New Therapeutic Options in Mild Moderate COVID-19 Outpatients
Abstract
1. Introduction
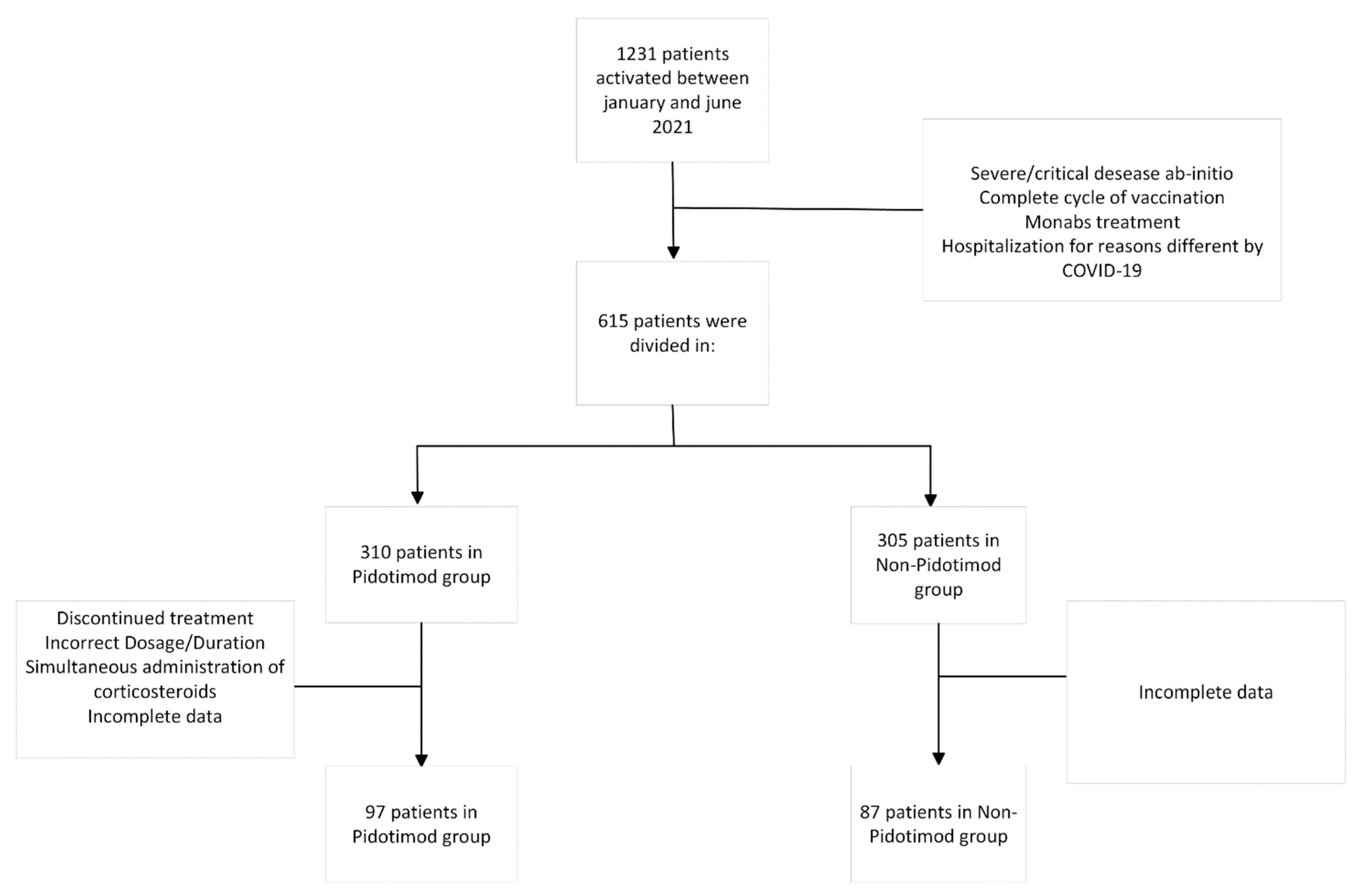
2. Materials and Methods
2.1. Study Procedures
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: www.coronavirus.jhu.edu/map.html (accessed on 29 September 2022).
