Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives
Abstract
1. Introduction
2. Materials and Methods
2.1. Olive Processing on a Pilot Plant Scale
2.1.1. Short-Term Incubation Tests in Anaerobiosis and spCO2 Conditions
2.1.2. SEM Observation
2.1.3. Long-Term Incubation Tests in Anaerobiosis and spCO2Conditions
2.1.4. Physico-Chemical Analysis of the Brine
2.1.5. Microbiological Analysis of the Brine
2.2. Laboratory Tests with Oleuropeinolytic Yeast Species
2.2.1. Yeast Growth under Different Conditions in the Absence of NaCl
2.2.2. Yeast Growth under Different Conditions in the Presence of NaCl
2.2.3. Enzymatic Activity of Yeasts Grown under Different Conditions
β-Glucosidase and Esterase Activities of Yeasts at pH 4 and 7 in the Absence of NaCl
β-Glucosidase and Esterase Activities of Yeasts Grown in Aerobic and spCO2 Conditions in the Presence of NaCl
2.3. Statistical Analysis
3. Results and Discussion
3.1. Pilot Plant Tests
3.1.1. Short-Term Fermentation of Naturally Processed Black Table Olive under Anaerobic and spCO2 Conditions
3.1.2. Long-Term Processing of Black Table Olives under Anaerobic and spCO2 Conditions
3.2. Laboratory Tests
3.2.1. Yeast Growth under Different Conditions in the Absence of NaCl
3.2.2. Yeast Growth under Different Conditions in the Presence of NaCl
3.2.3. Enzymatic Activity of Yeast Grown under Different Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dixon, N.M.; Kell, D.B. The inhibition by CO2 of the growth and metabolism of micro-organisms. J. Appl. Bacteriol. 1989, 67, 106–136. [Google Scholar] [CrossRef] [PubMed]
- Knoche, W. Chemical reactions of CO2 in water. In Biophysics and Physiology of Carbon Dioxide; Bauer, C., Gros, G., Bartels, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; pp. 3–11. [Google Scholar]
- Haas, G.J.; Prescott, H.E.; Dudley, E.; Dik, R.; Hintlian, C.; Keane, L. Inactivation of microorganisms by carbon dioxide under pressure. J. Food Saf. 1989, 9, 253–265. [Google Scholar] [CrossRef]
- Wei, C.I.; Balabam, M.O.; Fernando, S.Y.; Peplow, A.J. Bacterial effect of high pressure CO2 treatment on foods spiked with Listeria or Salmonella. J. Food Prot. 1991, 54, 189–193. [Google Scholar] [CrossRef] [PubMed]
- James, A.D.; Rajagopalan, K.; Syed, S.N.R. A review of effects of carbon dioxide on microbial growth and food quality. J. Food Prot. 1985, 48, 532–537. [Google Scholar]
- Yu, T.; Niu, L.; Iwahashi, H. High-pressure carbon dioxide used for pasteurization in food industry. Food Eng. Rev. 2020, 12, 364–380. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Sacconi, A.; Calamai, L.; Parenti, A. Effects of a small increase in carbon dioxide pressure during fermentation on wine aroma. Foods 2020, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, R.G. The inhibition of the growth of meat-attacking fungi by carbon dioxide. J. Soc. Chem. Ind. 1932, 51, 261–267. [Google Scholar]
- Wodzinski, R.J.; Frazier, W.C. Moisture requirements of bacteria IV: Influence of temperature and increased partial pressure of carbon dioxide on requirements of three species of bacteria. J. Bacteriol. 1961, 81, 401–408. [Google Scholar] [CrossRef]
- Garrido Fernández, A.; Fernández Diaz, M.J.; Adams, M.R. Table Olives: Production and Processing, 1st ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Ciafardini, G.; Zullo, B.A. Use of selected yeast starter cultures in industrial-scale processing of brined Taggiasca black table olives. Food Microbiol. 2019, 84, 103250. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Reducing Salt Intake in Populations: Report of a WHO Forum and Technical Meeting; WHO Document Publication Services: Geneva, Switzerland, 2007; Available online: https://www.who.int/dietphysicalactivity/Salt_Report_VC_april07.pdf (accessed on 10 July 2021).
