Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Soil Sampling and Preparation
2.3. Soil Chemical and Microbial Analyses
2.4. Data Analysis
3. Results
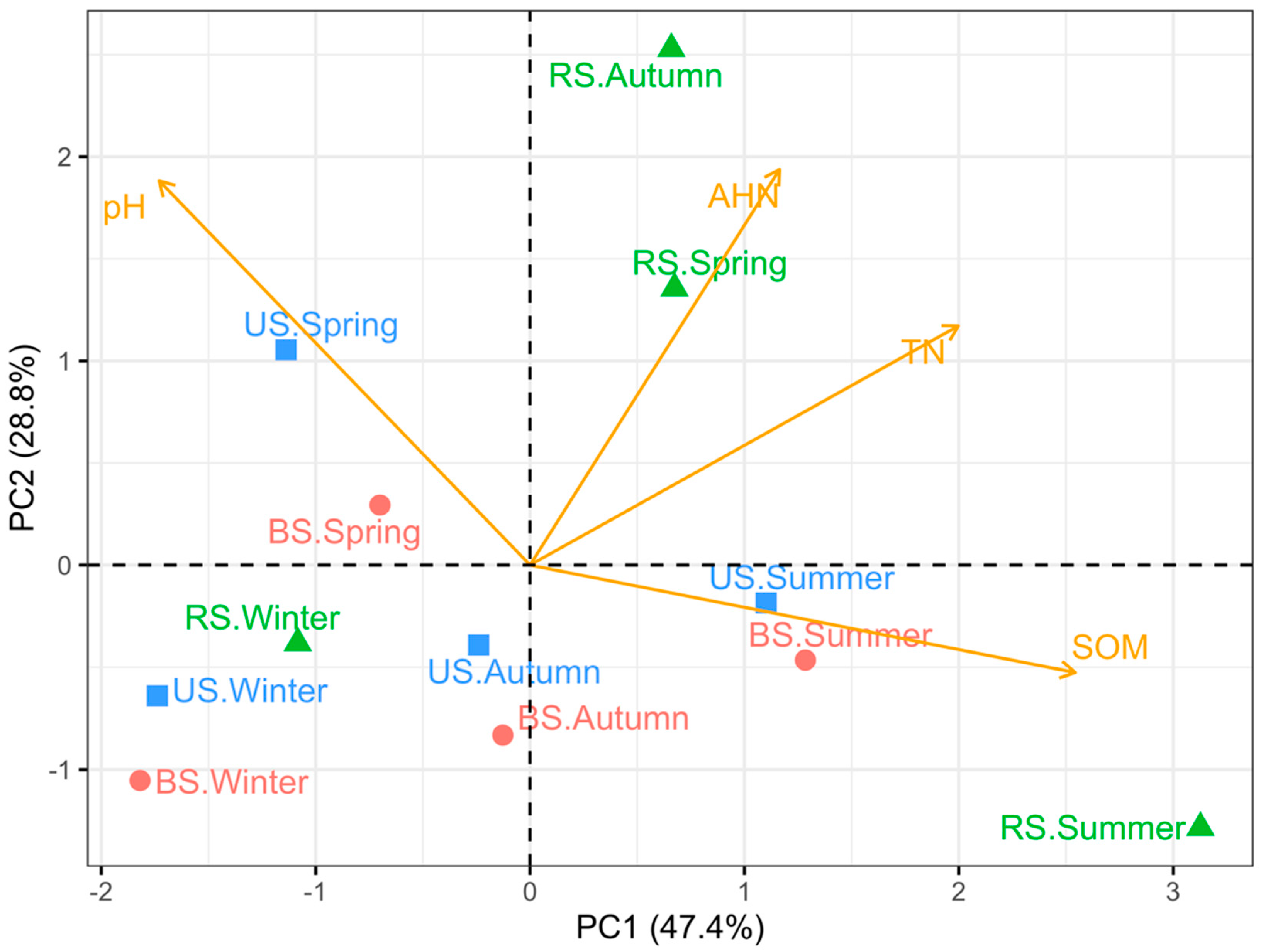
3.1. Soil Chemical Properties
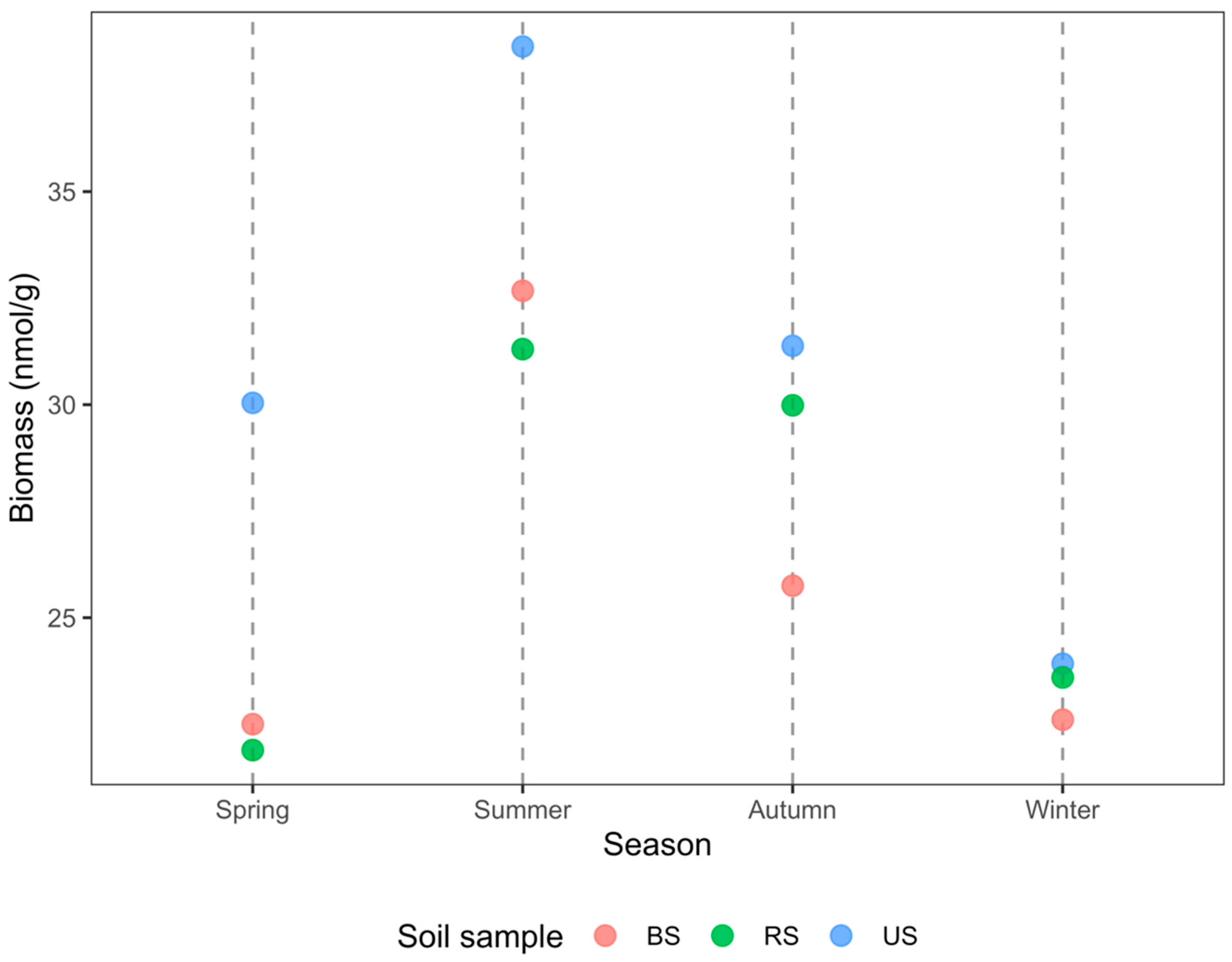
3.2. PLFA-Based Soil Microbial Biomass
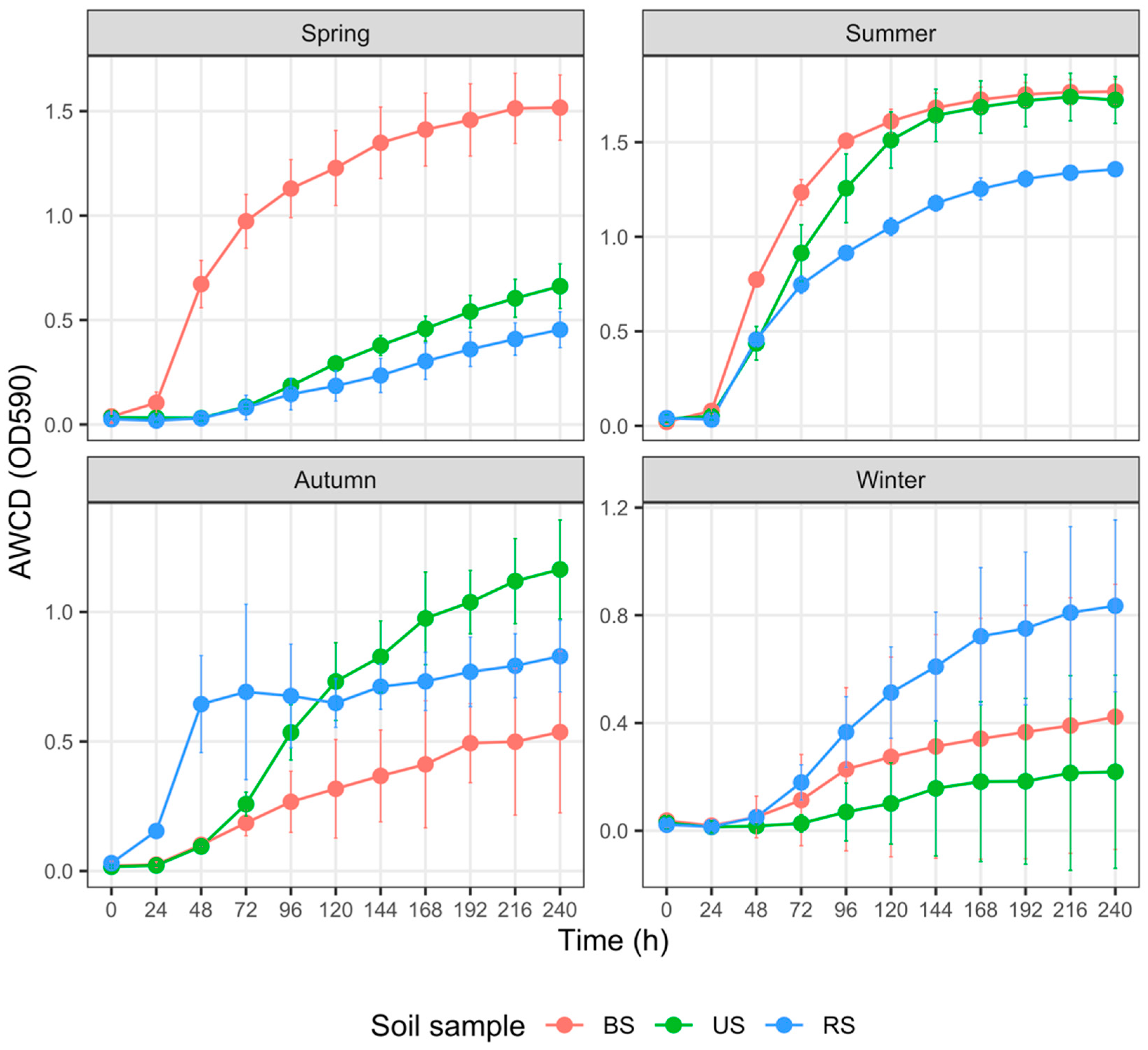
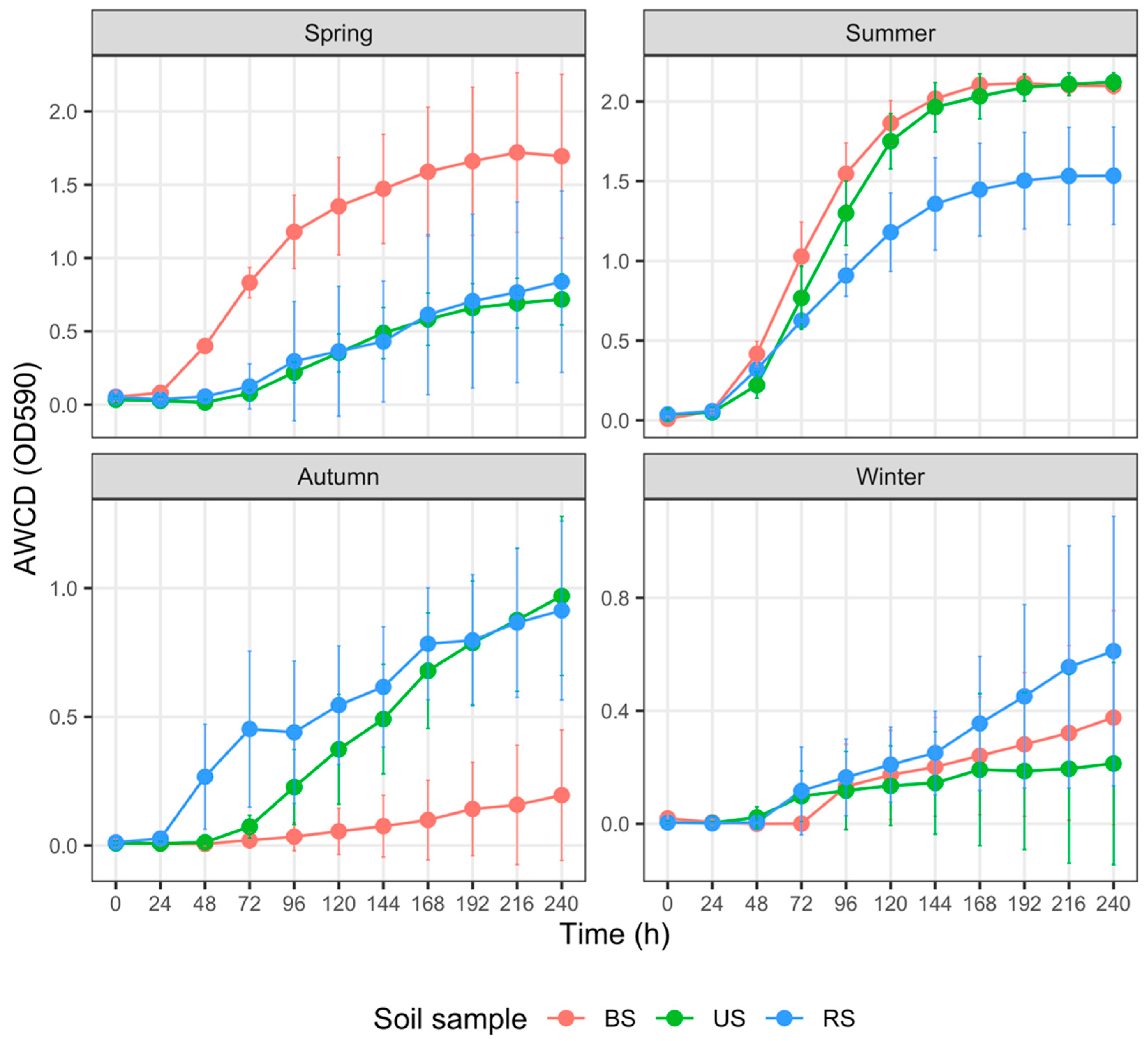
3.3. Carbon Metabolism Characteristics of Total Carbon Sources
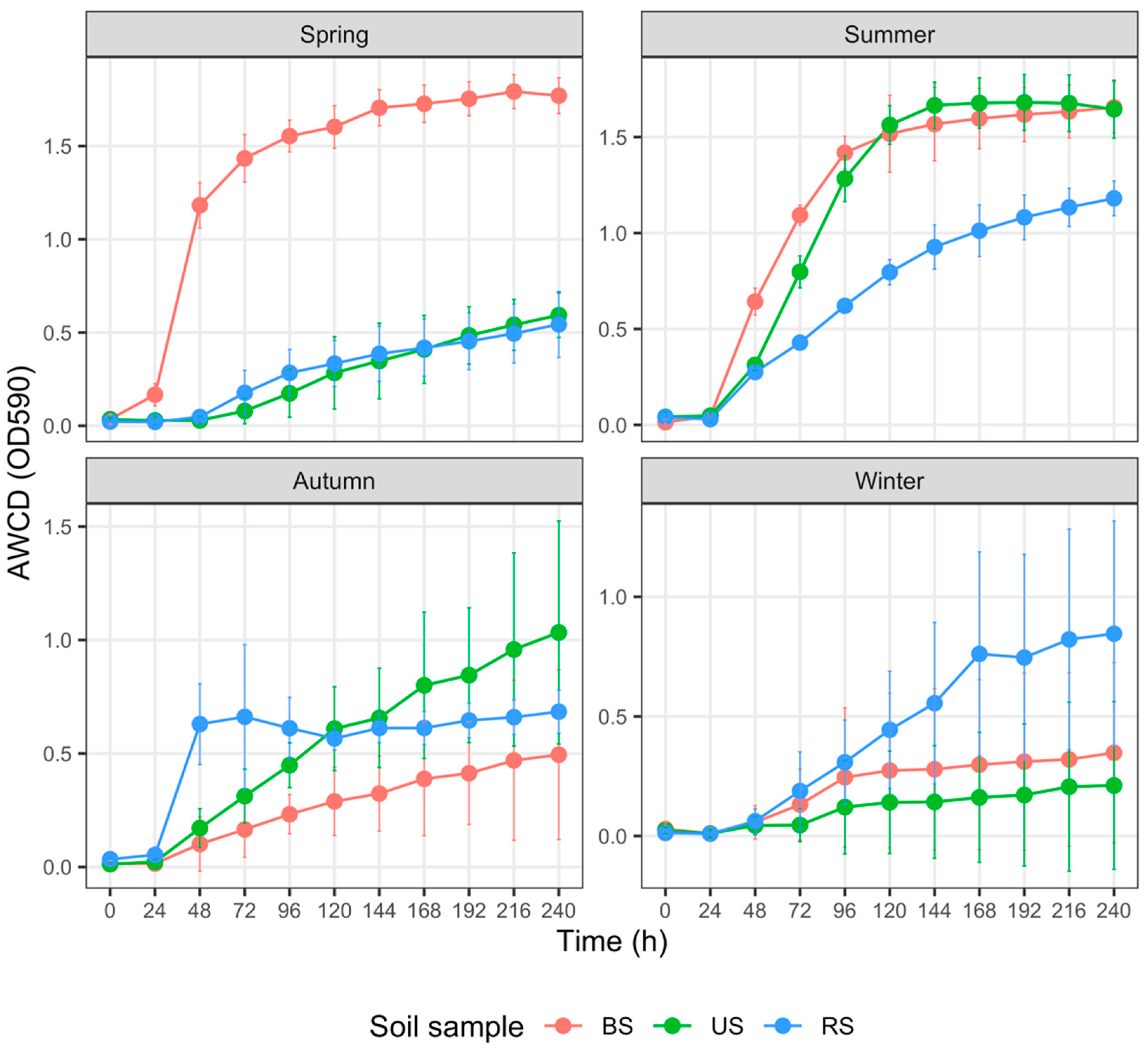
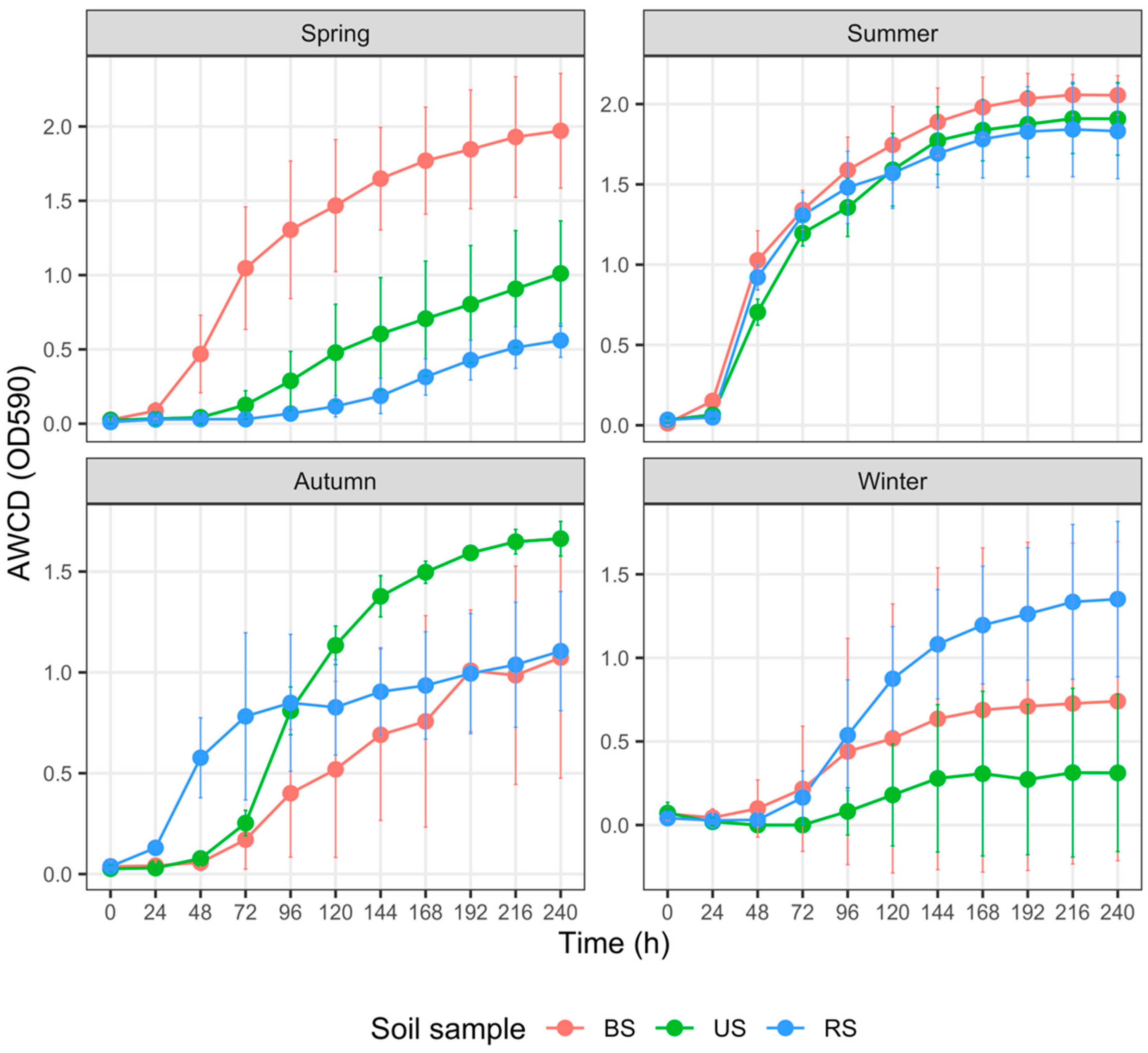
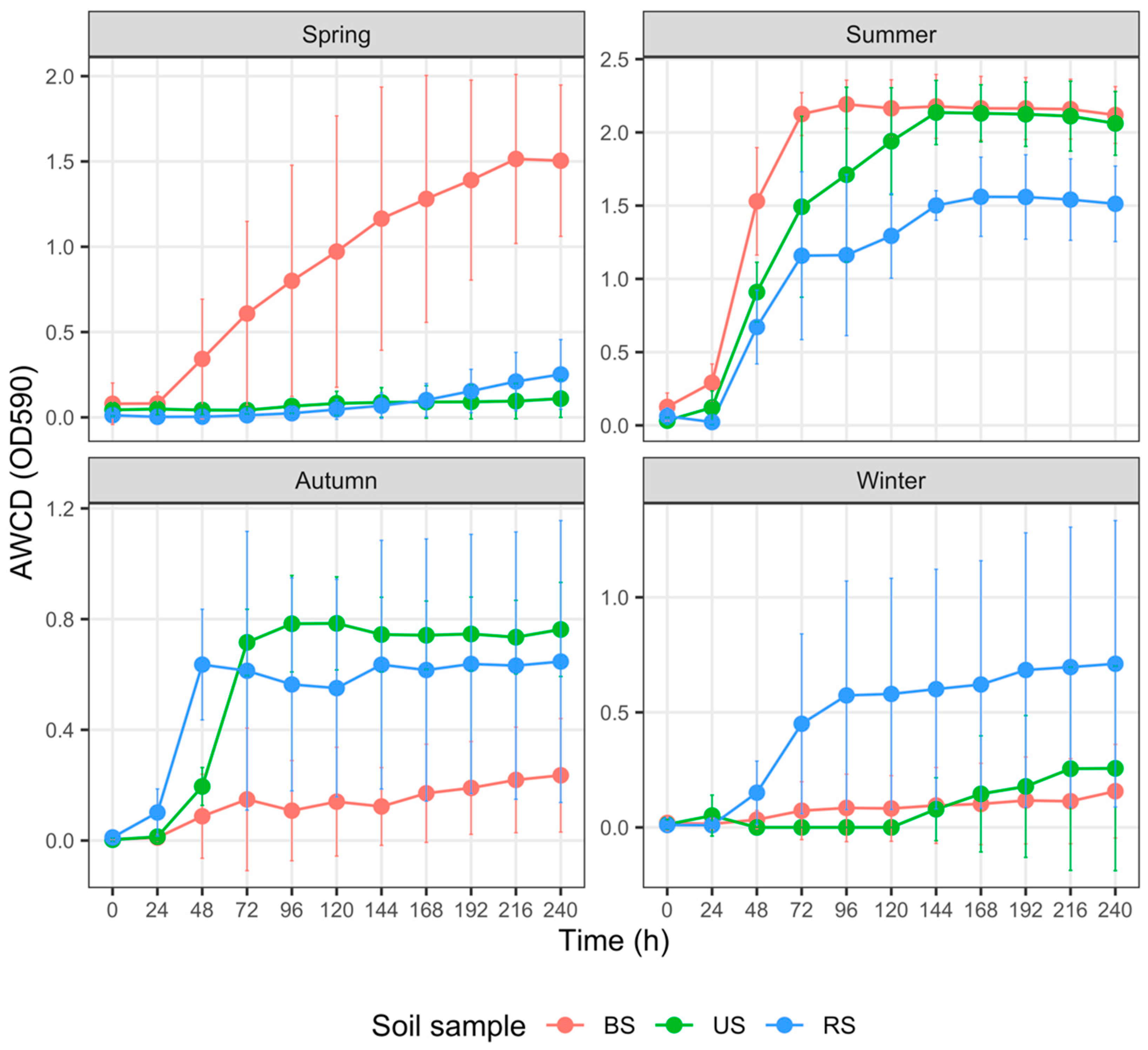
3.4. Carbon Metabolism Characteristics with Five Substrate Groups
4. Discussion
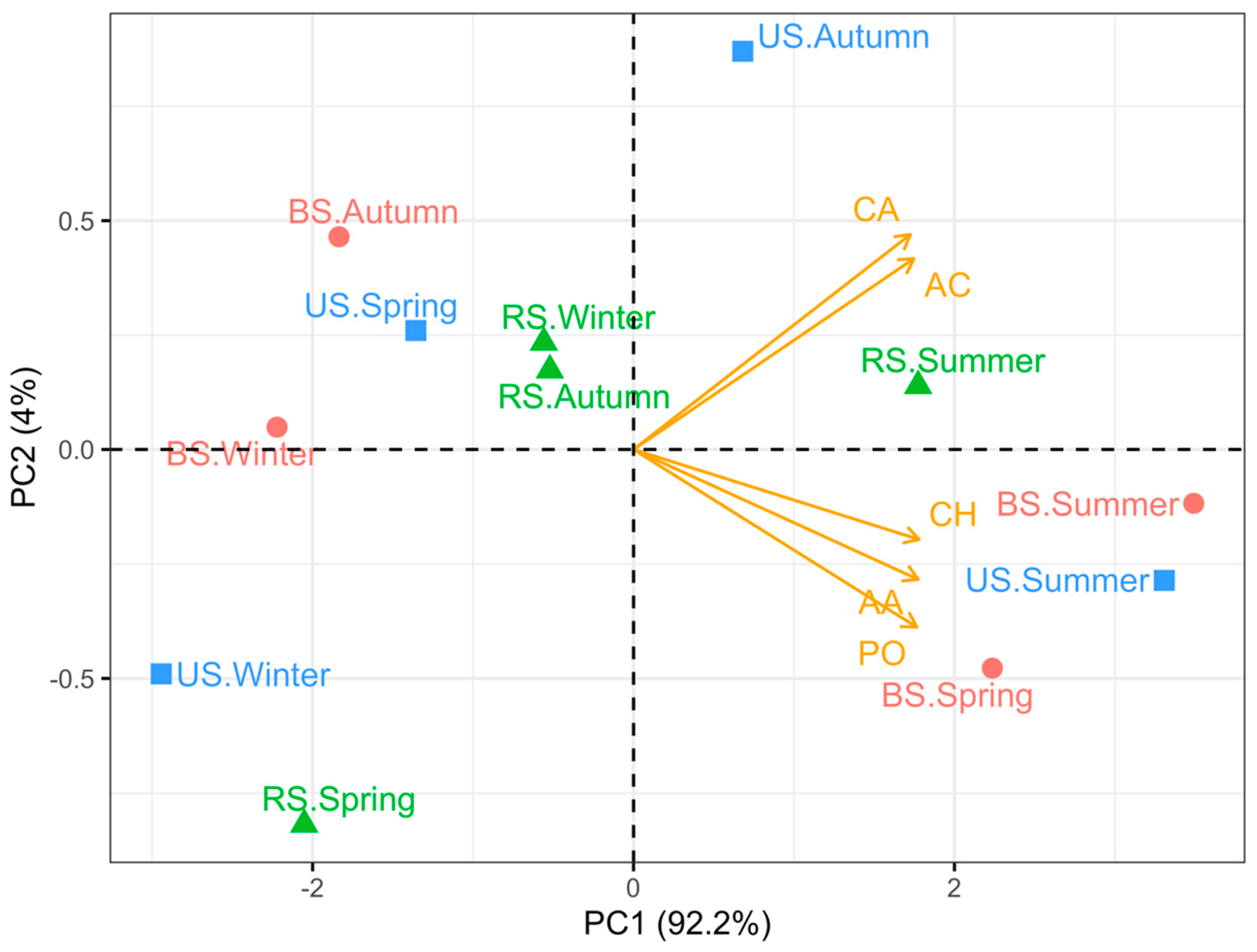
4.1. Rhizosphere Soil Possesses Superior Chemical Properties
4.2. Rice Cultivation Reduces Soil Microbial Biomass
4.3. Monoculture Practices Influence Soil Carbon Metabolism Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tellen, V.A.; Yerima, B.P.K. Effects of Land Use Change on Soil Physicochemical Properties in Selected Areas in the North West Region of Cameroon. Environ. Syst. Res. 2018, 7, 3. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, G.; Zhao, G.; le Master, D.C.; Parker, G.R.; Dunning, J.B.; Li, Q. China’s Forest Policy for the 21st Century. Science 2000, 288, 2135–2136. [Google Scholar] [CrossRef]
