; León-Rivera, R.; Li, M.; Gama, L.; Clements, J.E.; Berman, J.W. Mechanisms of CNS Viral Seeding by HIV(+) CD14(+) CD16(+) Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. mBio 2017, 8, e01280-17. [Google Scholar] [CrossRef]
- Campbell, J.H.; Ratai, E.M.; Autissier, P.; Nolan, D.J.; Tse, S.; Miller, A.D.; González, R.G.; Salemi, M.; Burdo, T.H.; Williams, K.C. Anti-α4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014, 10, e1004533. [Google Scholar] [CrossRef] [PubMed]
- Weed, M.R.; Gold, L.H.; Polis, I.; Koob, G.F.; Fox, H.S.; Taffe, M.A. Impaired performance on a rhesus monkey neuropsychological testing battery following simian immunodeficiency virus infection. AIDS Res. Hum. Retrovir. 2004, 20, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Courtney, S.M.; Petit, L.; Maisog, J.M.; Ungerleider, L.G.; Haxby, J.V. An area specialized for spatial working memory in human frontal cortex. Science 1998, 279, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, G.; Puce, A.; Constable, R.T.; Krystal, J.H.; Gore, J.C.; Goldman-Rakic, P. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cereb. Cortex 1996, 6, 600–611. [Google Scholar] [CrossRef]
- Miotto, E.C.; Bullock, P.; Polkey, C.E.; Morris, R.G. Spatial working memory and strategy formation in patients with frontal lobe excisions. Cortex 1996, 32, 613–630. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Owen, A.M.; Sahakian, B.J.; Semple, J.; Polkey, C.E.; Robbins, T.W. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1995, 33, 1–24. [Google Scholar] [CrossRef]
- Sahakian, B.J.; Elliott, R.; Low, N.; Mehta, M.; Clark, R.T.; Pozniak, A.L. Neuropsychological deficits in tests of executive function in asymptomatic and symptomatic HIV-1 seropositive men. Psychol. Med. 1995, 25, 1233–1246. [Google Scholar] [CrossRef]
- Bornstein, R.A.; Nasrallah, H.A.; Para, M.F.; Whitacre, C.C.; Rosenberger, P.; Fass, R.J. Neuropsychological performance in symptomatic and asymptomatic HIV infection. AIDS 1993, 7, 519–524. [Google Scholar] [CrossRef]
- Heaton, R.K.; Grant, I.; Butters, N.; White, D.A.; Kirson, D.; Atkinson, J.H.; McCutchan, J.A.; Taylor, M.J.; Kelly, M.D.; Ellis, R.J.; et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1995, 1, 231–251. [Google Scholar] [CrossRef]
- Maruff, P.; Currie, J.; Malone, V.; McArthur-Jackson, C.; Mulhall, B.; Benson, E. Neuropsychological characterization of the AIDS dementia complex and rationalization of a test battery. Arch. Neurol. 1994, 51, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef]
- Gold, R.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Giovannoni, G.; Selmaj, K.; Tornatore, C.; Sweetser, M.T.; Yang, M.; Sheikh, S.I.; et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl. J. Med. 2012, 367, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Miller, D.H.; Phillips, J.T.; Hutchinson, M.; Havrdova, E.; Kita, M.; Yang, M.; Raghupathi, K.; Novas, M.; Sweetser, M.T.; et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N. Engl. J. Med. 2012, 367, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.A.; Cook, D.R.; Chi, A.W.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: A novel candidate for HIV neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef]