- Chrysant, S.G. Effects of high salt intake on blood pressure and cardiovascular disease: The role of COX inhibitors. Clin. Cardiol. 2016, 39, 240–242. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Romero-Gil, V.; Rodríguez-Gómez, F.; García-García, P.; Garrido-Fernández, A. Microbial stability and quality of seasoned cracked green Aloreña table olives packed in diverse chloride salt mixtures. J. Food Prot. 2013, 76, 1923–1932. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Use of air-protected headspace to prevent yeast film formation on the brine of Leccino and Taggiasca black table olives processed in industrial-scale plastic barrels. Foods 2020, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Heperkan, D. Microbiota of the table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Zullo, B.A. Virgin olive oil yeasts: A review. Food Microbiol. 2018, 70, 245–253. [Google Scholar] [CrossRef]
- Ghoddusi, H.B.; Sherburn, R.E.; Aboaba, O.O. Growth limiting pH, water activity, and temperature for neuroxigenic strains of Clostridium butyricum. ISRN Microbiol. 2013, 2013, 731430. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Differential microbial composition of monovarietal and blended extra virgin olive oil determines oil quality during storage. Microorganisms 2020, 8, 402. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification of clinical important ascomycetous yeast based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997, 5, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Cadež, N.; Raspor, P.; Turchetti, B.; Cardinali, G.; Ciafardini, G.; Veneziani, G.; Péter, G. Candida adriatica sp. nov. and Candida molendinolei sp. nov., two yeast species isolated from olive oil ant its by-product. Int. Syst. Evol. Microbiol. 2012, 62, 2296–2302. [Google Scholar] [CrossRef]
- Montaño, A.; Cortés-Degado, A.; Sánchez, A.H.; Ruiz-Barba, J.L. Production of volatile compounds by wild-type yeasts in a natural olive-derived culture medium. Food Microbiol. 2021, 98, 103788. [Google Scholar] [CrossRef] [PubMed]
- Porru, C.; Rodríguez-Gómez, F.; Benítez-Cabello, A.; Jiménez-Díaz, R.; Zara, G.; Budroni, M.; Mannazzu, I.; Arroyo-López, F.N. Genotyping, identification and multifunctional features of yeasts associated to Bosana naturally black table olive fermentations. Food Microbiol. 2018, 69, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Paramithiotis, S.; Panagou, E.Z. Evolution of yeast consortia during the fermentation of Kalamata natural black olives upon two initial acidification treatments. Front. Microbiol. 2017, 8, 2673. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Venditti, G.; Zullo, B.A. Yeast dynamics in the black table olives processing using fermented brine as starter. Food Res. 2021, 5, 92–106. [Google Scholar] [CrossRef]
- Sidari, R.; Martorana, A.; De Bruno, A. Effect of brine composition on yeast biota associated with naturally fermented Nocellara messinese table olives. Food Sci. Technol. 2019, 109, 163–170. [Google Scholar] [CrossRef]