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Auricchio, A.; Di Nicola, M.; Potere, N.; Abbate, A.; Cipollone, F.; Vecchiet, J.; Falasca, K. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020, 2, e457–e458. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Mortara, A.; D’Alessio, A.; Morelli, M.; Tedeschi, A.; Festuccia, M.B.; Monforte, A.D.; Capochiani, E.; Selleri, C.; Simonetti, F.; et al. JAK Inhibition with Ruxolitinib in Patients with COVID-19 and Severe Pneumonia: Multicenter Clinical Experience from a Compassionate Use Program in Italy. J. Clin. Med. 2021, 10, 3752. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Zuccotti, G.V.; Mameli, C. Pidotimod: The past and the present. Ital. J. Pediatr. 2013, 39, 75. [Google Scholar] [CrossRef][Green Version]
- Chatterjee, A.N.; Al Basir, F. A Model for SARS-CoV-2 Infection with Treatment. Comput. Math. Methods Med. 2020, 2020, 1352982. [Google Scholar] [CrossRef]
- Giagulli, C.; Noerder, M.; Avolio, M.; Becker, P.D.; Fiorentini, S.; Guzman, C.A.; Caruso, A. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int. Immunopharmacol. 2009, 9, 1366–1373. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, C.; Zhu, C.; Dong, X.; Liu, X. Pidotimod: A review of its pharmacological features and clinical effectiveness in respiratory tract infections. Expert Rev. Anti-Infect. Ther. 2019, 17, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Radovanovic, D.; Garziano, M.; Pini, S.; Croce, G.; Fuccia, G.; Spitaleri, D.; Biasin, M.; Clerici, M.; Trabattoni, D. Anti-Inflammatory Effects of Immunostimulation in Patients with COVID-19 Pneumonia. J. Clin. Med. 2021, 10, 5765. [Google Scholar] [CrossRef] [PubMed]
- Ucciferri, C.; Mirko, B.; Vecchiet, J.; Falasca, K. Pidotimod in paucisymptomatic SARS-CoV2 infected patients. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020048. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Au, I.C.; Lau, K.T.; Lau, E.; Cowling, B.J.; Leung, G.M. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: An observational study. medRxiv 2022. [Google Scholar] [CrossRef]
- Vena, A.; Traman, L.; Bavastro, M.; Limongelli, A.; Dentone, C.; Magnè, F.; Giacobbe, D.R.; Mikulska, M.; Taramasso, L.; Di Biagio, A.; et al. Early Clinical Experience with Molnupiravir for Mild to Moderate Breakthrough COVID-19 among Fully Vaccinated Patients at Risk for Disease Progression. Vaccines 2022, 10, 1141. [Google Scholar] [CrossRef]
- Gentile, I.; Scotto, R.; Moriello, N.S.; Pinchera, B.; Villari, R.; Trucillo, E.; Ametrano, L.; Fusco, L.; Castaldo, G.; Buonomo, A.R.; et al. Nirmatrelvir/ritonavir and molnuipiravir in the treatment of mild/moderate COVID-19: Results of a real-life study. medRxiv 2022. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Lee, J.-C.; Chiu, C.-W.; Lee, C.-C.; Tsai, P.-J.; Hsu, I.-L.; Ko, W.-C. Oral Nirmatrelvir/Ritonavir Therapy for COVID-19: The Dawn in the Dark? Antibiotics 2022, 11, 220. [Google Scholar] [CrossRef]
- Takashita, E.; Yamayoshi, S.; Simon, V.; van Bakel, H.; Sordillo, E.M.; Pekosz, A.; Fukushi, S.; Suzuki, T.; Maeda, K.; Halfmann, P.; et al. Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants. N. Engl. J. Med. 2022, 387, 468–470. [Google Scholar] [CrossRef]
- Carta, S.; Silvestri, M.; Rossi, G.A. Modulation of airway epithelial cell functions by Pidotimod: NF-kB cytoplasmatic expression and its nuclear translocation are associated with an increased TLR-2 expression. Ital. J. Pediatr. 2013, 39, 29. [Google Scholar] [CrossRef]
- Zuccotti, G.V.; Mameli, C.; Trabattoni, D.; Beretta, S.; Biasin, M.; Guazzarotti, L.; Clerici, M. Immunomodulating activity of Pidotimod in children with Down syndrome. J. Biol. Regul. Homeost. Agents 2013, 27, 253–258. Available online: https://pubmed.ncbi.nlm.nih.gov/23489705/ (accessed on 30 August 2022).