- Gill, A.J.; Kovacsics, C.E.; Cross, S.A.; Vance, P.J.; Kolson, L.L.; Jordan-Sciutto, K.L.; Gelman, B.B.; Kolson, D.L. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J. Clin. Investig. 2014, 124, 4459–4472. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Xu, H.N.; Vance, P.; Gruenewald, A.L.; Garza, R.; Midkiff, C.; Alvarez-Hernandez, X.; Irwin, D.J.; Gill, A.J.; Kolson, D.L. Dimethyl Fumarate, an Approved Multiple Sclerosis Treatment, Reduces Brain Oxidative Stress in SIV-Infected Rhesus Macaques: Potential Therapeutic Repurposing for HIV Neuroprotection. Antioxidants 2021, 10, 416. [Google Scholar] [CrossRef]
- Barber, S.A.; Herbst, D.S.; Bullock, B.T.; Gama, L.; Clements, J.E. Innate immune responses and control of acute simian immunodeficiency virus replication in the central nervous system. J. Neurovirol. 2004, 10 (Suppl. 1), 15–20. [Google Scholar] [CrossRef]
- Akhtar, L.N.; Qin, H.; Muldowney, M.T.; Yanagisawa, L.L.; Kutsch, O.; Clements, J.E.; Benveniste, E.N. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J. Immunol. 2010, 185, 2393–2404. [Google Scholar] [CrossRef]
- Hua, L.L.; Liu, J.S.; Brosnan, C.F.; Lee, S.C. Selective inhibition of human glial inducible nitric oxide synthase by interferon-beta: Implications for multiple sclerosis. Ann. Neurol. 1998, 43, 384–387. [Google Scholar] [CrossRef]
- Fogarty, E.; Schmitz, S.; Tubridy, N.; Walsh, C.; Barry, M. Comparative efficacy of disease-modifying therapies for patients with relapsing remitting multiple sclerosis: Systematic review and network meta-analysis. Mult. Scler. Relat. Disord. 2016, 9, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Guo, M.L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Rawat, P.; Teodorof-Diedrich, C.; Spector, S.A. Human immunodeficiency virus Type-1 single-stranded RNA activates the NLRP3 inflammasome and impairs autophagic clearance of damaged mitochondria in human microglia. Glia 2019, 67, 802–824. [Google Scholar] [CrossRef] [PubMed]
- Mamik, M.K.; Power, C. Inflammasomes in neurological diseases: Emerging pathogenic and therapeutic concepts. Brain 2017, 140, 2273–2285. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Reinke, S.N.; Mamik, M.K.; McKenzie, B.A.; Maingat, F.; Branton, W.G.; Broadhurst, D.I.; Power, C. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology 2014, 11, 35. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Zeng, Z.; Wei, Y.; Gao, J.; Zhang, B.; Li, L.; Liu, L.; Wan, Y.; Zeng, Q.; et al. NLRP3-dependent pyroptosis is required for HIV-1 gp120-induced neuropathology. Cell Mol. Immunol. 2020, 17, 283–299. [Google Scholar] [CrossRef]
- Guthrie, L.N.; Abiraman, K.; Plyler, E.S.; Sprenkle, N.T.; Gibson, S.A.; McFarland, B.C.; Rajbhandari, R.; Rowse, A.L.; Benveniste, E.N.; Meares, G.P. Attenuation of PKR-like ER Kinase (PERK) Signaling Selectively Controls Endoplasmic Reticulum Stress-induced Inflammation Without Compromising Immunological Responses. J. Biol. Chem. 2016, 291, 15830–15840. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. PERK mediates eIF2α phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2272–2281. [Google Scholar] [CrossRef]