- Leventdurur, S.; Sert-Aydin, S.; Boyaci-Gunduz, C.P.; Agirman, B.; Ben Ghorbal, A.; Francesca, N.; Martorana, A.; Erten, H. Yeast biota of naturally fermented black olives in different brines made from cv. Gemlikgrown in various districts of the Cukurova region of Turkey. Yeast 2016, 33, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Zullo, B.A.; Antonielli, L.; Corte, L.; Roscini, L.; Cardinali, G. Yamadazyma terventina sp. nov., a yeast species of the Yamadazyma clade from Italian olive oils. Int. Syst. Evol. Microbiol. 2013, 63, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, S.; Mari, E.; Barbato, D.; Granchi, L. Extra virgin olive oil quality as affected by yeast species occurring in the extraction process. Foods 2019, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial application to improve olive mill wastewater phenolic extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Bremer, E.; Csonka, L.N.; Kraemer, R.; Poolman, B.; van der Heide, T.; Smith, L.T. Osmosensing and osmoreguladory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2001, 130, 437–460. [Google Scholar] [CrossRef]
- Csonka, L.N. Physiological and genetic response of bacteria to osmotic-stress. Microbiol. Rev. 1989, 53, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Poolman, B.; Blount, P.; Folgering, J.H.A.; Friesen, R.H.E.; Moe, P.C.; van der Heide, T. How do membrane proteins sense water stress? Mol. Microbiol. 2002, 44, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D. Formation of viable but not culturable cells. In Starvation in Bacteria; Kjelleberg, S., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 239–272. [Google Scholar]
- Branda, S.S.; Vik, A.; Friedman, L.; Kolter, R. Biofilms: The matrix revised. Trends Microbiol. 2020, 13, 2026. [Google Scholar]
- Debs-Louka, E.; Louka, N.; Abraham, G.; Chabot, V.; Allaf, K. Effect of compressed carbon dioxide on microbial cell viability. Appl. Environ. Microbiol. 1999, 65, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Oura, E.; Haarasilta, S.; Londesborough, J. Carbon dioxide fixation by baker’s yeast in a variety of growth conditions. J. Gen. Microbiol. 1980, 118, 51–58. [Google Scholar] [CrossRef][Green Version]
- Wei, C.J.; Tanner, R.D.; Malaney, G.W. Effect of sodium chloride on bakers’ yeast growing in gelatin. Appl. Environ. Microbiol. 1982, 43, 757–763. [Google Scholar] [CrossRef]
- Sirilun, S.; Chaiyavat, C.; Peng Kumsri, N.; Peerajan, S.; Chaiyasut, K.; Suwannalert, P.; Sivamaruthi, B.S. Screening of β-glucosidase production by Saccharomyces cerevisiae. J. Appl. Pharm. Sci. 2016, 6, 29–35. [Google Scholar] [CrossRef][Green Version]
- IOC, International Olive Council. Trade Standard Applying to Table Olives; International Olive Council: Madrid, Spain, 2004. [Google Scholar]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Bact. 1994, 77, 519–527. [Google Scholar] [CrossRef]

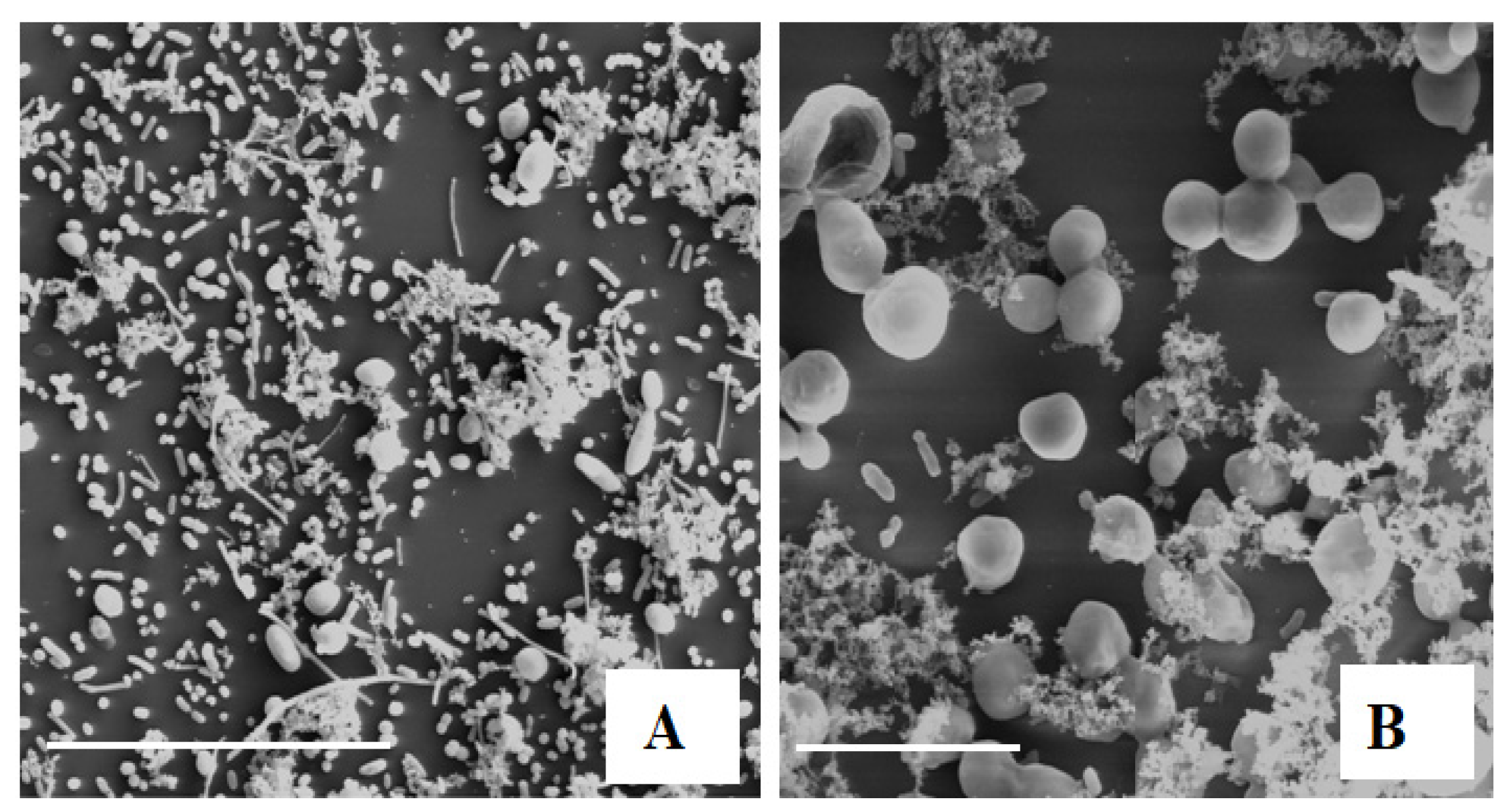
 ), anaerobiosis (
), anaerobiosis ( ), and slightly pressurized CO2 (
), and slightly pressurized CO2 ( ) conditions. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; and Y.t., Yamadazyma terventina.
) conditions. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; and Y.t., Yamadazyma terventina.
 ), anaerobiosis (
), anaerobiosis ( ), and slightly pressurized CO2 (
), and slightly pressurized CO2 ( ) conditions. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; and Y.t., Yamadazyma terventina.
