Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Extraction of Exopolysaccharides
2.2. Animal Model and Treatment
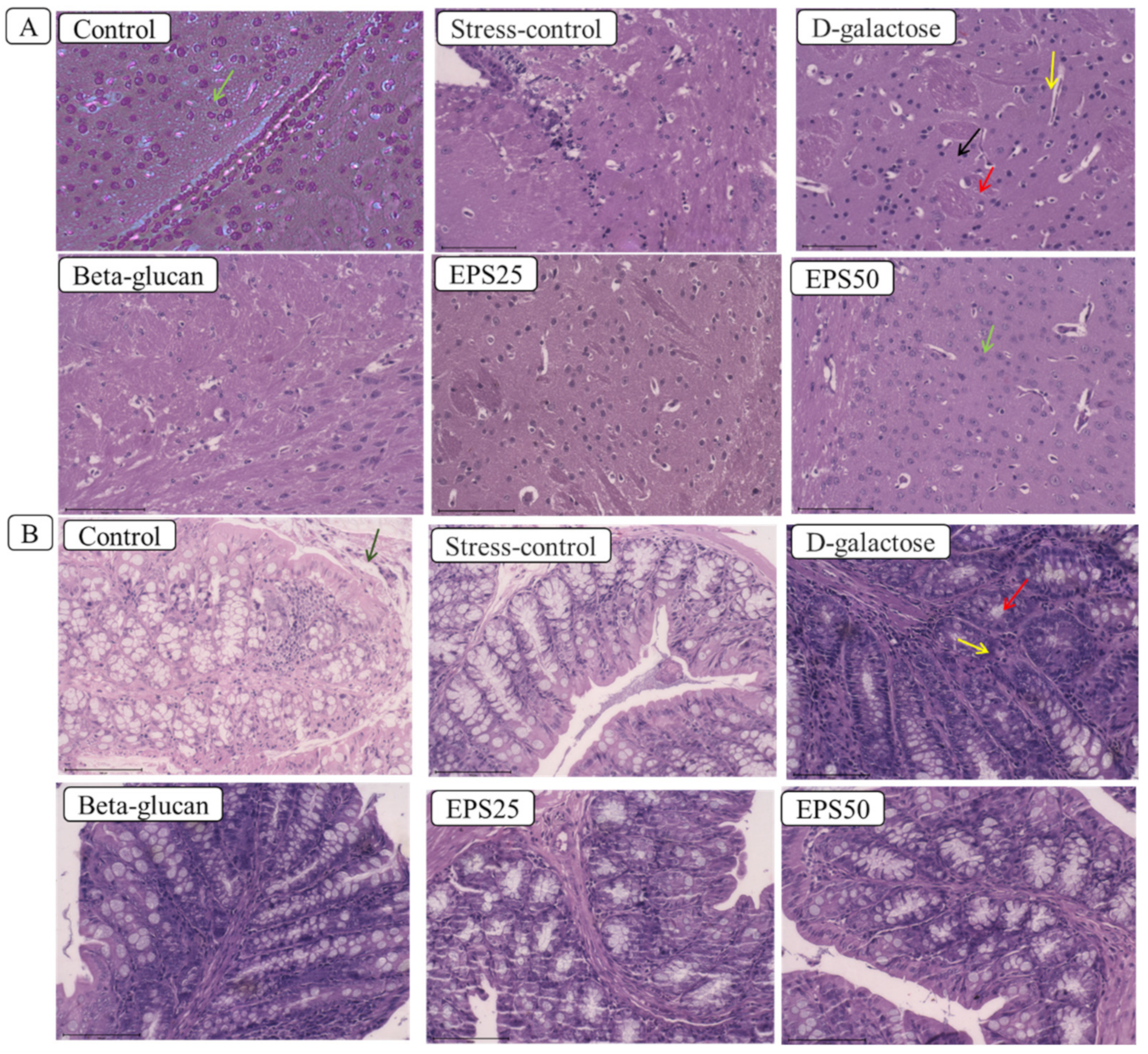
2.3. Histological Analysis
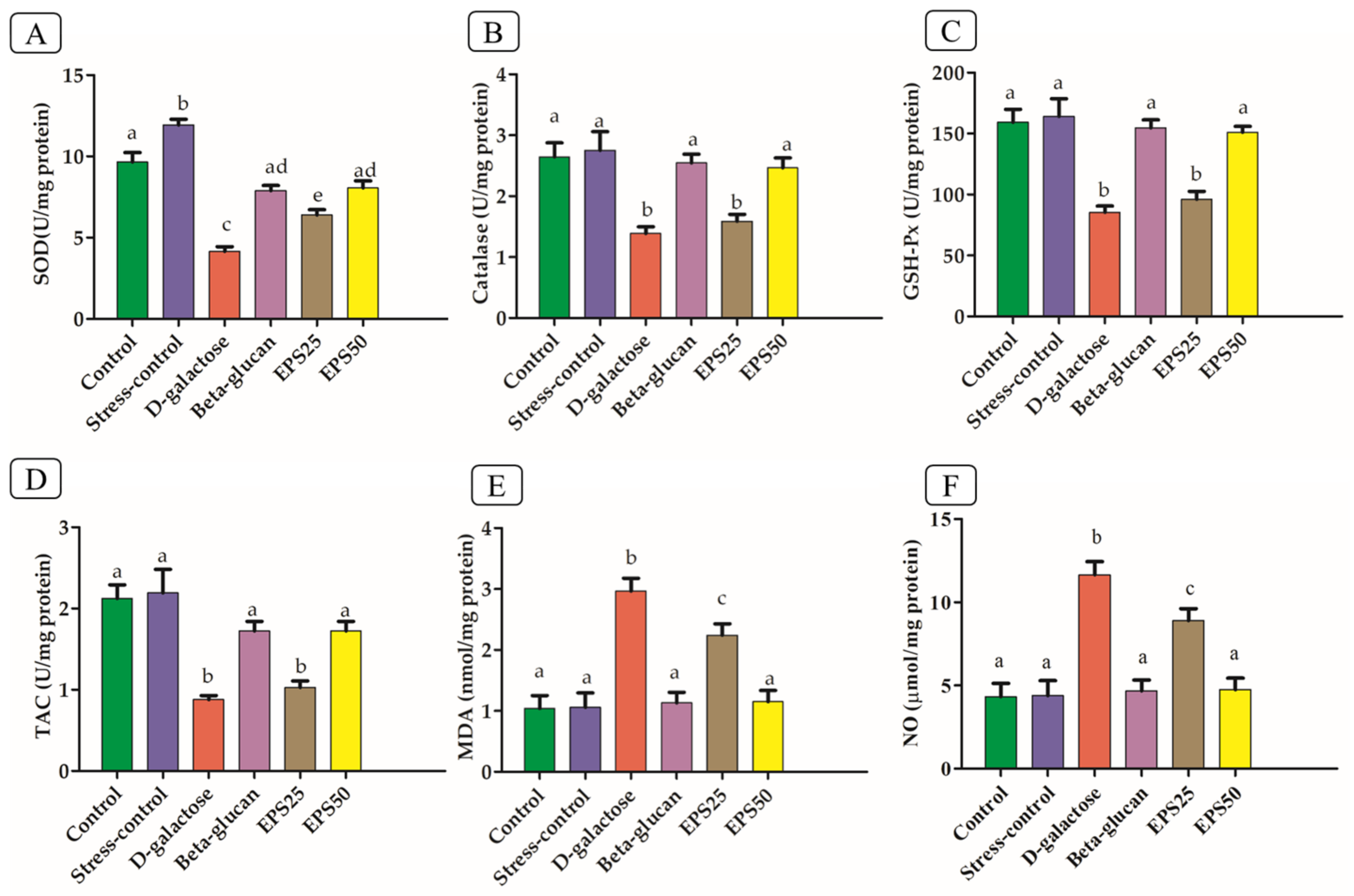
2.4. Determination of SOD, CAT, GSH-Px, TAC, MDA and NO
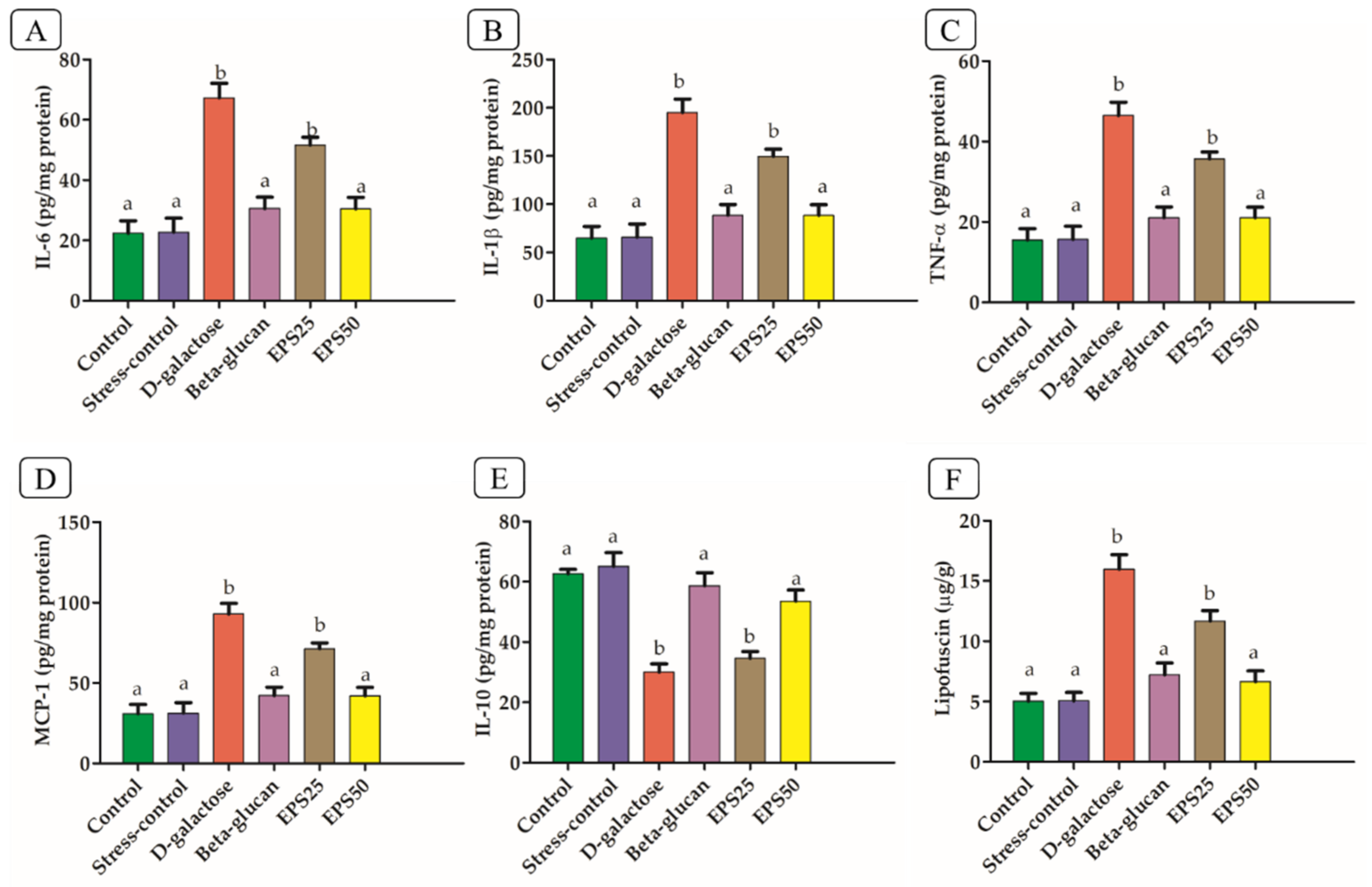
2.5. Measurement of IL- IL-6, IL-1β, TNF-α, MCP-1, and 1L-10
2.6. Measurement of Lipofuscin Content
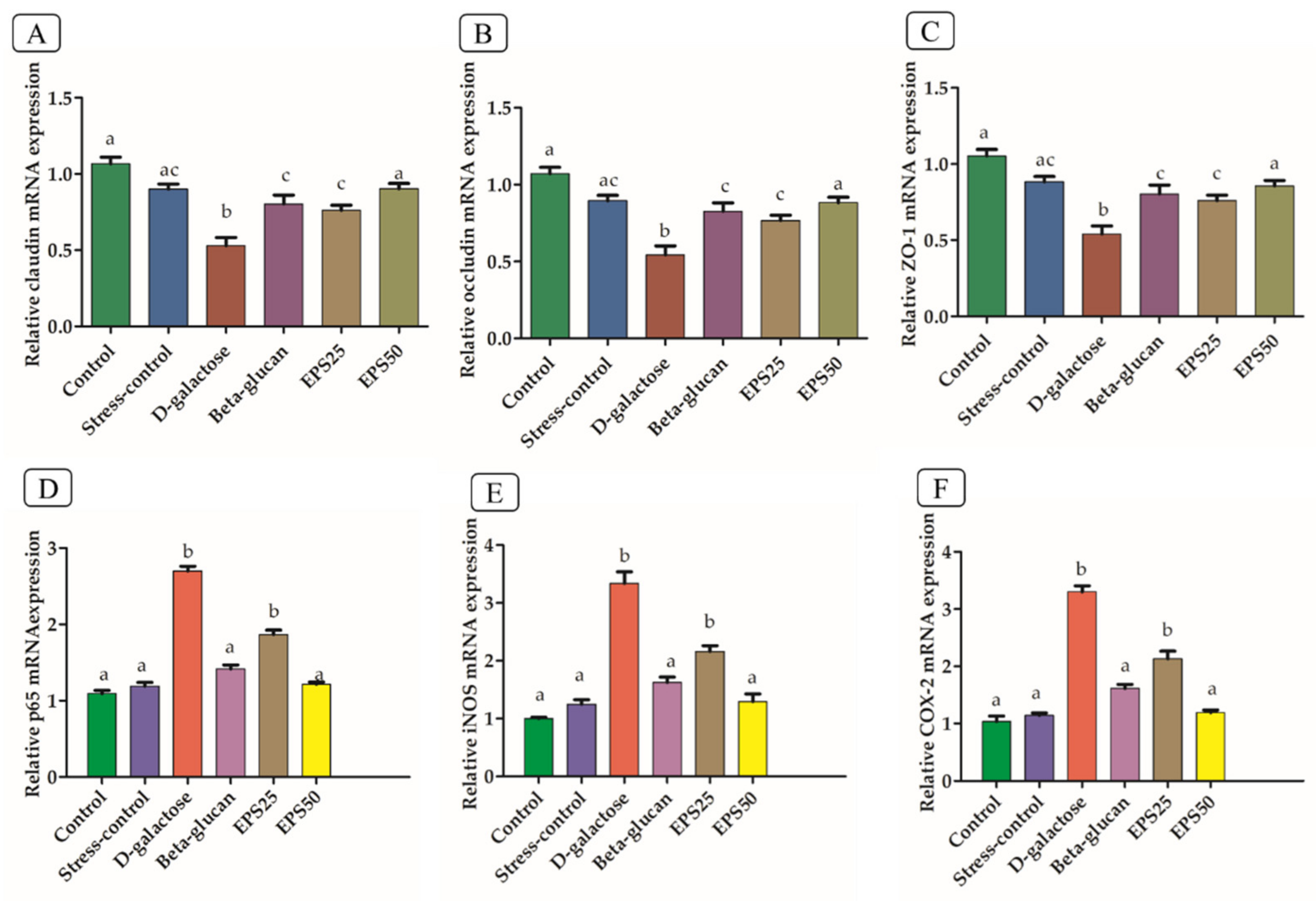
2.7. Effects of EPSRam12 on Intestinal Tight-Junction and Inflammatory Gene Expression by RT-qPCR
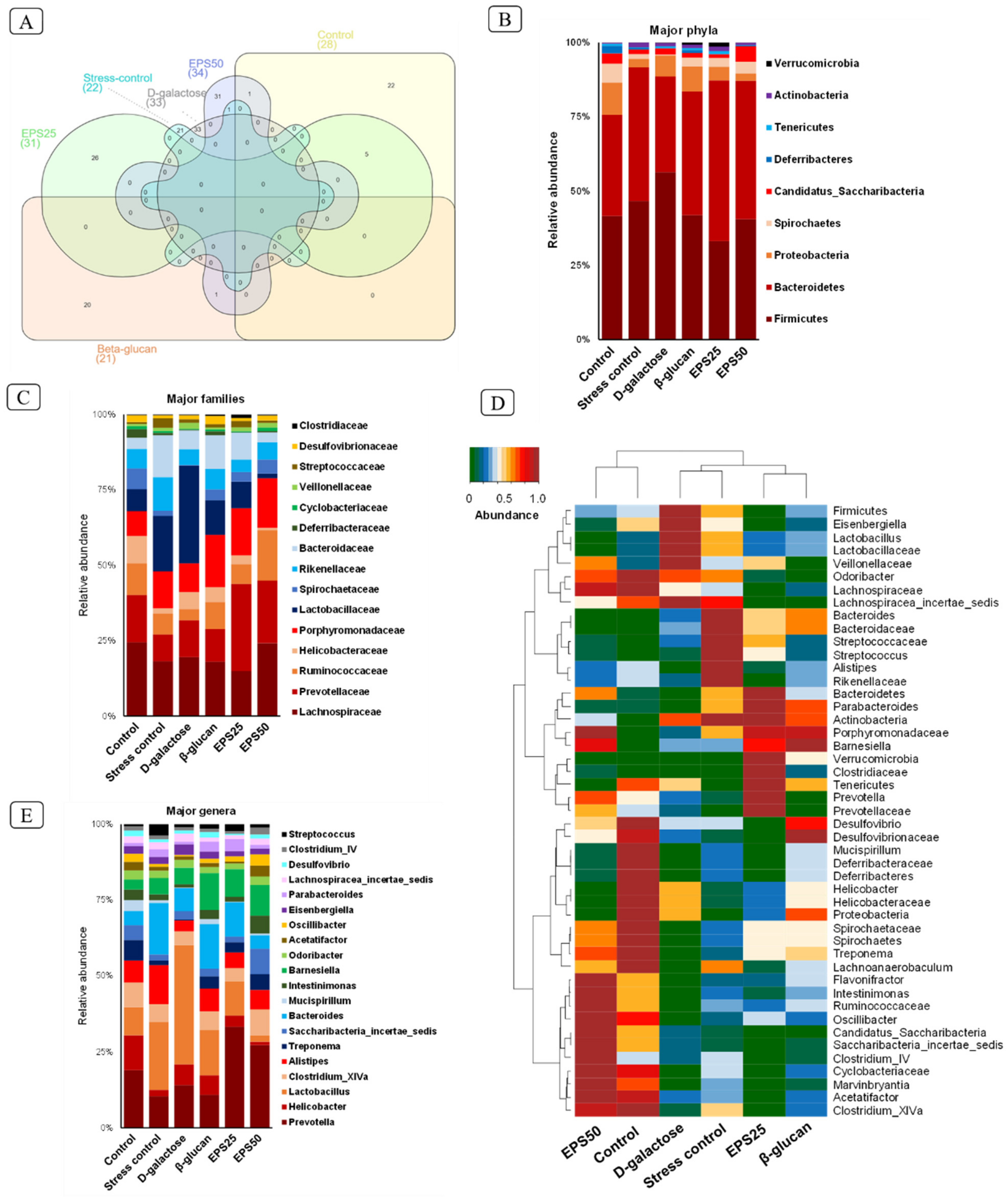
2.8. Microbiome Analysis
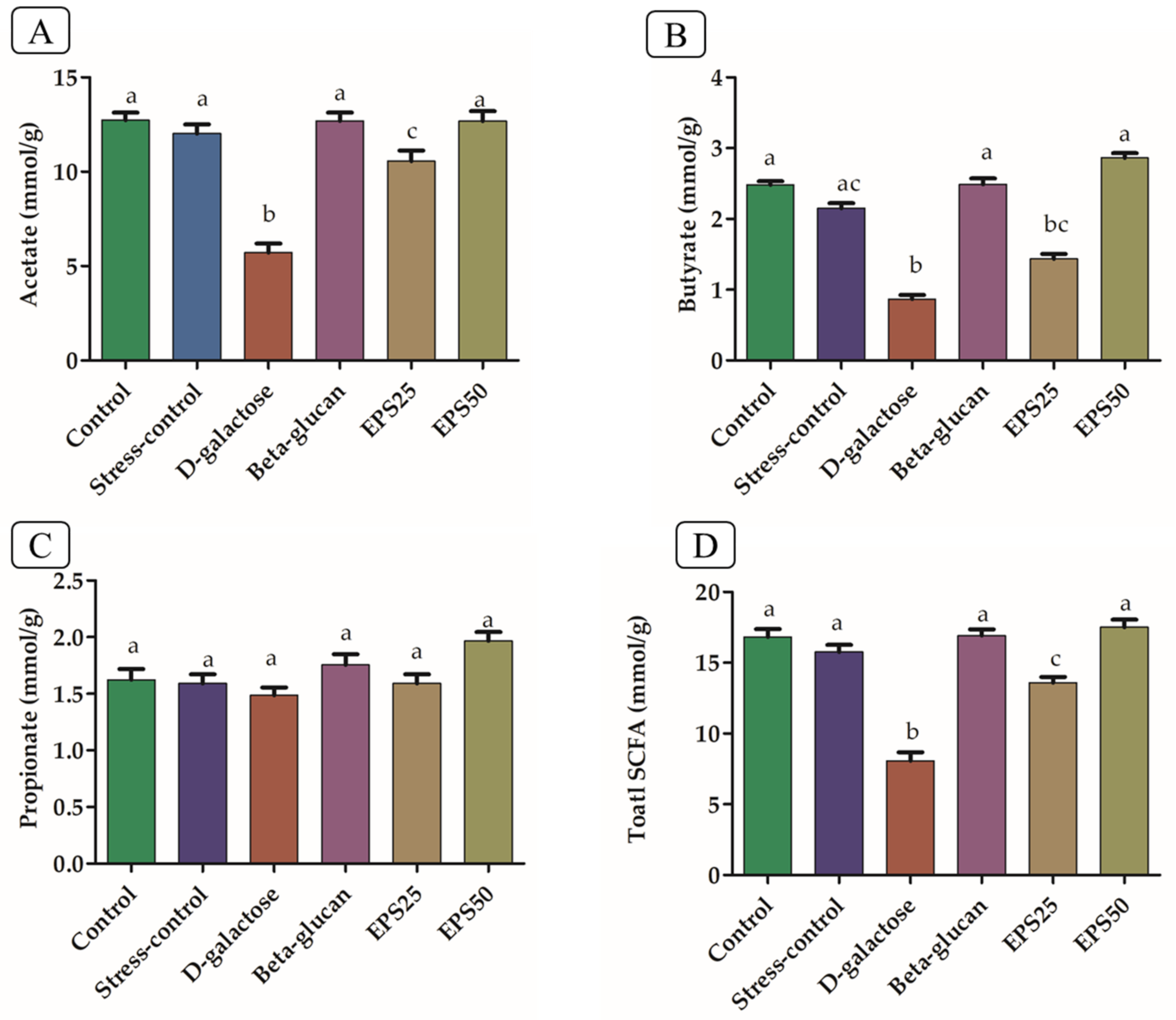
2.9. Cecal SCFA Levels
2.10. Statistical Analysis
3. Results and Discussion
3.1. Yield of EPSRam12
3.2. Effects of EPSRam12 on Histopathology
3.3. Effects of EPSRam12 on Oxidative Stress Markers
3.4. Effects of EPSRam12 on Inflammatory Markers
3.5. Effects of EPSRam12 on Lipofuscin Content
3.6. Effects of EPSRam12 on Intestinal Tight-Junction and Brain Inflammatory Genes
3.7. Gut Microbiome Modulation in Mice Treated with EPSRam12
3.8. Short-Chain Fatty Acid Analysis in Mice Treated with EPSRam12
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Therond, P. Oxidative stress and damages to biomolecules (lipids, proteins, DNA). Ann. Pharm. Fr. 2006, 64, 383–389. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Liu, J.-H.; Wu, R.-Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Wu, W.-M.; Hu, M.-L. Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with d-galactose. Food Chem. Toxicol. 2009, 47, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, S.; Kumar, S.; Jha, N.K.; Kesari, K.K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2021, 38, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Suganya, K.; Koo, B.-S. Gut–Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Budzyński, J.; Maria, K. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J. Gas-Troenterol. 2014, 20, 5212–5225. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Ali, S.A.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. Int. Rev. J. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, S.; Zhang, X. Modulation of Gut Microbiota–Brain Axis by Probiotics, Prebiotics, and Diet. J. Agric. Food Chem. 2015, 63, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Ma, F.; Song, Y.; Sun, M.; Wang, A.; Jiang, S.; Mu, G.; Tuo, Y. Exopolysaccharide Produced by Lactiplantibacillus plantarum-12 Alleviates Intestinal Inflammation and Colon Cancer Symptoms by Modulating the Gut Microbiome and Metabolites of C57BL/6 Mice Treated by Azoxymethane/Dextran Sulfate Sodium Salt. Foods 2021, 10, 3060. [Google Scholar] [CrossRef]
- Oerlemans, M.M.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2020, 76, 104289. [Google Scholar] [CrossRef]
- Behare, P.V.; Singh, R.; Nagpal, R.; Rao, K.H. Exopolysaccharides producing Lactobacillus fermentum strain for enhancing rheological and sensory attributes of low-fat dahi. J. Food Sci. Technol. 2013, 50, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Van Geel-Schutten, G.H.; Faber, E.J.; Smit, E.; Bonting, K.; Smith, M.R.; Brink, B.T.; Kamerling, J.P.; Vliegenthart, J.F.G.; Dijkhuizen, L. Biochemical and Structural Characterization of the Glucan and Fructan Exopolysaccharides Synthesized by the Lactobacillus reuteri Wild-Type Strain and by Mutant Strains. Appl. Environ. Microbiol. 1999, 65, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Bryan, N.S.; Grisham, M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007, 43, 645–657. [Google Scholar] [CrossRef]