- Ma, T.; Trinh, M.A.; Wexler, A.J.; Bourbon, C.; Gatti, E.; Pierre, P.; Cavener, D.R.; Klann, E. Suppression of eIF2α kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat. Neurosci. 2013, 16, 1299–1305. [Google Scholar] [CrossRef]
- Moreno, J.A.; Halliday, M.; Molloy, C.; Radford, H.; Verity, N.; Axten, J.M.; Ortori, C.A.; Willis, A.E.; Fischer, P.M.; Barrett, D.A.; et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 2013, 5, 206ra138. [Google Scholar] [CrossRef]
- Silveira, D.B.; Américo, M.F.; Flores, N.P.; Terenzi, H.; Pinto, A.R. Pharmacological inhibition of UPR sensor PERK attenuates HIV Tat-induced inflammatory M1 phenotype in microglial cells. Cell Biochem. Funct. 2022, 40, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.L.M. Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview. Cell Mol. Neurobiol. 2022, 42, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Ewers, M.; Bürger, K.; Annas, P.; Mörtberg, A.; Bogstedt, A.; Frölich, L.; Schröder, J.; Schönknecht, P.; Riepe, M.W.; et al. Lithium trial in Alzheimer’s disease: A randomized, single-blind, placebo-controlled, multicenter 10-week study. J. Clin. Psychiatry 2009, 70, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Coutinho, A.M.; Aprahamian, I.; Prando, S.; Mendes, L.L.; Diniz, B.S.; Gattaz, W.F.; Buchpiguel, C.A. Long-term lithium treatment reduces glucose metabolism in the cerebellum and hippocampus of nondemented older adults: An [¹⁸F]FDG-PET study. ACS Chem. Neurosci. 2014, 5, 484–489. [Google Scholar] [CrossRef]
- Devanand, D.P.; Strickler, J.G.; Huey, E.D.; Crocco, E.; Forester, B.P.; Husain, M.M.; Vahia, I.V.; Andrews, H.; Wall, M.M.; Pelton, G.H. Lithium Treatment for Agitation in Alzheimer’s disease (Lit-AD): Clinical rationale and study design. Contemp. Clin. Trials 2018, 71, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Everall, I.P.; Bell, C.; Mallory, M.; Langford, D.; Adame, A.; Rockestein, E.; Masliah, E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol. Cell Neurosci. 2002, 21, 493–501. [Google Scholar] [CrossRef]
- Maggirwar, S.B.; Tong, N.; Ramirez, S.; Gelbard, H.A.; Dewhurst, S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J. Neurochem. 1999, 73, 578–586. [Google Scholar] [CrossRef]
- Fields, J.; Dumaop, W.; Eleuteri, S.; Campos, S.; Serger, E.; Trejo, M.; Kosberg, K.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: Implications for HIV-associated neurocognitive disorders. J. Neurosci. 2015, 35, 1921–1938. [Google Scholar] [CrossRef]
- Cheney, L.; Guzik, H.; Macaluso, F.P.; Macian, F.; Cuervo, A.M.; Berman, J.W. HIV Nef and Antiretroviral Therapy Have an Inhibitory Effect on Autophagy in Human Astrocytes that May Contribute to HIV-Associated Neurocognitive Disorders. Cells 2020, 9, 1426. [Google Scholar] [CrossRef]
- Mehla, R.; Chauhan, A. HIV-1 differentially modulates autophagy in neurons and astrocytes. J. Neuroimmunol. 2015, 285, 106–118. [Google Scholar] [CrossRef]
- Bonam, S.R.; Tranchant, C.; Muller, S. Autophagy-Lysosomal Pathway as Potential Therapeutic Target in Parkinson’s Disease. Cells 2021, 10, 3547. [Google Scholar] [CrossRef] [PubMed]