- Bissett, A.; Richardson, A.E.; Baker, G.; Thrall, P.H. Long-Term Land Use Effects on Soil Microbial Community Structure and Function. Appl. Soil Ecol. 2011, 51, 66–78. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-Use History Has a Stronger Impact on Soil Microbial Community Composition than Aboveground Vegetation and Soil Properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long Term Tillage, Cover Crop, and Fertilization Effects on Microbial Community Structure, Activity: Implications for Soil Quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Gárriz, A.; Ruiz, O.A. Soil Bacterial and Fungal Community Structure of a Rice Monoculture and Rice-Pasture Rotation Systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; An, S.; Sun, H.; Bhople, P.; Chen, Z. Soil Physicochemical and Microbial Characteristics of Contrasting Land-Use Types along Soil Depth Gradients. Catena 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Arévalo-Gardini, E.; Canto, M.; Alegre, J.; Loli, O.; Julca, A.; Baligar, V. Changes in Soil Physical and Chemical Properties in Long Term Improved Natural and Traditional Agroforestry Management Systems of Cacao Genotypes in Peruvian Amazon. PLoS ONE 2015, 10, e0132147. [Google Scholar] [CrossRef]
- Carbonetto, B.; Rascovan, N.; Álvarez, R.; Mentaberry, A.; Vázquez, M.P. Structure, Composition and Metagenomic Profile of Soil Microbiomes Associated to Agricultural Land Use and Tillage Systems in Argentine Pampas. PLoS ONE 2014, 9, e99949. [Google Scholar] [CrossRef]