- Ma, J.; Wang, H.; Liu, B.; Shan, Y.; Zhou, H.; Qi, X.; Wu, W.; Jia, L. Combination of chick embryo and nutrient mixture prevent D-galactose-induced cognitive deficits, immune impairment and oxidative stress in aging rat model. Sci. Rep. 2019, 9, 4092. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Liang, Z.; Liu, A.; Chen, Z.; Tang, Y.; Xiao, M.; Chu, F.; Liu, W.; Jin, X.; et al. Musca domestica Cecropin (Mdc) Alleviates Salmonella typhimurium-Induced Colonic Mucosal Barrier Impairment: Associating With Inflammatory and Oxidative Stress Response, Tight Junction as Well as Intestinal Flora. Front. Microbiol. 2019, 10, 522. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Nagpal, R.; Indugu, N.; Singh, P. Distinct Gut Microbiota Signatures in Mice Treated with Commonly Used Food Preservatives. Microorganisms 2021, 9, 2311. [Google Scholar] [CrossRef]
- Clark, M.; Centner, A.M.; Ukhanov, V.; Nagpal, R.; Salazar, G. Gallic acid ameliorates atherosclerosis and vascular senescence and remodels the microbiome in a sex-dependent manner in ApoE−/− mice. J. Nutr. Biochem. 2022, 110, 109132. [Google Scholar] [CrossRef]
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A 2021, 76, 1895–1905. [Google Scholar] [CrossRef]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Filípek, J.; Dvorak, R. Determination of the Volatile Fatty Acid Content in the Rumen Liquid: Comparison of Gas Chromatography and Capillary Isotachophoresis. Acta Veter. Brno 2009, 78, 627–633. [Google Scholar] [CrossRef]
- Sims, I.M.; Frese, S.A.; Walter, J.; Loach, D.; Wilson, M.; Appleyard, K.; Eason, J.; Livingston, M.; Baird, M.; Cook, G.; et al. Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri 100-23. ISME J. 2011, 5, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, D.; Wang, Z.; Chen, C.; Ning, D.; Zhao, S. Protective Effect of Walnut on D-galactose-induced Aging Mouse Model. Food Sci. Nutr. 2019, 7, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Fang, X.; Miao, M.; Wang, T. Effects of Wuweizi syrup on brain aging mice model induced by d-galactose. J. King Saud Univ. Sci. 2020, 32, 2426–2431. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Xu, X.; Mao, G.; Sun, X.; Xu, N.; Wang, X. Gracilariopsis lemaneiformis Polysaccharide Attenuates D-Galactose–Induced Aging of Mice by Regulating Oxidative Stress and Gut Microbiota. Front. Mar. Sci. 2022, 9, 932147. [Google Scholar] [CrossRef]
- Sensini, F.; Inta, D.; Palme, R.; Brandwein, C.; Pfeiffer, N.; Riva, M.A.; Gass, P.; Mallien, A.S. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci. Rep. 2020, 10, 17281. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-H.; Zhang, Z.-F.; Zheng, Y.-L.; Lu, J.; Wu, D.-M.; Shan, Q.; Hu, B.; Wang, Y.-Y. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int. Immunopharmacol. 2009, 9, 91–96. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Askari, V.R.; Mousavi, S.H. Ellagic acid dose and time-dependently abrogates d-galactose-induced animal model of aging: Investigating the role of PPAR-γ. Life Sci. 2019, 232, 116595. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Zhang, M. The vivo antioxidant activity of self-made aged garlic extract on the d-galactose-induced mice and its mechanism research via gene chip analysis. RSC Adv. 2019, 9, 3669–3678. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Ma, F.; Sun, M.; Song, Y.; Xu, D.; Mu, G.; Tuo, Y. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct. 2021, 12, 373–386. [Google Scholar] [CrossRef]
- Wang, J.; Hu, J.-Q.; Song, Y.-J.; Yin, J.; Wang, Y.-Y.; Peng, B.; Zhang, B.-W.; Liu, J.-M.; Dong, L.; Wang, S. 2′-Fucosyllactose Ameliorates Oxidative Stress Damage in D-Galactose-Induced Aging Mice by Regulating Gut Microbiota and AMPK/SIRT1/FOXO1 Pathway. Foods 2022, 11, 151. [Google Scholar] [CrossRef]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef]