- León-Rivera, R.; Veenstra, M.; Donoso, M.; Tell, E.; Eugenin, E.A.; Morgello, S.; Berman, J.W. Central Nervous System (CNS) Viral Seeding by Mature Monocytes and Potential Therapies To Reduce CNS Viral Reservoirs in the cART Era. mBio 2021, 12, e03633-20. [Google Scholar] [CrossRef]
- Joseph, S.B.; Kincer, L.P.; Bowman, N.M.; Evans, C.; Vinikoor, M.J.; Lippincott, C.K.; Gisslén, M.; Spudich, S.; Menezes, P.; Robertson, K.; et al. Human Immunodeficiency Virus Type 1 RNA Detected in the Central Nervous System (CNS) After Years of Suppressive Antiretroviral Therapy Can Originate from a Replicating CNS Reservoir or Clonally Expanded Cells. Clin. Infect. Dis. 2019, 69, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Drozd, V.; Durygin, A.; Rodriguez, J.; Barber, P.; Atluri, V.; Liu, X.; Voss, T.G.; Saxena, S.; Nair, M. Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603. [Google Scholar] [CrossRef]
- Decloedt, E.H.; Maartens, G. Neuronal toxicity of efavirenz: A systematic review. Expert Opin. Drug Saf. 2013, 12, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Chowdhury, P.; Nagesh, P.K.B.; Rahman, M.A.; Zhi, K.; Yallapu, M.M.; Kumar, S. Novel elvitegravir nanoformulation for drug delivery across the blood-brain barrier to achieve HIV-1 suppression in the CNS macrophages. Sci. Rep. 2020, 10, 3835. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhi, K.; Nagesh, P.K.B.; Sinha, N.; Chowdhury, P.; Chen, H.; Gorantla, S.; Yallapu, M.M.; Kumar, S. An Elvitegravir Nanoformulation Crosses the Blood-Brain Barrier and Suppresses HIV-1 Replication in Microglia. Viruses 2020, 12, 564. [Google Scholar] [CrossRef]
- Kakuda, T.N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 2000, 22, 685–708. [Google Scholar] [CrossRef]
- Varatharajan, L.; Thomas, S.A. The transport of anti-HIV drugs across blood-CNS interfaces: Summary of current knowledge and recommendations for further research. Antiviral. Res. 2009, 82, A99–A109. [Google Scholar] [CrossRef]
- Gerson, T.; Makarov, E.; Senanayake, T.H.; Gorantla, S.; Poluektova, L.Y.; Vinogradov, S.V. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine 2014, 10, 177–185. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R., Jr.; Deutsch, R.; Letendre, S.; Ellis, R.J.; Casaletto, K.; Marquine, M.J.; Woods, S.P.; Vaida, F.; Atkinson, J.H.; et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin. Infect. Dis. 2015, 60, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.H.; Maki, P.M.; Springer, G.; Benning, L.; Anastos, K.; Gustafson, D.; Villacres, M.C.; Jiang, X.; Adimora, A.A.; Waldrop-Valverde, D.; et al. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 2017, 89, 1594–1603. [Google Scholar] [CrossRef]
- Spudich, S.; Robertson, K.R.; Bosch, R.J.; Gandhi, R.T.; Cyktor, J.C.; Mar, H.; Macatangay, B.J.; Lalama, C.M.; Rinaldo, C.; Collier, A.C.; et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J. Clin. Investig. 2019, 129, 3339–3346. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Zaunders, J.; Levert, A.; Butterly, S.; Liu, Z.; Ishida, T.; Huang, C.; Gates, T.; Rae, C.; Jugé, L.; et al. Neuron Damage and Reservoir are Secondary to HIV Transcripts Despite Suppressive ART. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021. [Google Scholar]