- Ferrario, B.E.; Garuti, S.; Braido, F.; Canonica, G.W. Pidotimod: The state of art. Clin. Mol. Allergy 2015, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wang, Z.; Qu, Y.; Zhu, H.; Zhu, Q.; Tong, W.; Bao, L.; Lv, Q.; Cong, J.; Li, D.; et al. Distinct uptake, amplification, and release of SARS-CoV-2 by M1 and M2 alveolar macrophages. Cell Discov. 2021, 7, 24. [Google Scholar] [CrossRef]
- Trabattoni, D.; Clerici, M.; Centanni, S.; Mantero, M.; Garziano, M.; Blasi, F. Immunomodulatory effects of pidotimod in adults with community-acquired pneumonia undergoing standard antibiotic therapy. Pulm. Pharmacol. Ther. 2017, 44, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Garziano, M.; Rainone, V.; Trabattoni, D.; Biasin, M.; Senatore, L.; Marchisio, P.G.; Rossi, M.; Principi, N.; Clerici, M. Immunomodulatory activity of pidotimod administered with standard antibiotic therapy in children hospitalized for community-acquired pneumonia. J. Transl. Med. 2015, 13, 288. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Peri, C.; Raynal, M.E.; Defilippi, A.-C.; Risso, F.M.; Schenone, G.; Pallestrini, E.; Melioli, G. Naturally occurring immune response against bacteria commonly involved in upper respiratory tract infections: Analysis of the antigen-specific salivary IgA levels. Immunol. Lett. 2003, 86, 85–91. [Google Scholar] [CrossRef]
- Hu, S.; Fu, X.; Fu, A.; Du, W.; Ji, J.; Li, W. The regulatory peptide pidotimod facilitates M2 macrophage polarization and its function. Amino Acids 2014, 46, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Chun, W.; Lee, H.; Min, J.-H.; Kim, S.-M.; Seo, J.-Y.; Ahn, K.-S.; Oh, S.-R. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells 2021, 10, 897. [Google Scholar] [CrossRef]
- Mantha, S.; Tripuraneni, S.L.; Roizen, M.F.; Fleisher, L.A. Proposed Modifications in the 6-Minute Walk Test for Potential Application in Patients With Mild COVID-19: A Step to Optimize Triage Guidelines. Anesthesia Analg. 2020, 131, 398–402. [Google Scholar] [CrossRef]
- Singh, S.J.; Puhan, M.; Andrianopoulos, V.; Hernandes, N.A.; Mitchell, K.E.; Hill, C.J.; Lee, A.L.; Camillo, C.A.; Troosters, T.; Spruit, M.A.; et al. An official systematic review of the European Respiratory Society/American Thoracic Society: Measurement properties of field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1447–1478. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Dexamethasone for COVID-19? Not so fast. J. Biol. Regul. Homeost. Agents 2020, 34, 1241–1243. [Google Scholar] [CrossRef]
