CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective
Abstract
:1. Introduction
2. Relationship between CRISPR-Cas and Phages
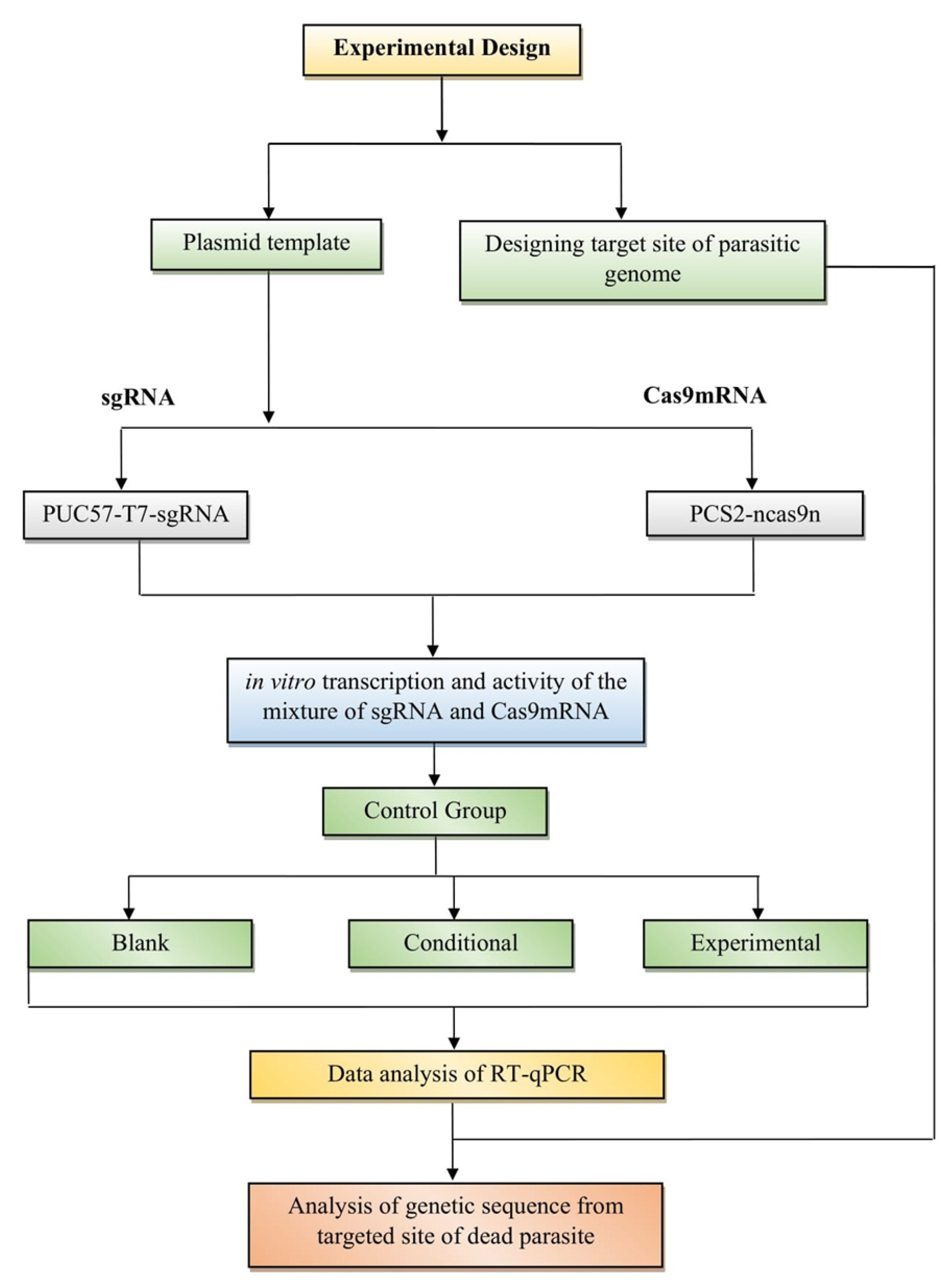
3. CRISPR-Cas for Anti-Parasitic Action
4. CRISPR-Cas for Developing RNA-Guided Immunity against RNA Viruses in Fish
5. Application of CRISPR-Cas in Fish Disease
6. Advances in Bioinformatics in CRISPR-Cas
7. Limitations of CRISPR-Cas for Aquatic Disease Perspective
8. Future Perspective and Approaches
- Antibiotics’ massive selection pressures brought on by antibiotic exposure cause commensal and pathogenic microorganisms to develop and propagate antibiotic resistance. This strategy is paradoxical for preventing the fast evolution of new antibiotic-resistant organisms because of the lengthy process of discovering new antibiotics. To deal with diseases brought on by resistant superbugs, alternative strategies including creating nucleic acid-based anti-bacterial therapeutics, anti-bacterial peptides, bacteriocins, anti-virulence chemicals, and bacteriophage therapies should be used. To address antibiotic resistance in this situation, scientists have already begun to use the recently popular CRISPR-Cas system [90]. Antibiotic-resistant superbugs are one of the major concerns today, but CRISPR technology is expected to protect from this problem if used properly;
- Antibiotics target cellular processes or activities, such as nucleic acid synthesis and cell membrane formation, to impact specialized bacterial mechanisms. These processes cannot destroy specific pathogens in the diverse microbial community—antibiotics damage both the members of the beneficial microbiota and the bacteria that cause infections. There is currently no antibiotic method that targets exclusively pathogenic bacteria. The use of antibiotics nowadays is not species-specific. The CRISPR-Cas9 gene-editing technique and its applications against bacteria will be a crucial strategy to stop the clonal proliferation of dangerous bacteria, offering a novel remedy to the world-wide issue [91];
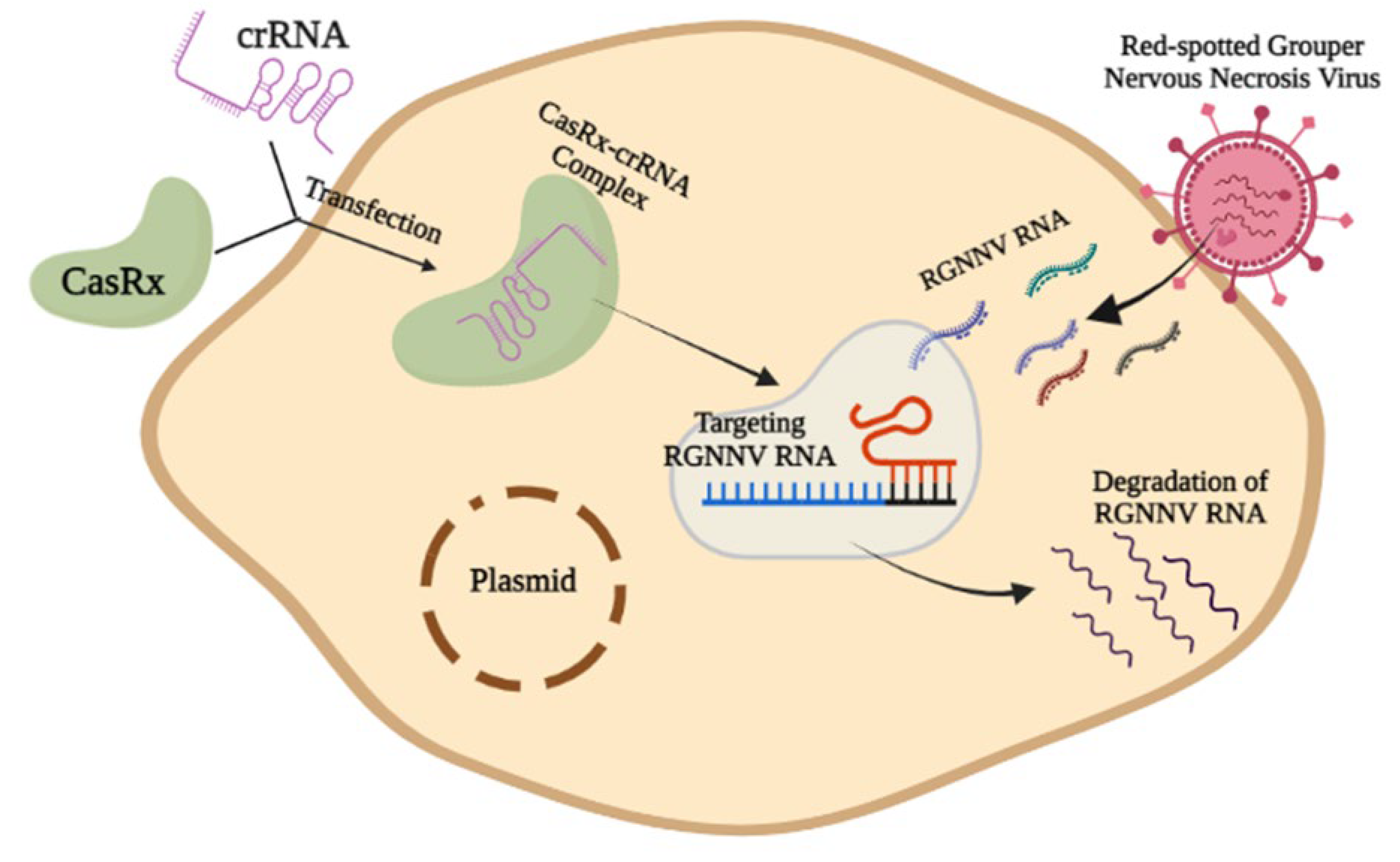