- Agnihotri, S.P.; Wuthrich, C.; Dang, X.; Nauen, D.; Karimi, R.; Viscidi, R.; Bord, E.; Batson, S.; Troncoso, J.; Koralnik, I.J. A fatal case of JC virus meningitis presenting with hydrocephalus in a human immunodeficiency virus-seronegative patient. Ann. Neurol. 2014, 76, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, A.D.; Skolasky, R.L.; Moxley, R.T.; McArthur, J.; Rubin, L.; Haughey, N.D.; Sacktor, N. Intranasal Insulin Improves Attention and Memory in People with HIV. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Virtual, 6–11 March 2021. [Google Scholar]
- Hoang, T.N.; Pino, M.; Boddapati, A.K.; Viox, E.G.; Starke, C.E.; Upadhyay, A.A.; Gumber, S.; Nekorchuk, M.; Busman-Sahay, K.; Strongin, Z.; et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2021, 184, 460–475.e421. [Google Scholar] [CrossRef] [PubMed]
| Therapeutic | Anti-Inflammatory in CNS | AntiHIV in CNS | Other Effects | Randomized-Controlled? | Group n (Analyzed) and Summary Stats * | Outcome(s) |
|---|---|---|---|---|---|---|
| Antiretroviral | ||||||
| Maraviroc | No decrease in CSF neopterin and β2-microglobulin. Ref [92] | NM | High EC50 in microglia in vitro. Ref [91] | ✓ | Maraviroc arm n = 9 Control arm n = 5 Time-treatment arm interaction: (p < 0.05) Effect size: (d = 0.77) and (d = 0.55) after 6 months or 12 months, respectively. | Improved Cognition in PWH based on time-treatment arm interaction. Ref [92] |
| Cenicriviroc | NM | NM | N/A | Open label | Single arm (Cenicriviroc) n = 17 Cognitive domain of attention (p = 0.011) and working memory (p = 0.017). Decreased plasma levels of sCD163, sCD14 and neopterin (p < 0.01). | Improved Cognition; decrease in myeloid activation markers. Ref [94] |
| Dolutegravir + Maraviroc | NM | NM | Increased CD4+ and CD8+ cell counts. | ✓ | Dual placebo n = 63 Dolutegravir and placebo n = 67 Dolutegravir and Maraviroc n = 60 cognitive testing (p > 0.10). | No improvement in cognition in PWH + NCI. Ref [96] |
| Misc. drugs | ||||||
| Lithium | NM | NM | Lithium is well tolerated with ART. | ✓ | Placebo arm n = 31 Lithium arm n = 30 Summary Global Deficit Score-24 Weeks (p = 0.329) | No improvement in cognition in PWH + NCI vs. placebo arm. Ref [97] |
| Selegiline transdermal system (STS) | NM | NM | Improvement in psychomotor speed in two prior pilot studies. See reference [98]. | ✓ | 3 mg/24 h STS (n = 42) 6 mg/24 h STS (n = 43) Placebo (n = 43) NPZ-8 score p = 0.35. NPZ total score p = 0.88. (Oxidative stress) CSF protein carbonyl concentration (p = 0.260) | No improvement in cognition in STS vs placebo arm. No effect on oxidative stress. Ref [98,99] |
| Paroxetine, Fluconazole, or both | NM | NM | N/A | ✓ | Paroxetine + Fluconazole n = 11 Paroxetine + Placebo n = 11 Placebo + Fluconazole n = 9 Placebo + Placebo n = 10 Paroxetine arms improved summary NPZ-8 score (p = 0.023) | Fluconazole did not have an observable additive effect with Paroxetine. Paroxetine in general improved cognition in PWH. Ref [100] |