- Spagnuolo, V.; Guffanti, M.; Galli, L.; Poli, A.; Querini, P.R.; Ripa, M.; Clementi, M.; Scarpellini, P.; Lazzarin, A.; Tresoldi, M.; et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 2020, 10, 21291. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Tajuddin, S.; Khaskheli, S.; Khan, N.; Niamatullah, H.; Nasir, N. Clinical outcomes of immunomodulatory therapies in the management of COVID-19: A tertiary-care experience from Pakistan. PLoS ONE 2022, 17, e0262608. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Zha, W.; Zhou, N.; Hong, X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: A dose-response meta-analysis. Metabolism 2020, 117, 154373. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Acute Respiratory Distress Syndrome: Diagnosis and Management. Am. Fam. Physician 2020, 101, 730–738. [Google Scholar] [CrossRef]
- Trigo, J.; García-Azorín, D.; Planchuelo-Gómez, Á.; Martínez-Pías, E.; Talavera, B.; Hernández-Pérez, I.; Valle-Peñacoba, G.; Simón-Campo, P.; De Lera, M.; Chavarría-Miranda, A.; et al. Factors associated with the presence of headache in hospitalized COVID-19 patients and impact on prognosis: A retrospective cohort study. J. Headache Pain 2020, 21, 94. [Google Scholar] [CrossRef]
- Shapiro, R.E.; Gallardo, V.J.; Caronna, E.; Pozo-Rosich, P. The impact of headache disorders on COVID-19 survival: A world population-based analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Gil-Rodrigo, A.; Miró, Ò.; Piñera, P.; Burillo-Putze, G.; Jiménez, S.; Martín, A.; Martín-Sánchez, F.J.; Jacob, J.; Guardiola, J.M.; García-Lamberechts, E.J.; et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departmen. Emergencias 2020, 32, 233–241. Available online: https://pubmed.ncbi.nlm.nih.gov/32692000/ (accessed on 13 October 2022).
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admission |Read by QxMD. Qjm 2001, 94, 521–526. Available online: https://read.qxmd.com/read/11588210/validation-of-a-modified-early-warning-score-in-medical-admissions (accessed on 23 August 2022). [CrossRef]