) conditions. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; and Y.t., Yamadazyma terventina.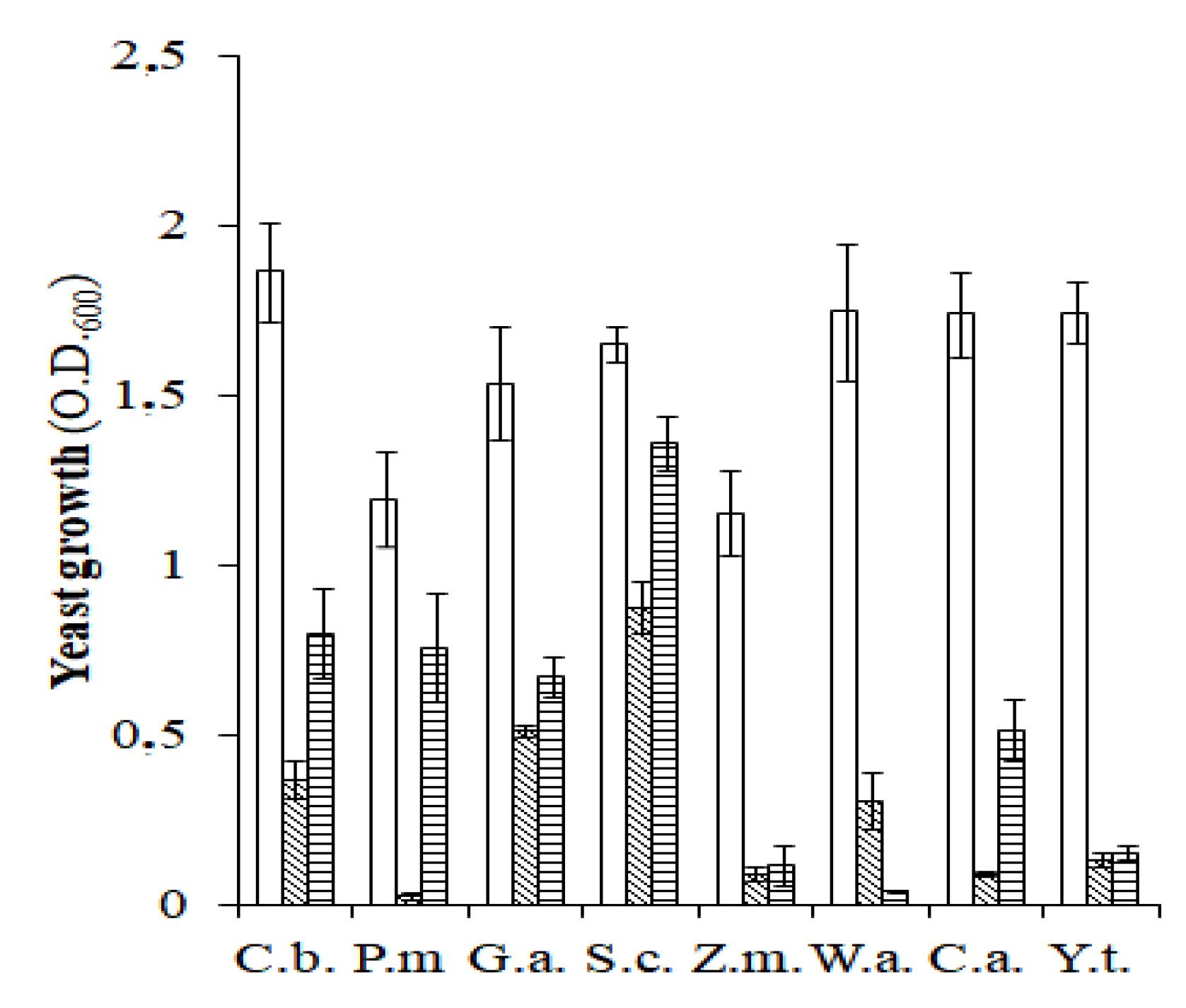
 ), 5 (
), 5 ( ), 8 (
), 8 ( ), and 11% (
), and 11% ( ) of NaCl. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; Y.t., Yamadazyma terventina.
) of NaCl. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; Y.t., Yamadazyma terventina.
 ), 5 (
), 5 ( ), 8 (
), 8 ( ), and 11% (
), and 11% ( ) of NaCl. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; Y.t., Yamadazyma terventina.
) of NaCl. C.b., Candida boidinii; P.m., Pichia manshurica; G.a., Groenewaldozyma auringiensis; S.c., Saccharomyces cerevisiae; Z.m., Zygotorulaspora mrakii; W.a., Wickerhamomyces anomalus; C.a., Candida adriatica; Y.t., Yamadazyma terventina.