| Atorvastatin | X | X | Reduction of blood lipids. | Open Label | Atorvastatin (no ART) single arm n = 7 viral load and CSF inflammatory markers (p > 0.05) Reduced cholesterol and LDL at 4–8 weeks (p < 0.01) or triglycerides at 4 weeks (p < 0.05). | No effect on viral load or inflammatory markers in CSF. Ref [106] |
| Memantine | NM | NM | Significant increase in NAA/Cr in FWM (p= 0.040) and Parietal Cortex (p= 0.023) via MRS. | ✓ | Memantine arm n = 54 Placebo arm n = 56 NPZ-8 score p = 0.585 (week 16) | No improvement in cognition in PWH. Ref [110] |
| Anti-inflammatory | ||||||
| Minocycline | NM | NM | Decrease in oxidative stress markers after 24 weeks. Ref [108] Decrease in various oxidative stress lipid markers (p ≤ 0.024). Ref [108] No effect on various markers of inflammation (p > 0.05) Ref [108]. | ✓ | Minocycline arm n = 26 Placebo arm n = 26 Cognitive analysis by UNP Sum criteria (p = 0.37) Ref [109]. Minocycline arm n = 8 Ref [108] Placebo arm n = 13 Ref [108] | No improvement in cognition in PWH. Ref [109] |
| Treatment | System | Anti-Inflammatory Properties? | Anti-Retroviral Properties? | Other Effects | Recommended for Future Clinical Trials? |
|---|---|---|---|---|---|
| Antiretroviral | |||||
| Nanodiamond carrier + efavirenz | Human neuroblastoma cells; primary human macrophages | NM | ✓ | Prolonged retention in HIV-infected macrophages. Non-toxic to neuroblastoma cells. Ref [250] | Needs further study in animal models. |
| PLGA encapsulated elvitegravir | In vitro human BBB model, primary human MDMs; SCID mouse model. | NM | ✓ | Enhanced HIV suppression in MDMs and mouse model. Pronounced penetration in BBB model in vitro. Ref [252,253] | Needs further study in animal models with larger groups. |
| Nano-NRTIs | Human primary MDMs injected intracerebrally in SCID mice; primary rat neuron culture. | ✓ | ✓ | Less toxic than free NRTIs. Ref [256] | Various nano delivery systems (like the two formulations above) are a potential avenue of research. |
| Miscellaneous treatments | |||||
| JHU083 | EcoHIV Mouse Model | NM | NM | Neuroprotectant and reverses cognitive dysfunction in infected mice. Ref [160] | ✓ |
| Didehydro-Cortistatin A (dCA) | BLT mice Ref [173]; Tat Transgenic mice + glia cell lines. Ref [174] | In glia cell line. NM in BLT model. | ✓ | HIV Tat inhibitor. Reverses dopaminergic system dysfunction linked to Tat neurotoxicity. Ref [173] | Further studies examining cognitive impairment in vivo warranted. |
| Natalizumab | SIV Model | NM | ✓ | Neuroprotective. Inhibited trafficking of SIV infected monocytes to CNS and gut. Ref [207] | Further animal studies that investigate blockage of infected monocyte trafficking is worth consideration. |
| anti-JAM-A or anti-ALCAM antibodies | in vitro human BBB model | NM | ✓ | Blocks transmigration of infected monocytes across BBB model. Ref [248] | Warrants testing in animal models. |
| Anti-inflammatory | |||||
| B18R | Human primary MDMs injected intracerebrally in SCID mice. | ✓ | NM | Treatment reversed cognitive deficits in HIV infected mice. Ref [137] | ✓ |
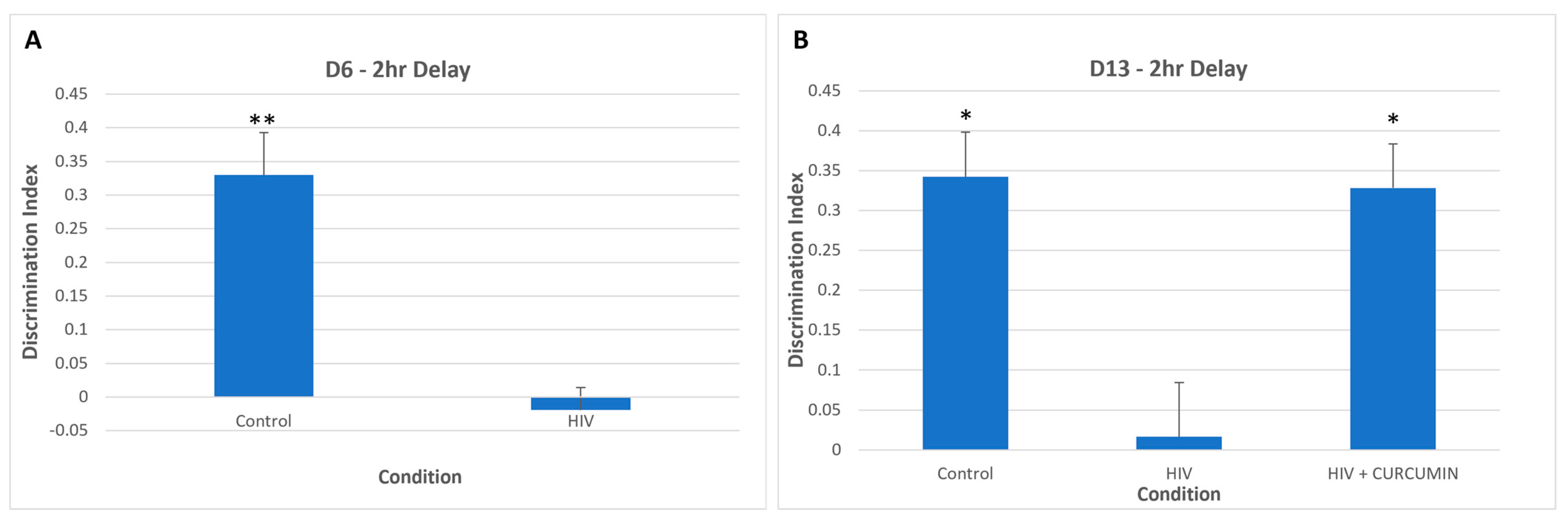