- Stenhouse, C.; Coates, S.; Tivey, M.; Allsop, P.; Parker, T. Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a general surgical ward. Br. J. Anaesth. 2000, 84, 663P. [Google Scholar] [CrossRef]
- Özdemir, S.; Algın, A.; Akça, H.; Altunok, I.; Kokulu, K.; Eroğlu, S.E.; Aksel, G. Predictive Ability of the MEWS, REMS, and RAPS in Geriatric Patients With SARS-CoV-2 Infection in the Emergency Department. Disaster Med. Public Health Prep. 2022, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Grignaschi, A.; Lanotte, A.M.G.; Cocco, G.; Vidili, G.; Giostra, F.; Schiavone, C. Role of Lung Ultrasound in the Management of Patients with Suspected SARS-CoV-2 Infection in the Emergency Department. J. Clin. Med. 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Habib, K.; Khanum, I.; Khan, N.; Muhammad, Z.A.; Mahmood, S.F. Clinical characteristics and outcomes of COVID-19: Experience at a major tertiary care center in Pakistan. J. Infect. Dev. Ctries. 2021, 15, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Rommasi, F.; Nasiri, M.J.; Mirsaeidi, M. Immunomodulatory agents for COVID-19 treatment: Possible mechanism of action and immunopathology features. Mol. Cell Biochem. 2022, 477, 711–726. [Google Scholar] [CrossRef]
| Gender | |
|---|---|
| F | 100 (54.3%) |
| M | 84 (45.7%) |
| Age (years) | 53.0 (42.0–62.5) |
| Illness Duration (days) | 22.0 (18.0–28.0) |
| Comorbidity | |
| No | 59 (34.3%) |
| Yes | 113 (65.7%) |
| BMI (kg/m2) | 27.1 (23.8–29.4) |
| Fever | |
| No | 51 (27.9%) |
| Yes | 132 (72.1%) |
| Maximum Temperature | 38.0 (37.6–38.5) |
| Days of Fever | 3.0 (0.0–6.0) |
| Cough | |
| No | 24 (13.0%) |
| Yes | 160 (87.0%) |
| Dyspnea | |
| No | 139 (75.5%) |
| Yes | 45 (24.5%) |
| Ageusia or Anosmia | |
| No | 88 (47.8%) |
| Yes | 96 (52.2%) |
| Fatigue | |
| No | 41 (22.3%) |
| Yes | 143 (77.7%) |
| Myalgia | |
| No | 64 (34.8%) |
| Yes | 120 (65.2%) |
| Headache | |
| No | 104 (56.5%) |
| Yes | 80 (43.5%) |
| Others | |
| No | 86 (47.3%) |
| Yes | 96 (52.7%) |
| Number of Symptoms | 4.0 (3.0–6.0) |
| SpO2 at Enrolment | 98.0 (97.0–99.0) |
| Minimum SpO2 | 96.0 (94.0–97.0) |
| Mews | 1.0 (0.0–3.0) |
| LUS | 6.0 (2.0–10.0) |
| Walking test | 97.0 (95.0–99.0) |
| Pidotimod Therapy | |
| No | 87 (47.3%) |
| Yes | 97 (52.7%) |