- Puissant, J.; Villenave, C.; Chauvin, C.; Plassard, C.; Blanchart, E.; Trap, J. Quantification of the Global Impact of Agricultural Practices on Soil Nematodes: A Meta-Analysis. Soil Biol. Biochem. 2021, 161, 108383. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of No-Tillage and Conventional Tillage Systems on Soil Microbial Communities. Appl. Environ. Soil Sci. 2012, 2012, 548620. [Google Scholar] [CrossRef]
- Maroušek, J.; Gavurová, B. Recovering Phosphorous from Biogas Fermentation Residues Indicates Promising Economic Results. Chemosphere 2022, 291, 133008. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A. Economic Considerations on Nutrient Utilization in Wastewater Management. Energy 2021, 14, 3468. [Google Scholar] [CrossRef]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An Overview of Global Rice Production, Supply, Trade, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef]
- Linh, T.B.; Sleutel, S.; Vo Thi, G.; Le Van, K.; Cornelis, W.M. Deeper Tillage and Root Growth in Annual Rice-Upland Cropping Systems Result in Improved Rice Yield and Economic Profit Relative to Rice Monoculture. Soil Tillage Res. 2015, 154, 44–52. [Google Scholar] [CrossRef]
- Linh, T.B.; Le Van, K.; Van Elsacker, S.; Cornelis, W.M. Effect of Cropping System on Physical Properties of Clay Soil Under Intensive Rice Cultivation. Land Degrad Dev. 2016, 27, 973–982. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Zoubek, T.; Bartoš, P. Economic Impacts of Soil Fertility Degradation by Traces of Iron from Drinking Water Treatment. Environ. Dev. Sustain. 2022, 24, 4835–4844. [Google Scholar] [CrossRef]