| Curcumin | Human primary MDMs injected intracerebrally in SCID mice. | Reduction in mouse myeloid activation markers (trend). | X | Treatment reversed cognitive deficits in HIV infected mice. Ref [154] | Further testing in HIV + NCI animal models warranted. |
| Ruxolitinib | Human primary MDMs injected intracerebrally in SCID mice. | ✓ | ✓ | N/A Ref [155] | See Ref [111]. Authors did not examine cognition. |
| Baricitinib | Human primary MDMs injected intracerebrally in SCID mice. | ✓ | ✓ | Treatment reversed cognitive impairment in HIV infected mice. Ref [120] | ✓ |
| Intranasal Insulin | EcoHIV Mouse Model | ✓ | ✓ | Treatment reversed cognitive impairment in HIV infected mice. Ref [159] | ✓ |
| Intranasal Insulin | FIV Model | ✓ | ✓ | Treatment improved neurobehavioral performance in FIV infected cats. Ref [191] | ✓ |
| PPARγ agonists | EcoHIV Mouse Model; gp120 neurotoxicity mouse model | ✓ | ✓ | See Ref [161,166,167]. | ✓ |
| Immunophilin Ligand FK506 | Gp120 transgenic mouse model | ✓ | NM | Mitigates mitochondrial dysfunction and synaptodendritic injury. Ref [175] | ✓ |
| Dimethyl fumarate (DMF) | SIV model; primary rat cortex neurons + conditioned media. | In vitro. Ref [221,222] | In vitro. Ref [221,222] | Neither anti-inflammatory nor anti-SIV in SIV model. Ref [223] | Future studies combining DMF with ART in animal model worth considering. |
| IFN-β | gp120 transgenic mouse model; primary rat and mouse neurons. | ✓ | NM | Reduces synaptodendritic injury in vivo. Ref [83] | ✓ |
| MCC950 | gp120 transgenic mouse model; primary mouse neurons and microglia. | ✓ | NM | Reduces synaptodendritic injury in vivo. Ref [232] | ✓ |
| PERK inhibitor GSK2606414 | Mouse microglia cell line. | ✓ | NM | N/A Ref [237] | Warrants further studies in animal models. |
| Rapamycin (autophagy inducer) | Primary human fetal neurons and human astrocytes. | NM | NM | Neuroprotective in vitro. Ref [246] | Autophagy inducers warrants further studies in animal models. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanlan, A.; Zhang, Z.; Koneru, R.; Reece, M.; Gavegnano, C.; Anderson, A.M.; Tyor, W. A Rationale and Approach to the Development of Specific Treatments for HIV Associated Neurocognitive Impairment. Microorganisms 2022, 10, 2244. https://doi.org/10.3390/microorganisms10112244
Scanlan A, Zhang Z, Koneru R, Reece M, Gavegnano C, Anderson AM, Tyor W. A Rationale and Approach to the Development of Specific Treatments for HIV Associated Neurocognitive Impairment. Microorganisms. 2022; 10(11):2244. https://doi.org/10.3390/microorganisms10112244
Chicago/Turabian StyleScanlan, Aaron, Zhan Zhang, Rajeth Koneru, Monica Reece, Christina Gavegnano, Albert M. Anderson, and William Tyor. 2022. "A Rationale and Approach to the Development of Specific Treatments for HIV Associated Neurocognitive Impairment" Microorganisms 10, no. 11: 2244. https://doi.org/10.3390/microorganisms10112244
APA StyleScanlan, A., Zhang, Z., Koneru, R., Reece, M., Gavegnano, C., Anderson, A. M., & Tyor, W. (2022). A Rationale and Approach to the Development of Specific Treatments for HIV Associated Neurocognitive Impairment. Microorganisms, 10(11), 2244. https://doi.org/10.3390/microorganisms10112244