| Pidotimod Start Day | 3.0 (2.0–6.0) |
| Pidotimod Duration | 3.0 (0.0–10.0) |
| Corticosteroid Therapy | |
| No | 119 (65.0%) |
| Yes | 64 (35.0%) |
| Corticosteroid Start Day | 8.0 (7.0–10.0) |
| ER Access/Hospitalization | |
| No | 150 (81.5%) |
| Yes | 34 (18.5%) |
| Pidotimod Therapy | |||
|---|---|---|---|
| No (n = 87) | Yes (n = 97) | * p-Value | |
| Gender | |||
| F | 47 (54.0%) | 53 (54.6%) | 0.526 |
| M | 40 (46.0%) | 44 (45.4%) | |
| Age (years) | 53.0 (44.0–64.0) | 53.0 (40.0–61.0) | 0.240 |
| Illness Duration (days) | 23.0 (20.0–31.0) | 21.0 (17.0–27.0) | 0.005 |
| Comorbidity | |||
| No | 19 (24.4%) | 40 (42.6%) | 0.012 |
| Yes | 59 (75.6%) | 54 (57.4%) | |
| BMI (kg/m2) | 27.1 (26.0–29.4) | 26.9 (23.5–29.4) | 0.508 |
| Fever during the course | |||
| No | 26 (29.9%) | 25 (26.0%) | 0.563 |
| Yes | 61 (70.1%) | 71 (74.0%) | |
| Maximum Temperature | 38.0 (37.5–38.5) | 38.0 (37.6–38.5) | 0.914 |
| Days of Fever | 3.0 (0.0–6.0) | 3.0 (0.0–6.0) | 0.438 |
| Cough ** | |||
| No | 14 (16.1%) | 10 (10.3%) | 0.245 |
| Yes | 73 (83.9%) | 87 (89.7%) | |
| Dyspnea ** | |||
| No | 64 (73.6%) | 75 (77.3%) | 0.753 |
| At rest and in motion | 11 (12.6%) | 9 (9.3%) | |
| Only in motion | 12 (13.8%) | 13 (13.4%) | |
| Ageusia or Anosmia ** | |||
| No | 46 (52.9%) | 42 (43.3%) | 0.194 |
| Yes | 41 (47.1%) | 55 (56.7%) | |
| Fatigue ** | |||
| No | 24 (27.6%) | 17 (17.5%) | 0.102 |
| Yes | 63 (72.4%) | 80 (82.5%) | |
| Myalgia ** | |||
| No | 37 (42.5%) | 27 (27.8%) | 0.037 |
| Yes | 50 (57.5%) | 70 (72.2%) | |
| Headache ** | |||
| No | 57 (65.5%) | 47 (48.5%) | 0.020 |
| Yes | 30 (34.5%) | 50 (51.5%) | |
| Others ** | |||
| No | 49 (56.3%) | 37 (38.9%) | 0.113 |
| Yes | 28 (43.7%) | 57 (61.1%) | |
| Number of Symptoms ** | 4.0 (3.0–5.0) | 5.0 (3.0–6.0) | <0.001 |
| SpO2 at Enrolment | 98.0 (96.0–99.0) | 98.0 (97.0–99.0) | 0.103 |
| Minimum SpO2 during the course | 95.0 (93.0–97.0) | 96.0 (94.0–97.0) | 0.046 |
| Mews at Enrolment | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 0.062 |
| Maximum Mews during the course | 3.0 (0.0–5.0) | 1.0 (0.0–3.0) | 0.770 |
| Maximum LUS during the course | 7.0 (2.0–12.0) | 4.5 (1.5–8.0) | 0.128 |
| Minimum SpO2 Walking Test | 97.0 (93.0–98.0) | 97.0 (96.0–99.0) | 0.010 |
| Corticosteroid Therapy | |||