- Xuan, D.T.; Guong, V.T.; Rosling, A.; Alström, S.; Chai, B.; Högberg, N. Different Crop Rotation Systems as Drivers of Change in Soil Bacterial Community Structure and Yield of Rice, Oryza Sativa. Biol. Fertil. Soils 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Luo, X.; Fu, X.; Yang, Y.; Cai, P.; Peng, S.; Chen, W.; Huang, Q. Microbial Communities Play Important Roles in Modulating Paddy Soil Fertility. Sci. Rep. 2016, 6, 20326. [Google Scholar] [CrossRef]
- Breidenbach, B.; Pump, J.; Dumont, M.G. Microbial Community Structure in the Rhizosphere of Rice Plants. Front. Microbiol. 2016, 6, 1537. [Google Scholar] [CrossRef]
- Perera, T.A.; Tirimanne, S. Role of Microbial Communities in Sustainable Rice Cultivation. In Role of Microbial Communities for Sustainability; Seneviratne, G., Zavahir, J.S., Eds.; Springer: Singapore, 2021; pp. 189–223. ISBN 978-981-15-9912-5. [Google Scholar]
- Maroušek, J.; Kolář, L.; Vochozka, M.; Stehel, V.; Maroušková, A. Novel Method for Cultivating Beetroot Reduces Nitrate Content. J. Clean. Prod. 2017, 168, 60–62. [Google Scholar] [CrossRef]
- Xiang, H.; Lan, N.; Wang, F.; Zhao, B.; Wei, H.; Zhang, J. Reduced Pests, Improved Grain Quality and Greater Total Income: Benefits of Intercropping Rice with Pontederia Cordata. J. Sci. Food Agric. 2021, 101, 5907–5917. [Google Scholar] [CrossRef]