- Zhou, X.; Qi, W.; Hong, T.; Xiong, T.; Gong, D.; Xie, M.; Nie, S.-P. Exopolysaccharides from Lactobacillus plantarum NCU116 Regulate Intestinal Barrier Function via STAT3 Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9719–9727. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Su, Z.-R.; Chen, J.-N.; Lai, X.-P.; Mao, Q.-Q.; Cheng, C.H.; Ip, S.-P.; Lin, Z.-X. Isorhynchophylline improves learning and memory impairments induced by D-galactose in mice. Neurochem. Int. 2014, 76, 42–49. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lin, H.-Y.; Su, K.-Y.; Chen, C.-H.; Yu, Y.-L.; Lin, C.-C.; Yu, S.-L.; Yan, H.-Y.; Su, K.-J.; Chen, Y.-L.S. Rutin, a Flavonoid That Is a Main Component ofSaussurea involucrata, Attenuates the Senescence Effect in D-Galactose Aging Mouse Model. Evidence-Based Complement. Altern. Med. 2012, 2012, 980276. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Jiang, Y.; Zhao, W.; Guo, T.; Cao, Y.; Teng, J.; Hao, X.; Zhao, J.; Yang, Z. Antioxidant status and gut microbiota change in an aging mouse model as influenced by exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibetan kefir. J. Dairy Sci. 2017, 100, 6025–6041. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Cao, X.; Ye, Y.; Yang, L.; Wang, J.; Liu, H.; Sun, H. Potential prebiotic activities of soybean peptides Maillard reaction products on modulating gut microbiota to alleviate aging-related disorders in D-galactose-induced ICR mice. J. Funct. Foods 2020, 65, 103729. [Google Scholar] [CrossRef]
- Lemos, L.N.; Fulthorpe, R.R.; Triplett, E.W.; Roesch, L.F. Rethinking microbial diversity analysis in the high throughput sequencing era. J. Microbiol. Methods 2011, 86, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, J.; Zhang, J.; Wang, X.; Hao, L.; Jia, L. Purification, in vitro antioxidant and in vivo anti-aging activities of exopolysaccharides by Agrocybe cylindracea. Int. J. Biol. Macromol. 2017, 102, 351–357. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Bhadada, S.K.; Sharma, S.S.; Kaur, J.