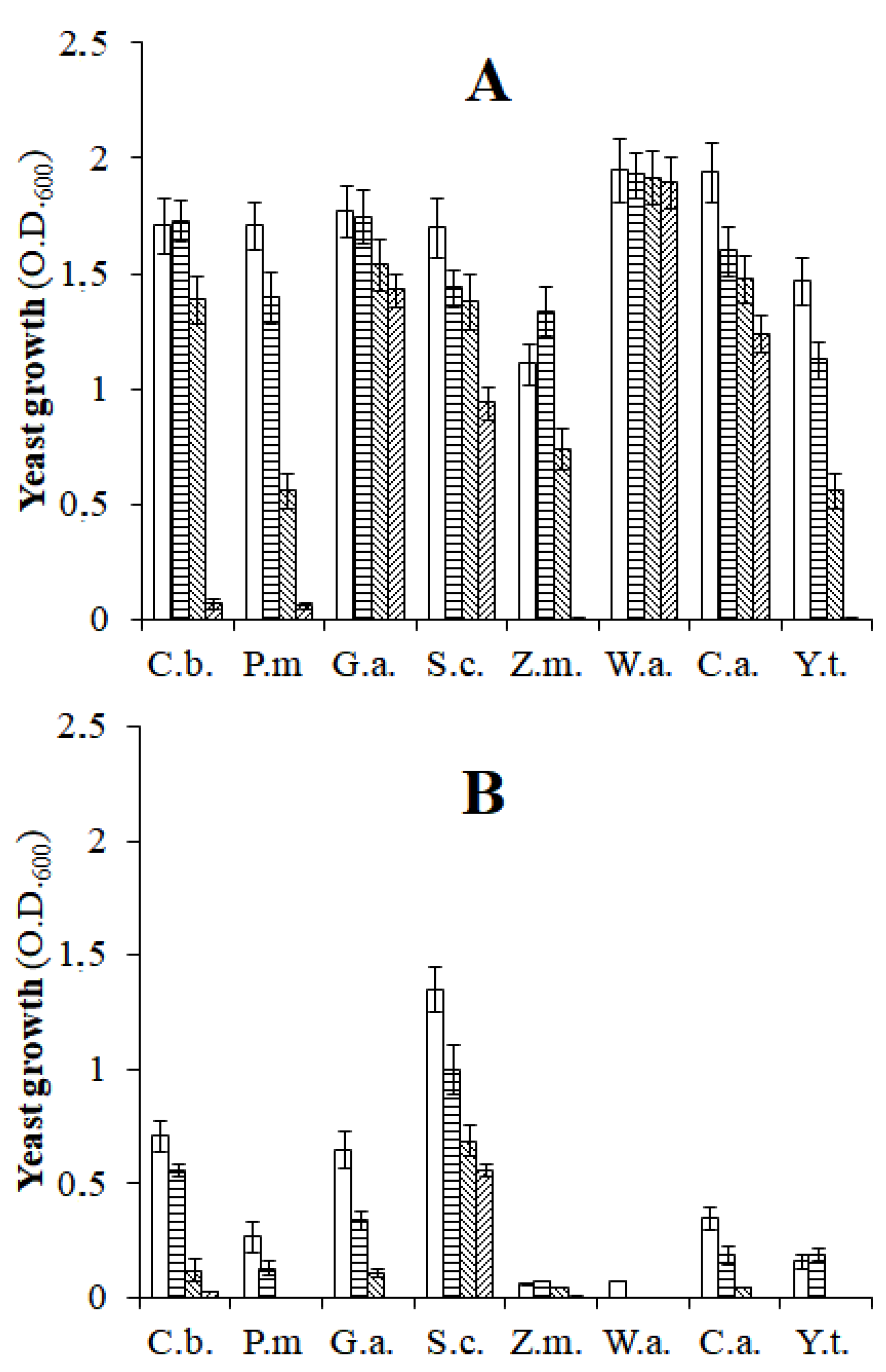
 ) and 7 (
) and 7 ( ) of six yeast strains from naturally processed black table olives.
) of six yeast strains from naturally processed black table olives.
 ) and 7 (
) and 7 ( ) of six yeast strains from naturally processed black table olives.
) of six yeast strains from naturally processed black table olives.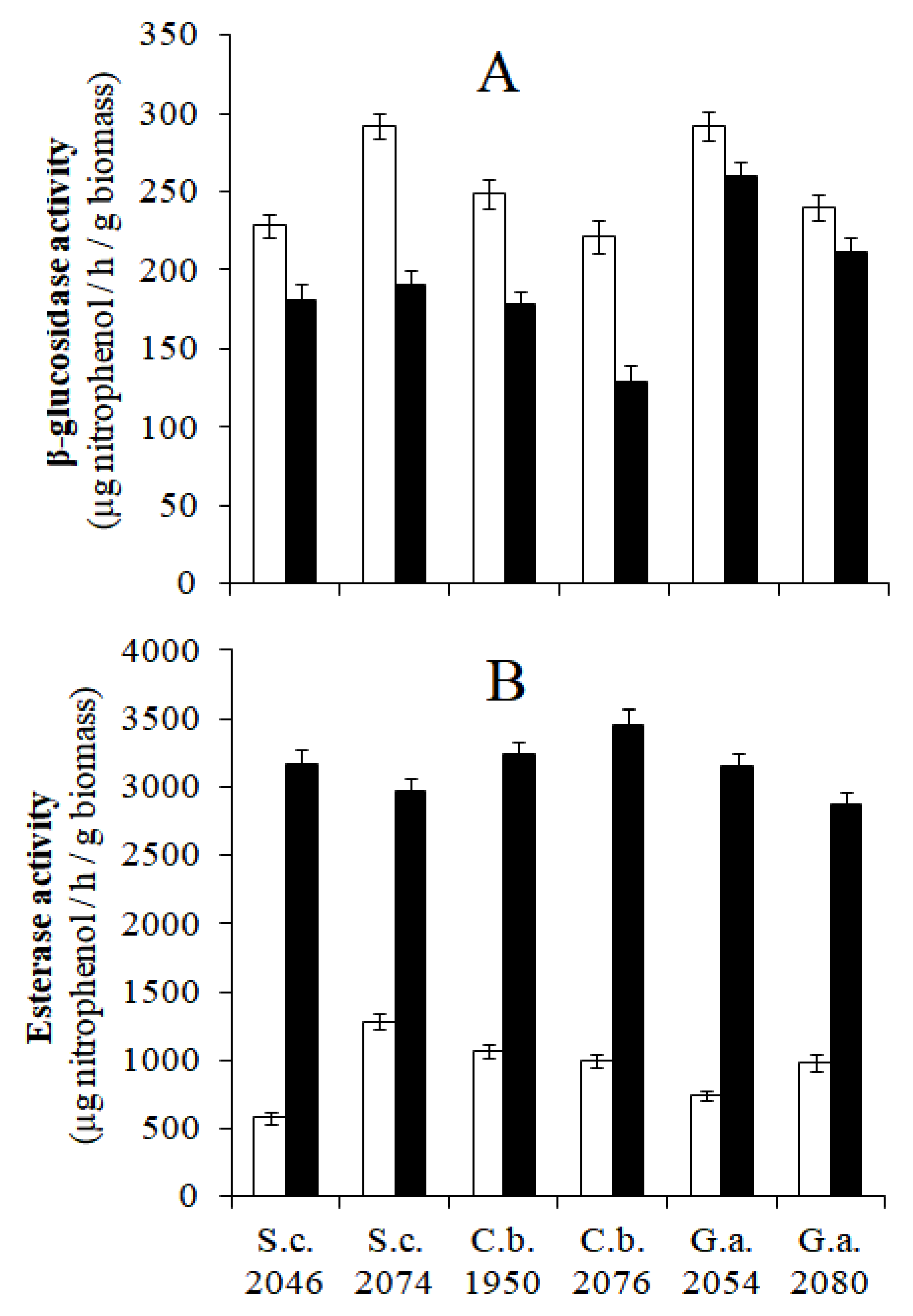
 , S. cerevisiae 2046;
, S. cerevisiae 2046;  , S. cerevisiae 2074;
, S. cerevisiae 2074;  , C. boidinii 1950;
, C. boidinii 1950;  , C. boidinii 2076;
, C. boidinii 2076;  , G. auringiensis 2054;
, G. auringiensis 2054;  , G. auringiensis 2080).