- Nie, L.; Peng, S. Rice Production in China. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 33–52. ISBN 978-3-319-47516-5. [Google Scholar]
- Mei, F.; Wu, X.; Yao, C.; Li, L.; Wang, L.; Chen, Q. Rice Cropping Regionalization in China. Chin. J. Rice Sci. 1988, 2, 97–110. [Google Scholar]
- Tang, S.; Ding, L.; Bonjean, A.P.A. Rice Production and Genetic Improvement in China. In Cereals in China; He, Z., Bonjean, A.P.A., Eds.; CIMMYT: Mexico City, Mexico, 2010; ISBN 978-970-648-177-1. [Google Scholar]
- Qian, L.; Zuo, F.; Liu, H.; Zhang, C.; Chi, X.; Zhang, D. Determination of Geographical Origin of Wuchang Rice with the Geographical Indicator by Multielement Analysis. J. Food Qual. 2019, 2019, 8396865. [Google Scholar] [CrossRef]
- Cooperative Research Group of Chinese Soil Taxonomy (CRG-CST). Chinese Soil Taxonomy, 3rd ed.; China Science-Technology University Publishing House: Hefei, China, 2001. [Google Scholar]
- Tan, K.; Wang, S.; Song, Y.; Liu, Y.; Gong, Z. Estimating Nitrogen Status of Rice Canopy Using Hyperspectral Reflectance Combined with BPSO-SVR in Cold Region. Chemom. Intell. Lab. Syst. 2018, 172, 68–79. [Google Scholar] [CrossRef]
- Lu, R. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Frostegård, A.; Bååth, E. The Use of Phospholipid Fatty Acid Analysis to Estimate Bacterial and Fungal Biomass in Soil. Biol. Fertil. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids. Lab. Tech. Biochem. Mol. Biol. 1972, 3, 268–618. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Microbial Biomass Measured as Total Lipid Phosphate in Soils of Different Organic Content. J. Microbiol. Methods 1991, 14, 151–163. [Google Scholar] [CrossRef]