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.H.; Oh, T.; Ahn, B.; Unno, T. Codium Fragile Ameliorates High-fat diet-induced Metabolism by Modulating the Gut Microbiota in Mice. Nutrients 2020, 12, 1848. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Bang, C.; Rühlemann, M.C.; Starke, A.; Sicks, F.; Kaspari, V.; Jandowsky, A.; Frölich, K.; Ismer, G.; Bernhard, A.; et al. Ecology impacts the decrease of Spirochaetes and Prevotella in the fecal gut microbiota of urban humans. BMC Microbiol. 2021, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yan, H.; Yu, B.; Walsh, M.C.; Yu, J.; Zheng, P.; Huang, Z.; Luo, J.; Mao, X.; He, J.; et al. Prevotella-rich enterotype may benefit gut health in finishing pigs fed diet with a high amylose-to-amylopectin ratio. Anim. Nutr. 2021, 7, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Di Paola, M.; Pavarini, L.; Strati, F.; Pindo, M.; Sanchez, M.; Cavalieri, D.; Boirivant, M.; De Filippo, C. Nod2 Deficiency in mice is Associated with Microbiota Variation Favouring the Expansion of mucosal CD4+ LAP+ Regulatory Cells. Sci. Rep. 2018, 8, 14241. [Google Scholar] [CrossRef] [PubMed]
- Dziarski, R.; Park, S.Y.; Kashyap, D.; Dowd, S.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Ravn, J.L.; Thøgersen, J.C.; Eklöf, J.; Pettersson, D.; Ducatelle, R.; van Immerseel, F.; Pedersen, N.R. GH11 xylanase increases prebiotic oligosaccharides from wheat bran favouring butyrate-producing bacteria in vitro. Anim. Feed Sci. Technol. 2017, 226, 113–123. [Google Scholar] [CrossRef]
- Wang, F.; Xu, T.; Zhang, Y.; Zheng, T.; He, Y.; He, F.; Jiang, Y. Long-term combined administration of Bifidobacterium bifidum TMC3115 and Lactobacillus plantarum 45 alleviates spatial memory impairment and gut dysbiosis in APP/PS1 mice. FEMS Microbiol. Lett. 2020, 367, fnaa048. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose Glycerol Monolaurate Up-Regulated Beneficial Indigenous Microbiota without Inducing Metabolic Dysfunction and Systemic Inflammation: New Insights into Its Antimicrobial Potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef]