, G. auringiensis 2080).
 , S. cerevisiae 2046;
, S. cerevisiae 2046;  , S. cerevisiae 2074;
, S. cerevisiae 2074;  , C. boidinii 1950;
, C. boidinii 1950;  , C. boidinii 2076;
, C. boidinii 2076;  , G. auringiensis 2054;
, G. auringiensis 2054;  , G. auringiensis 2080).
, G. auringiensis 2080).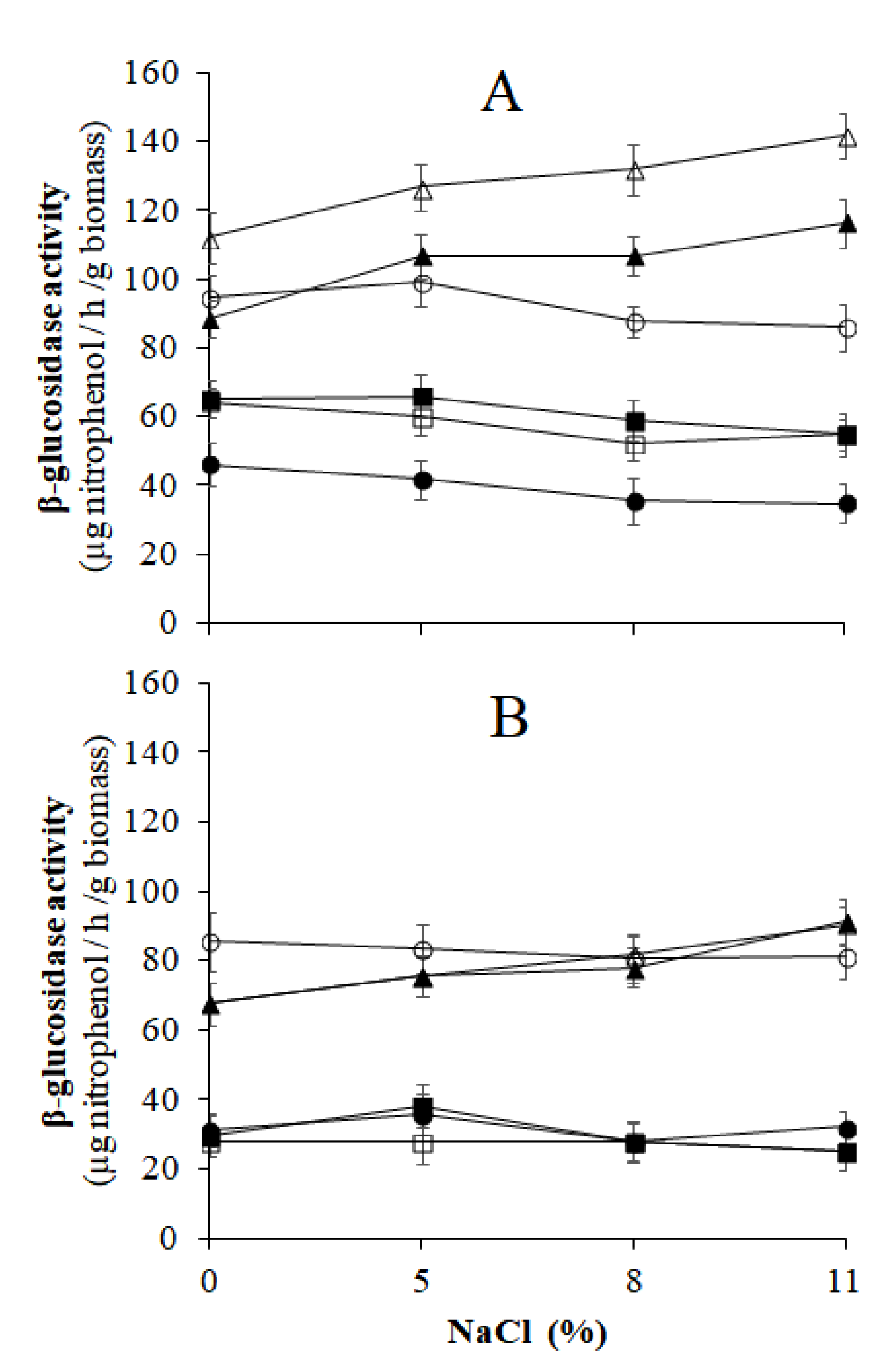
 , S. cerevisiae 2046;
, S. cerevisiae 2046;  , S. cerevisiae 2074;
, S. cerevisiae 2074;  , C. boidinii 1950;
, C. boidinii 1950;  , C. boidinii 2076;
, C. boidinii 2076;  , G. auringiensis 2054;
, G. auringiensis 2054;  , G. auringiensis 2080).