- Boyle, S.A.; Yarwood, R.R.; Bottomley, P.J.; Myrold, D.D. Bacterial and Fungal Contributions to Soil Nitrogen Cycling under Douglas Fir and Red Alder at Two Sites in Oregon. Soil Biol. Biochem. 2008, 40, 443–451. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. The Application of the Biolog EcoPlate Approach in Ecotoxicological Evaluation of Dairy Sewage Sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Jałowiecki, Ł.; Chojniak, J.M.; Dorgeloh, E.; Hegedusova, B.; Ejhed, H.; Magnér, J.; Płaza, G.A. Microbial Community Profiles in Wastewaters from Onsite Wastewater Treatment Systems Technology. PLoS ONE 2016, 11, e0147725. [Google Scholar]
- Sofo, A.; Ricciuti, P. A Standardized Method for Estimating the Functional Diversity of Soil Bacterial Community by Biolog® EcoPlatesTM Assay—The Case Study of a Sustainable Olive Orchard. Appl. Sci. 2019, 9, 4035. [Google Scholar] [CrossRef]
- Ge, Z.; Du, H.; Gao, Y.; Qiu, W. Analysis on Metabolic Functions of Stored Rice Microbial Communities by BIOLOG ECO Microplates. Front. Microbiol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- Pohlert, T. PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums Extended. Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 25 September 2022).