- Among all of the pathogens that cause disease in the fish body, viral diseases can be considered the most dangerous. In particular, fish diseases by RNA viruses cause the most suffering to scientists and fish farmers. From that point of view, since RNA viruses show mutations or Single Nucleotide Polymorphisms (SNP) so frequently, any preventive measures designed to target a particular virus may no longer work after the mutation or SNP. The CRISPR-Cas method can play a vital role in solving this problem in the future. CRISPR-Cas technology has already experimented with the RNA virus targeting method for red-spotted grouper nervous necrosis virus (RGNNV) in fish [13]. Scientists found success in this experiment by using the CasRx-crRNA complex;
- Apart from these, CRISPR has also been applied for anti-parasitic action. Scientists are also succeeding in this area [25]. Moreover, using this technology, genetically improved or modified species can be created that will be born with high immunity from the beginning of life.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodger, H.D. Fish Disease Causing Economic Impact in Global Aquaculture. In Fish Vaccines; Adams, A., Ed.; Springer Basel: Basel, Switzerland, 2016; pp. 1–34. [Google Scholar]
- Peeler, E.; Feist, S. Human intervention in freshwater ecosystems drives disease emergence. Freshw. Biol. 2011, 56, 705–716. [Google Scholar] [CrossRef]
- Diwan, A.D.; Ninawe, A.S.; Harke, S.N. Gene editing (CRISPR-Cas) technology and fisheries sector. Can. J. Microbiol. 2017, 1, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Paladini, G.; Longshaw, M.; Gustinelli, A.; Shinn, A.P. Parasitic Diseases in Aquaculture: Their Biology, Diagnosis, and Control; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 37–107. [Google Scholar]
- Frantz, A.; Perga, M.E.; Guillard, J. Parasitic versus nutritional regulation of natural fish populations. Ecol. Evol. 2018, 8, 8713–8725. [Google Scholar] [CrossRef] [PubMed]
- Orobets, V.; Lisovets, E.; Zabashta, S.; Ermakov, A. Control of fish parasites in aquaculture. IOP Conf. Ser. Earth Environ. Sci. 2019, 403, 012065. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate, and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; de Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.F.; Gaze, W.H.; Kuroda, M.; et al. Critical knowledge gaps and research need related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Seruggia, D.; Montoliu, L. The new CRISPR-Cas system: RNA-guided genome engineering to efficiently produce any desired genetic alteration in animals. Transgenic Res. 2014, 23, 707–716. [Google Scholar] [CrossRef]