- Hong, Y.; Sheng, L.; Zhong, J.; Tao, X.; Zhu, W.; Ma, J.; Yan, J.; Zhao, A.; Zheng, X.; Wu, G.; et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 2021, 13, 1930874. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-L.; Wu, T.-C.; Chan, S.-T.; Hong, M.-J.; Chen, H.-L. Fructo-oligosaccharide attenuates the production of pro-inflammatory cytokines and the activation of JNK/Jun pathway in the lungs of d-galactose-treated Balb/cJ mice. Eur. J. Nutr. 2014, 53, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Álvarez-Mercado, A.I.; Plaza-Diaz, J. Dietary Polysaccharides as Modulators of the Gut Microbiota Ecosystem: An Update on Their Impact on Health. Nutrients 2022, 14, 4116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, L.; Liu, Y.; Zhang, R.; Wu, Z.; Weng, P.; Zhang, P.; Zhang, X. Polysaccharide Regulation of Intestinal Flora: A Viable Approach to Maintaining Normal Cognitive Performance and Treating Depression. Front. Microbiol. 2022, 13, 807076. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Song, Z.; Xie, P.; Li, L.; Wang, B.; Peng, D.; Zhu, G. Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 2021, 275, 114164. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, T.; Fan, K.; Wang, M.; Qi, Y.; Xiao, F.; Bi, K.; Jia, Y. Polysaccharide of Schisandra Chinensis Fructus ameliorates cognitive decline in a mouse model of Alzheimer’s disease. J. Ethnopharmacol. 2019, 237, 354–365. [Google Scholar] [CrossRef]
- Patel, M.; Prasad, W.; Naithani, H.; Nataraj, B.H.; Arora, S.; Behare, P.V. Comparative evaluation of in situ and ex-situ iron-complexing ability of exopolysaccharides producing lactic acid bacteria in whey medium. LWT 2021, 147, 111598. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Product Size (bp) |