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra (Vol. 2); Sthda. 2017. Available online: http://www.sthda.com/english/articles/31-principal-component-methods-in-r-practical-guide/112-pca-principal-component-analysis-essentials/ (accessed on 18 October 2022).
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 19 August 2021).
- Lê, S.; Josse, J.; Rennes, A.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Bender, S.F.; Hartman, K.; Hydbom, S.; Lima, R.A.A.; Loaiza, V.; Nemecek, T.; Oehl, F.; Olsson, P.A.; Petchey, O.; et al. Organic and Conservation Agriculture Promote Ecosystem Multifunctionality. Sci. Adv. 2021, 7, eabg6995. [Google Scholar] [CrossRef] [PubMed]
- Manoharachary, C.; Mukerji, K.G. Rhizosphere Biology—An Overview. In Microbial Activity in the Rhizoshere; Mukerji, K.G., Manoharachary, C., Singh, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–15. ISBN 978-3-540-29420-7. [Google Scholar]
- Huo, C.; Luo, Y.; Cheng, W. Rhizosphere Priming Effect: A Meta-Analysis. Soil Biol. Biochem. 2017, 111, 78–84. [Google Scholar] [CrossRef]
- Nguyen, C. Rhizodeposition of Organic C by Plant: Mechanisms and Controls. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 97–123. ISBN 978-90-481-2666-8. [Google Scholar]
- Wichern, F.; Eberhardt, E.; Mayer, J.; Joergensen, R.G.; Müller, T. Nitrogen Rhizodeposition in Agricultural Crops: Methods, Estimates and Future Prospects. Soil Biol. Biochem. 2008, 40, 30–48. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, K.; Ni, F. Measuring the Rhizodeposition of Carbon by Rice: An Approach Based on Carbon Flux Observations. Soil Sci. Plant Nutr. 2017, 63, 499–506. [Google Scholar] [CrossRef]
- Lu, Y.; Watanabe, A.; Kimura, M. Input and Distribution of Photosynthesized Carbon in a Flooded Rice Soil. Glob. Biogeochem. Cycles 2002, 16, 32-1–32-38. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Kolář, L.; Vochozka, M.; Kopecký, M.; Maroušková, A.; Batt, J.; Poliak, M.; Šoch, M.; Bartoš, P.; et al. Advances in Nutrient Management Make It Possible to Accelerate Biogas Production and Thus Improve the Economy of Food Waste Processing. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–10. [Google Scholar] [CrossRef]
- Hupe, A.; Schulz, H.; Bruns, C.; Haase, T.; Heß, J.; Joergensen, R.G.; Wichern, F. Even Flow? Changes of Carbon and Nitrogen Release from Pea Roots over Time. Plant Soil 2018, 431, 143–157. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, X.; Shi, Y.; Lu, C.; Miao, Y.; Chen, Z.; Han, S. Quantitative Evidence of Underestimated Nitrogen Rhizodeposition Using 15N Split-Root Labeling during Spring Wheat Developmental Period. Catena 2021, 207, 105618. [Google Scholar] [CrossRef]
- Zheng, X.; Yuan, J.; Zhang, T.; Hao, F.; Jose, S.; Zhang, S. Soil Degradation and the Decline of Available Nitrogen and Phosphorus in Soils of the Main Forest Types in the Qinling Mountains of China. Forests 2017, 8, 460. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Wang, Y.; Shen, Q.; Mu, L.; Liu, Z. Spatiotemporal Heterogeneity of Soil Available Nitrogen During Crop Growth Stages on Mollisol Slopes of Northeast China. Land Degrad. Dev. 2017, 28, 856–869. [Google Scholar] [CrossRef]
- Chen, F.S.; Zeng, D.H.; Zhou, B.; Singh, A.N.; Fan, Z.P. Seasonal Variation in Soil Nitrogen Availability under Mongolian Pine Plantations at the Keerqin Sand Lands, China. J. Arid. Environ. 2006, 67, 226–239. [Google Scholar] [CrossRef]
- Prasad, J.K.; Dey, R.; Gupta, S.K.; Raghuwanshi, R. Portraying Microbial Beneficence for Ameliorating Soil Health and Plant Growth. In Soil Health; Giri, B., Varma, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 287–312. ISBN 978-3-030-44364-1. [Google Scholar]
- Kavamura, V.N.; Aono, A.H.; Esposito, E. 6.18—Biotechnological Strategies Applied to the Decontamination of Soils Polluted With Heavy Metals☆. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 240–252. ISBN 978-0-444-64047-5. [Google Scholar]
- DeLuca, T.H.; Pingree, M.R.A.; Gao, S. Chapter 16—Assessing Soil Biological Health in Forest Soils. In Developments in Soil Science; Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 397–426. ISBN 0166-2481. [Google Scholar]
- Kandeler, E. 3—Physiological and Biochemical Methods for Studying Soil Biota and Their Function. In Soil Microbiology, Ecology and Biochemistry, 3rd ed.; PAUL, E.A., Ed.; Academic Press: San Diego, CA, USA, 2007; pp. 53–83. ISBN 978-0-12-546807-7. [Google Scholar]
- Zhao, J.; Li, Y.; Wang, B.; Huang, X.; Yang, L.; Lan, T.; Zhang, J.; Cai, Z. Comparative Soil Microbial Communities and Activities in Adjacent Sanqi Ginseng Monoculture and Maize-Sanqi Ginseng Systems. Appl. Soil Ecol. 2017, 120, 89–96. [Google Scholar] [CrossRef]