, G. auringiensis 2080).
 , S. cerevisiae 2046;
, S. cerevisiae 2046;  , S. cerevisiae 2074;
, S. cerevisiae 2074;  , C. boidinii 1950;
, C. boidinii 1950;  , C. boidinii 2076;
, C. boidinii 2076;  , G. auringiensis 2054;
, G. auringiensis 2054;  , G. auringiensis 2080).
, G. auringiensis 2080).
| Yeast | Source | Oleuropeinolytic Properties | Reference |
|---|---|---|---|
| Candida adriatica | Leccino virgin olive oil | + | [21,22], this study |
| Manzanilla, Leccino table olive brine | |||
| Candida boidinii | Bosana, Manzanilla, Taggiasca table olive brine | ++ | [22,23], unpublished data |
| Groenewaldozyma auringiensis | Taggiasca table olive brine | + | [Unpublished data] |
| Pichia manshurica | Kalamata, Taggiasca table olive brine | ++ | [24,25] |
| Saccharomyces cerevisiae | Bosana, Nocellara messinese, Taggiasca, Leccino table olive brine | + | [23,25,26], this study |
| Wickerhamomyces anomalus | Gemlik, Bosana, Nocellara messinese, Taggiasca table olive brine | + | [23,26,27], unpublished data |
| Yamadazyma terventina | Leccino, Frantoio, and Moraiolo virgin olive oil | + | [28,29] |
| Zygotorulaspora mrakii | Bosana, Manzanilla, Taggiasca, Leccino table olive brine | + | [22,23,25], this study |
| NaCl Concentration (%, w v−1) | Anaerobiosis | spCO2 2 | ||||
|---|---|---|---|---|---|---|
| Total Yeasts 1 | Total Bacteria | Total Moulds | Total Yeasts | Total Bacteria | Total Moulds | |
| 0 | 5.77±0.18 a | 6.34 ± 0.25 a | 1.85 ± 0.19 | 3.74 ± 0.07 a | n.d. | n.d. |
| 2 | 5.96 ± 0.01 a | 5.94 ± 0.12 ab | n.d. | 2.65 ± 0.09 b | n.d. | n.d. |
| 5 | 4.88 ± 0.13 ab | 4.37 ± 0.14 b | n.d. | 1.65 ± 0.03 c | n.d. | n.d. |
| 8 | 4.19 ± 0.07 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 11 | 3.62 ± 0.08 c | n.d. | n.d. | n.d. | n.d. | n.d. |
| Month | Brine Temperature (°C) | Total Yeasts (Log CFU/mL) | Preeminent Yeast Species 2 | Total Aerobic Bacteria (Log CFU/mL) | Total Molds (Log CFU/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Anaerobiosis | spCO2 1 | Anaerobiosis | spCO2 | Anaerobiosis | spCO2 | Anaerobiosis | spCO2 | ||
| 0 | 0.89 ± 0.12 d | 0.89 ± 0.12 d | 2.49 ± 0.02 a | 2.49 ± 0.02 | 1.09 ± 0.20 b | 1.09 ± 0.20 | |||
| 1 | 10.5 | 0.58 ± 0.06 d | n.d. | n.d. | 1.05 ± 0.03 b | n.d. | n.d. | n.d. | |
| 2 | 9.3 | 0.70 ± 0.02 d | n.d. | Z.m. (60) | n.d. | 1.25 ± 0.08 b | n.d. | n.d. | n.d. |
| 3 | 9.7 | 0.80 ± 0.02 d | n.d. | C.a. (50) | n.d. | 0.75 ± 0.01 c | n.d. | n.d. | n.d. |
| 4 | 12.2 | 1.60 ± 0.10 c | n.d. | C.a. (52) | n.d. | 0.62 ± 0.05 c | n.d. | 1.47 ± 0.29 ab | n.d. |
| 5 | 14.5 | 1.88 ± 0.23 c | n.d. | Z.m. (51) | n.d. | n.d. | n.d. | 1.90 ± 0.19 a | n.d. |
| 6 | 15.3 | 3.60 ± 0.32 b | 2.30 ± 0.22 b | C.a. (58) | S.c. (80) | n.d. | n.d. | 2.03 ± 0.23 a | n.d. |
| 7 | 18.5 | 4.33 ± 0.15 ab | 3.48 ± 0.11 a | C.a. (54) | S.c. (85) | n.d. | n.d. | 1.87 ± 0.10 a | n.d. |