|---|---|---|
| ZO-1 | F: 5′-CCAGCAACTTTCAGACCACC-3′ R: 5′-TTGTGTACGGCTTTGGTGTG-3′ | 154 |
| Claudin | F: 5′-TGCACAGAGAGCAAGGGTATAG-3′ R: 5′-GAGCCGATCCATCCCAGAGA-3′ | 193 |
| Occludin | F: 5′-GCTTACAGGCAGAACTAGACG-3′ R: 5′-TCTGCAGATCCCTTAACTTGC-3′ | 142 |
| P65 | F: 5′-TCTTCTTGCTGTGCGACAAG-3′ R: 5′-GCATGGAGACTCGAACAGGA-3′ | 177 |
| INOS | F: 5′-ACAGGAACCTACCAGCTCAC-3′ R: 5′-CGACCTGATGTTGCCATTGT-3′ | 201 |
| COX2 | F: 5′-AGGTCATTGGTGGAGAGGTG-3′ R: 5′-CCTGCTTGAGTATGTCGCAC-3′ | 192 |
| β-actin | F: 5′-AGAGGGAAATCGTGCGTGAC-3′ R: 5′-CAATAGTGATGACCTGGCCGT-3′ | 138 |
| GAPDH | F: 5′-GCAAGAGAGAGGCCCTCAG-3′ R: 5′-TGTGAGGGAGATGCTCAGTG-3′ | 74 |
| Group | Number of ASVs | Chao1 Index | Shannon Index |
|---|---|---|---|
| Control | 22 | 675 | 3.56 |
| Stress-control | 24 | 579 | 3.25 |
| D-galactose | 21 | 725 | 3.18 |
| Beta-glucan | 17 | 797 | 3.77 |
| EPS25 | 15 | 640 | 3.39 |
| EPS50 | 28 | 647 | 3.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, M.; Dasriya, V.L.; Nataraj, B.H.; Nagpal, R.; Behare, P.V. Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms 2022, 10, 2046. https://doi.org/10.3390/microorganisms10102046
Kumari M, Dasriya VL, Nataraj BH, Nagpal R, Behare PV. Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms. 2022; 10(10):2046. https://doi.org/10.3390/microorganisms10102046
Chicago/Turabian StyleKumari, Manorama, Vaishali L. Dasriya, Basavaprabhu H. Nataraj, Ravinder Nagpal, and Pradip V. Behare. 2022. "Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model" Microorganisms 10, no. 10: 2046. https://doi.org/10.3390/microorganisms10102046
APA StyleKumari, M., Dasriya, V. L., Nataraj, B. H., Nagpal, R., & Behare, P. V. (2022). Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms, 10(10), 2046. https://doi.org/10.3390/microorganisms10102046