- Galdos, M.v.; Pires, L.F.; Cooper, H.v.; Calonego, J.C.; Rosolem, C.A.; Mooney, S.J. Assessing the Long-Term Effects of Zero-Tillage on the Macroporosity of Brazilian Soils Using X-Ray Computed Tomography. Geoderma 2019, 337, 1126–1135. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar Farming: Defining Economically Perspective Applications. Clean. Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- Stávková, J.; Maroušek, J. Novel Sorbent Shows Promising Financial Results on P Recovery from Sludge Water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Mishra, A.K.; Singh, B.; Singh, R.P.; Patra, D.D. Tillage Effects on Crop Yield and Physicochemical Properties of Sodic Soils. Land Degrad. Dev. 2016, 27, 223–230. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of Ecosystem Nitrogen Cycle to Nitrogen Addition: A Meta-Analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent Responses of Soil Microbial Communities to Elevated Nutrient Inputs in Grasslands across the Globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967. [Google Scholar] [CrossRef] [PubMed]
- Dornbush, M.E.; von Haden, A.C. Chapter 8—Intensified Agroecosystems and Their Effects on Soil Biodiversity and Soil Functions. In Soil Health and Intensification of Agroecosytems; Al-Kaisi, M.M., Lowery, B., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 173–193. ISBN 978-0-12-805317-1. [Google Scholar]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen Fertilization Directly Affects Soil Bacterial Diversity and Indirectly Affects Bacterial Community Composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-Term Fertilizer and Crop-Rotation Treatments Differentially Affect Soil Bacterial Community Structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Lafond, G.P.; Ziadi, N.; Grant, C.A. Soil Microbial Response to Nitrogen Fertilizer and Tillage in Barley and Corn. Soil Tillage Res. 2012, 118, 139–146. [Google Scholar] [CrossRef]
- Bissett, A.; Richardson, A.E.; Baker, G.; Kirkegaard, J.; Thrall, P.H. Bacterial Community Response to Tillage and Nutrient Additions in a Long-Term Wheat Cropping Experiment. Soil Biol. Biochem. 2013, 58, 281–292. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Meng, M.; Zhai, L.; Zhang, B.; Jia, Z.; Gu, Z.; Liu, Q.; Zhang, Y.; Zhang, J. The Use of Biolog Eco Microplates to Compare the Effects of Sulfuric and Nitric Acid Rain on the Metabolic Functions of Soil Microbial Communities in a Subtropical Plantation within the Yangtze River Delta Region. Catena 2021, 198, 105039. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors Affecting Soil Microbial Biomass and Functional Diversity with the Application of Organic Amendments in Three Contrasting Cropland Soils during a Field Experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Lacoux, J.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J.-E.; et al. Functional Response of Soil Microbial Communities to Tillage, Cover Crops and Nitrogen Fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, B.; Hou, Y.; Liu, S.; Wang, Y. Impact on the Microbial Biomass and Metabolic Function of Carbon Source by Black Soil during Rice Cultivation. Environ. Sci. 2015, 36, 3011–3017. [Google Scholar] [CrossRef]
- Dou, S.; Guo, D. Soil Type Distribution and Black Soil Land Protection in Jilin Province. J. Jilin Agric. Univ. 2018, 40, 449–456. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Dick, R.P. PLFA Profiling of Microbial Community Structure and Seasonal Shifts in Soils of a Douglas-Fir Chronosequence. Microb. Ecol. 2008, 55, 500–511. [Google Scholar] [CrossRef]

| Seasons | Samples | pH | SOM (g/kg) | TN (g/kg) | AHN (mg/kg) |
|---|---|---|---|---|---|
| Spring | RS | 6.3 ± 0.1 | 33.6 ± 3.0 | 1.45 ± 0.4 | 197 ± 15 |
| BS | 6.2 ± 0.1 | 25.8 ± 3.0 | 1.28 ± 0.1 | 157 ± 12 | |
| US | 6.4 ± 0.2 | 26.6 ± 3.0 | 1.24 ± 0.2 | 162 ± 13 | |
| Summer | RS | 5.8 ± 0.2 | 41.1 ± 4.0 | 1.86 ± 0.3 | 145 ± 12 |
| BS | 6.1 ± 0.1 | 36.2 ± 2.0 | 1.66 ± 0.4 | 133 ± 12 | |
| US | 6.2 ± 0.3 | 36.3 ± 2.0 | 1.77 ± 0.2 | 122 ± 11 | |
| Autumn | RS | 6.5 ± 0.1 | 29.5 ± 3.0 | 2.38 ± 0.2 | 157 ± 13 |
| BS | 6.2 ± 0.2 | 31.6 ± 2.0 | 1.53 ± 0.3 | 94.2 ± 10 | |
| US | 6.3 ± 0.1 | 32.2 ± 1.0 | 1.56 ± 0.2 | 97.2 ± 11 | |
| Winter | RS | 6.4 ± 0.1 | 31.6 ± 3.0 | 1.23 ± 0.2 | 97 ± 9 |
| BS | 6.2 ± 0.2 | 23.4 ± 1.0 | 1.14 ± 0.1 | 91 ± 8 | |
| US | 6.3 ± 0.1 | 25.2 ± 3.0 | 1.20 ± 0.2 | 93 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Wang, H.; Ma, C.; Li, S.; Wu, H.; Yuan, K.; Meng, X.; Song, Z.; Fang, X.; Zhao, Z. Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate. Microorganisms 2022, 10, 2153. https://doi.org/10.3390/microorganisms10112153
Wei Z, Wang H, Ma C, Li S, Wu H, Yuan K, Meng X, Song Z, Fang X, Zhao Z. Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate. Microorganisms. 2022; 10(11):2153. https://doi.org/10.3390/microorganisms10112153
Chicago/Turabian StyleWei, Zhanxi, Hao Wang, Chao Ma, Shuyuan Li, Haimiao Wu, Kaini Yuan, Xiangyuan Meng, Zefeng Song, Xiaofeng Fang, and Zhirui Zhao. 2022. "Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate" Microorganisms 10, no. 11: 2153. https://doi.org/10.3390/microorganisms10112153
APA StyleWei, Z., Wang, H., Ma, C., Li, S., Wu, H., Yuan, K., Meng, X., Song, Z., Fang, X., & Zhao, Z. (2022). Unraveling the Impact of Long-Term Rice Monoculture Practice on Soil Fertility in a Rice-Planting Meadow Soil: A Perspective from Microbial Biomass and Carbon Metabolic Rate. Microorganisms, 10(11), 2153. https://doi.org/10.3390/microorganisms10112153