| 8 | 20.8 | 4.71 ± 0.22 ab | 3.05 ± 0.02 a | C.a. (57) | S.c. (87) | n.d. | n.d. | 1.97 ± 0.12 a | n.d. |
| 9 | 22.5 | 5.31 ± 0.22 a | 3.32 ± 0.12 a | S.c. (60) | S.c. (100) | n.d. | n.d. | 1.77 ± 0.09 a | n.d. |
| 10 | 16.5 | 5.60 ± 0.12 a | 3.41 ± 0.10 a | S.c. (62) | S.c. (100) | n.d. | n.d. | 1.80 ± 0.20 a | n.d. |
| 11 | 12.5 | 5.80 ± 0.09 a | 3.65 ± 0.07 a | S.c. (70) | S.c. (100) | n.d. | n.d. | 1.74 ± 0.11 a | n.d. |
| 12 | 11.8 | 5.98 ± 0.05 a | 3.76 ± 0.11 a | S.c. (75) | S.c. (100) | n.d. | n.d. | 1.84 ± 0.11 a | n.d. |
| Month | pH | Titratable Acidity (g Lactic Acid/L) | Total Phenols (mg CAE 1/mL) | ||||
|---|---|---|---|---|---|---|---|
| Anaerobiosis | spCO2 2 | Anaerobiosis | spCO2 | Anaerobiosis | spCO2 | ∆ 3 | |
| 1 | 4.10 ± 0.10 | 3.44 ± 0.09 | 7.16 ± 0.12 | 7.47 ± 0.11 | 2.46 ± 0.07 a 4 a 5 | 1.08 ± 0.09 b b | −56 |
| 2 | 4.09 ± 0.08 | 3.83 ± 0.13 | 7.29 ± 0.12 | 7.48 ± 0.13 | 2.49 ± 0.01 a a | 2.21 ± 0.01 ab a | −11 |
| 3 | 3.98 ± 0.08 | 3.90 ± 0.10 | 7.40 ± 0.15 | 7.35 ± 0.12 | 2.50 ± 0.03 a a | 2.45 ± 0.04 a a | −2 |
| 4 | 4.08 ± 0.08 | 3.93 ± 0.10 | 7.35 ± 0.12 | 7.25 ± 0.11 | 1.90 ± 0.02 a b | 2.50 ± 0.02 a a | 32 |
| 5 | 4.00 ± 0.08 | 3.95 ± 0.11 | 7.43 ± 0.17 | 7.45 ± 0.15 | 1.98 ± 0.04 a b | 2.65 ± 0.03 a a | 34 |
| 6 | 3.88 ± 0.08 | 3.98 ± 0.10 | 7.40 ± 0.13 | 7.55 ± 0.12 | 2.00 ± 0.01 a b | 2.80 ± 0.01 a a | 40 |
| 7 | 3.96 ± 0.12 | 4.00 ± 0.11 | 7.77 ± 0.19 | 7.73 ± 0.20 | 2.30 ± 0.03 a b | 2.90 ± 0.01 a a | 26 |
| 8 | 3.90 ± 0.04 | 4.02 ± 0.07 | 7.70 ± 0.17 | 7.23 ± 0.14 | 2.23 ± 0.04 a b | 3.08 ± 0.03 a a | 38 |
| 9 | 3.88 ± 0.08 | 3.93 ± 0.10 | 7.43 ± 0.12 | 7.34 ± 0.10 | 2.20 ± 0.02 a b | 2.75 ± 0.02 a a | 25 |
| 10 | 3.98 ± 0.08 | 3.93 ± 0.10 | 7.30 ± 0.17 | 7.05 ± 0.15 | 1.90 ± 0.025 ab b | 2.65 ± 0.03 a a | 39 |
| 11 | 4.20 ± 0.06 | 4.00 ± 0.11 | 6.98 ± 0.10 | 7.29 ± 0.14 | 1.85 ± 0.02 b c | 2.53 ± 0.01 a a | 37 |
| 12 | 4.23 ± 0.10 | 4.16 ± 0.13 | 7.40 ± 0.14 | 8.10 ± 0.12 | 1.72 ± 0.01 b c | 2.50 ± 0.04 a a | 45 |
| Mean value | 4.02 ± 0.12 | 3.92 ± 0.17 | 7.38 ± 0.21 | 7.44 ± 0.27 | 2.13 ± 0.27 | 2.51 ± 0.50 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zullo, B.A.; Ciafardini, G. Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives. Microorganisms 2022, 10, 2049. https://doi.org/10.3390/microorganisms10102049
Zullo BA, Ciafardini G. Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives. Microorganisms. 2022; 10(10):2049. https://doi.org/10.3390/microorganisms10102049
Chicago/Turabian StyleZullo, Biagi Angelo, and Gino Ciafardini. 2022. "Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives" Microorganisms 10, no. 10: 2049. https://doi.org/10.3390/microorganisms10102049
APA StyleZullo, B. A., & Ciafardini, G. (2022). Use of Slightly Pressurized Carbon Dioxide to Enhance the Antimicrobial Properties of Brines in Naturally Processed Black Table Olives. Microorganisms, 10(10), 2049. https://doi.org/10.3390/microorganisms10102049