| No | 34 (39.1%) | 85 (88.5%) | <0.001 |
| Yes | 53 (60.9%) | 11 (11.5%) | |
| Corticosteroid Start Day | 8.0 (7.0–9.0) | 8.0 (7.0–12.0) | 0.282 |
| Corticosteroid Duration | 3.5 (0.0–11.0) | 0.0 (0.0–0.0) | <0.001 |
| ER Access/Hospitalization | |||
| No | 64 (73.6%) | 86 (88.7%) | 0.008 |
| Yes | 23 (26.4%) | 11 (11.3%) | |
| ER Access/Hospitalization | |||
|---|---|---|---|
| No (n = 150) | Yes (n = 34) | * p-Value | |
| Gender | |||
| F | 84 (56.0%) | 16 (47.1%) | 0.225 |
| M | 66 (44.0%) | 18 (52.9%) | |
| Age (years) | 50.5 (39.0–60.0) | 66.0 (54.0–79.0) | <0.001 |
| Illness Duration (days) | 22.0 (18.0–27.0) | 28.0 (23.0–38.0) | 0.002 |
| Comorbidity | |||
| No | 55 (39.6%) | 4 (12.1%) | 0.003 |
| Yes | 84 (60.4%) | 29 (87.9%) | |
| BMI (kg/m2) | 26.2 (23.5–29.1) | 33.3 (27.1–40.8) | 0.010 |
| Fever during the course | |||
| No | 46 (30.9%) | 5 (14.7%) | 0.058 |
| Yes | 103 (69.1%) | 29 (85.3%) | |
| Maximum Temperature | 38.0 (37.5–38.5) | 38.2 (37.8–38.9) | 0.183 |
| Days of Fever | 3.0 (0.0–5.0) | 4.0 (2.0–7.0) | 0.031 |
| Cough ** | |||
| No | 21 (14.0%) | 3 (8.8%) | 0.418 |
| Yes | 129 (86.0%) | 31 (91.2%) | |
| Dyspnea ** | |||
| No | 121 (80.7%) | 18 (52.9%) | <0.001 |
| At rest and in motion | 9 (6.0%) | 11 (32.4%) | |
| Only in motion | 20 (13.3%) | 5 (14.7%) | |
| Ageusia or Anosmia ** | |||
| No | 65 (43.3%) | 23 (67.6%) | 0.010 |
| Yes | 85 (56.7%) | 11 (32.4%) | |
| Fatigue ** | |||
| No | 30 (20.0%) | 11 (32.4%) | 0.118 |
| Yes | 120 (80.0%) | 23 (67.6%) | |
| Myalgia ** | |||
| No | 49 (32.7%) | 15 (44.1%) | 0.206 |
| Yes | 101 (67.3%) | 19 (55.9%) | |
| Headache ** | |||
| No | 78 (52.0%) | 26 (76.5%) | 0.009 |
| Yes | 72 (48.0%) | 8 (23.5%) | |
| Others ** | |||
| No | 67 (45.3%) | 19 (55.9%) | 0.336 |
| Yes | 81 (54.7%) | 21 (44.1%) | |
| Number of Symptoms ** | 4.0 (3.0–6.0) | 4.0 (3.0–5.0) | 0.127 |
| SpO2 at Enrolment | 98.0 (97.0–100.0) | 96.0 (95.0–98.0) | <0.001 |
| Minimum SpO2 during the course | 96.0 (95.0–97.0) | 93.0 (90.0–94.0) | <0.001 |
| Maximum Mews during the course | 1.0 (0.0–2.0) | 5.0 (3.0–7.0) | 0.001 |
| Maximum LUS during the course | 4.5 (1.5–6.5) | 12.0 (8.0–14.0) | 0.003 |
| Minimum SpO2 at Walking Test | 97.0 (95.0–99.0) | 93.0 (90.5–97.0) | <0.001 |
| Pidotimod Therapy | |||
| No | 64 (42.7%) | 23 (67.6%) | 0.008 |
| Yes | 86 (57.3%) | 11 (32.4%) | |
| Pidotimod Start Day | 3.0 (2.0–6.0) | 4.0 (1.0–6.0) | 0.792 |
| Pidotimod Duration | 7.0 (0.0–10.0) | 0.0 (0.0–2.0) | <0.001 |
| Corticosteroid Therapy | |||
| No | 102 (68.5%) | 17 (50.0%) | 0.042 |
| Yes | 47 (31.5%) | 17 (50.0%) | |
| Corticosteroid Start Day | 8.5 (8.0–11.0) | 7.0 (4.5–8.0) | <0.001 |
| ORc | 95%CI | p-Value | |
|---|---|---|---|
| Gender (M vs. F) | 1.431 | [0.678, 3.020] | 0.346 |
| Age (years) | 1.064 | [1.036, 1.092] | <0.0001 |
| BMI (kg/m2) | 1.220 | [1.059, 1.427] | 0.007 |
| Illness Duration (days) | 1.073 | [1.031, 1.116] | <0.0001 |
| Comorbidity (Yes vs. No) | 4.747 | [1.581, 14.250] | 0.005 |
| Dyspnea (Yes vs. No) | 1.648 | [1.034, 2.624] | 0.035 |
| Ageusia or Anosmia (Yes vs. No) | 0.365 | [0.166, 0.804] | 0.012 |
| Headache (Yes vs. No) | 0.333 | [0.141, 0.783] | 0.012 |
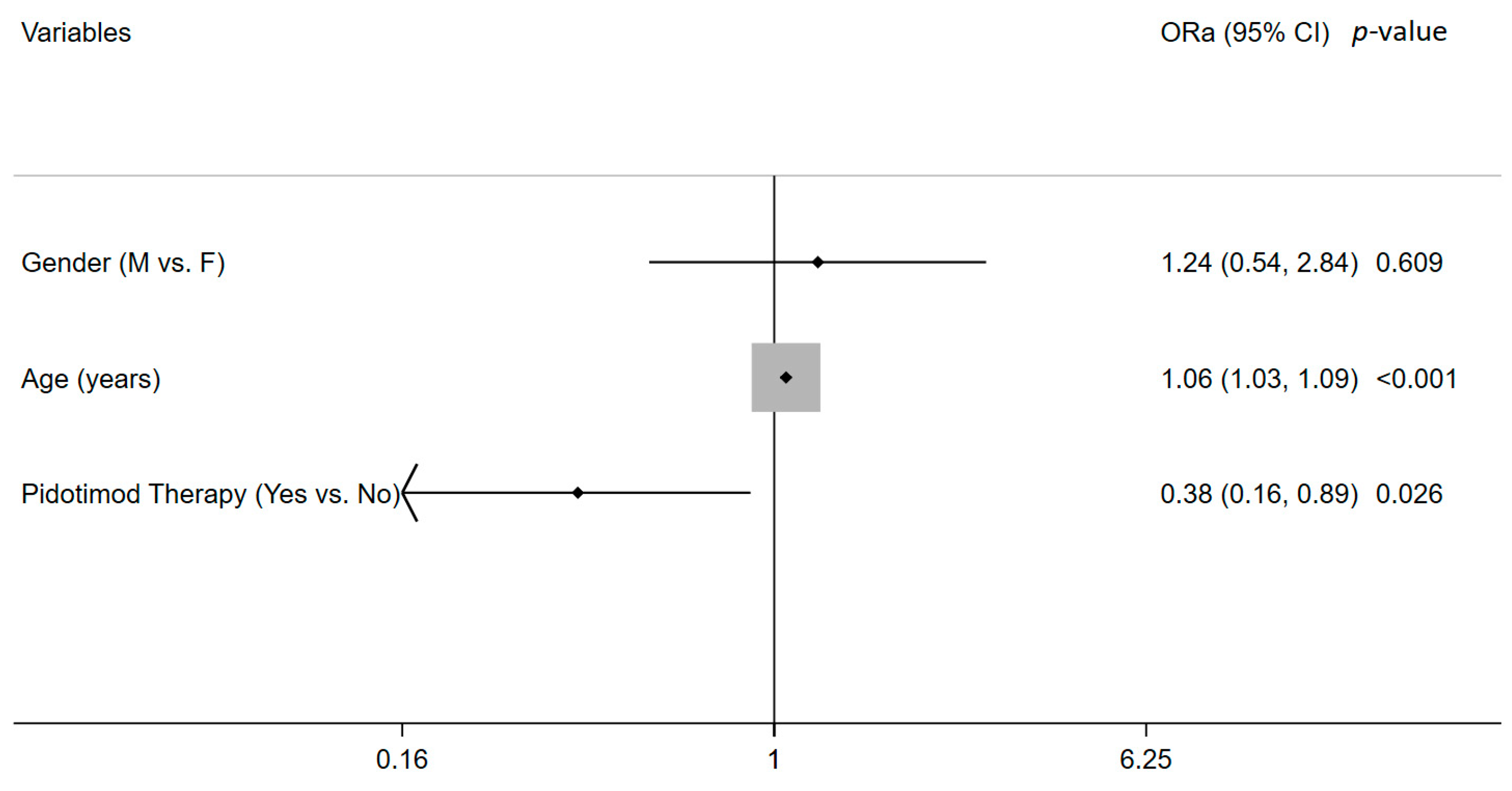
| Pidotimod Therapy (Yes vs. No) | 0.355 | [0.161, 0.782] | 0.010 |
| Corticosteroid Therapy (Yes vs. No) | 2.170 | [1.019, 4.621] | 0.045 |
| Coeff | 95%CI | p-Value | |
|---|---|---|---|
| Gender | −0.970 | [−3.825, 1.879] | 0.502 |
| Age (years) | 0.163 | [0.078, 0.248] | <0.0001 |
| Pidotimod Therapy (Yes) | −4.190 | [−7.041, −1.340] | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ucciferri, C.; Di Gasbarro, A.; Borrelli, P.; Di Nicola, M.; Vecchiet, J.; Falasca, K. New Therapeutic Options in Mild Moderate COVID-19 Outpatients. Microorganisms 2022, 10, 2131. https://doi.org/10.3390/microorganisms10112131
Ucciferri C, Di Gasbarro A, Borrelli P, Di Nicola M, Vecchiet J, Falasca K. New Therapeutic Options in Mild Moderate COVID-19 Outpatients. Microorganisms. 2022; 10(11):2131. https://doi.org/10.3390/microorganisms10112131
Chicago/Turabian StyleUcciferri, Claudio, Alessandro Di Gasbarro, Paola Borrelli, Marta Di Nicola, Jacopo Vecchiet, and Katia Falasca. 2022. "New Therapeutic Options in Mild Moderate COVID-19 Outpatients" Microorganisms 10, no. 11: 2131. https://doi.org/10.3390/microorganisms10112131
APA StyleUcciferri, C., Di Gasbarro, A., Borrelli, P., Di Nicola, M., Vecchiet, J., & Falasca, K. (2022). New Therapeutic Options in Mild Moderate COVID-19 Outpatients. Microorganisms, 10(11), 2131. https://doi.org/10.3390/microorganisms10112131