- Ansai, S.; Mochida, K.; Fujimoto, S.; Mokodongan, D.F.; Sumarto, B.K.; Masengi, K.W.; Hadiaty, R.K.; Nagano, A.J.; Toyoda, A.; Naruse, K.; et al. Genome editing reveals fitness effects of a gene for sexual dichromatism in Sulawesian fishes. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Han, C.; Yang, M.; Huang, F.; Duan, X.; Wang, S.; Yu, Y.; Liu, J.; Yang, H.; et al. Efficient RNA Virus Targeting via CRISPR/CasRx in Fish. J. Virol. 2021, 95, e0046121. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, T.; Liu, C.; Xu, Q.; Liu, Q. Pathogen detection strategy based on CRISPR. Microchem. J. 2022, 174, 107036. [Google Scholar] [CrossRef]
- Gratacap, R.L.; Wargelius, A.; Edvardsen, R.B.; Houston, R.D. Potential of Genome Editing to Improve Aquaculture Breeding and Production. Trends Genet. 2019, 35, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wargelius, A. Application of genome editing in aquatic farm animals: Atlantic salmon. Transgenic Res. 2019, 28, 101–105. [Google Scholar] [CrossRef]
- Henry, M.; Debarbieux, L. Tools from viruses: Bacteriophage successes and beyond. Virology 2012, 434, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Touchon, M.; Bernheim, A.; Rocha, E.P. Genetic and life-history traits associated with the distribution of prophages in bacteria. ISME J. 2016, 10, 2744–2754. [Google Scholar] [CrossRef] [Green Version]
- Castillo, D.; Espejo, R.; Middelboe, M. Genomic structure of bacteriophage 6H and its distribution as prophage in Flavobacterium psychrophilum strains. FEMS Microbiol. Lett. 2014, 351, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Kalatzis, P.G.; Rørbo, N.; Castillo, D.; Mauritzen, J.J.; Jørgensen, J.; Kokkari, C.; Middelboe, M. Stumbling across the same phage: Comparative genomics of widespread temperate phages infecting the fish pathogen Vibrio anguillarum. Viruses 2017, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Almeida, G.M.; Laanto, E.; Ashrafi, R.; Sundberg, L.R. Bacteriophage adherence to mucus mediates preventive protection against pathogenic bacteria. MBio 2019, 10, e01984-19. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, Y.; Wang, Y.; Zhang, Z. Barcode sequence could be a good target for developing a species-specific anti-parasite agent based on CRISPR-Cas9. FASEB J. 2020, 34, 9393–9404. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Alcalá, X.X.; Knight, R.; Katz, L.A. Exploration of the germline genome of the ciliate Chilodonella uncinata through single-cell omics (transcriptomics and genomics). MBio 2018, 9, e01836-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lai, J.; Zhang, X.; Wang, Y.; Zhang, Z. Development of an RNA anti-parasite based on CRISPR-Cas9 against Chilodonella piscicola. Aquaculture 2022, 552, 738025. [Google Scholar] [CrossRef]
- Majeed, M.; Soliman, H.; Kumar, G.; El-Matbouli, M.; Saleh, M. Editing the genome of Aphanomyces invadans using CRISPR/Cas9. Parasites Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR-Cas13a. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D.B.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable inhibition and detection of RNA viruses using Cas13. Mol. Cell 2019, 76, 826–837.e11. [Google Scholar] [CrossRef] [Green Version]
- Makarova, K.S.; Haft, D.H.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Horvath, P.; Moineau, S.; Mojica, F.J.; Wolf, Y.I.; Yakunin, A.F.; et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 2011, 9, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Konermann, S.; Joung, J.; Slaymaker, I.M.; Cox, D.B.; Shmakov, S.; Makarova, K.S.; Semenova, E.; Minakhin, L.; et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 2016, 353, aaf5573. [Google Scholar] [CrossRef]
- Horigome, C.; Oma, Y.; Konishi, T.; Schmid, R.; Marcomini, I.; Hauer, M.H.; Dion, V.; Harata, M.; Gasser, S.M. SWR1. chromatin remodelers contribute to DNA double-strand break perinuclear anchorage site choice. Mol. Cell 2014, 55, 626–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 2018, 173, 665–676.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandín, I.; Souto, S. Betanodavirus and VER Disease: A 30-year. Pathogens 2020, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, T.; Furuhashi, M.; Nagai, T.; Nakai, T.; Muroga, K. Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl. Environ. Microbiol. 1997, 63, 1633–1636. [Google Scholar] [CrossRef] [Green Version]
- Sahul Hameed, A.S.; Ninawe, A.S.; Nakai, T.; Chi, S.C.; Johnson, K.L. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Nodaviridae. J. Gen. Virol. 2019, 100, 3–4. [Google Scholar] [CrossRef]
- Chi, S.; Lo, B.; Lin, S. Characterization of grouper nervous necrosis virus (GNNV). J. Fish Dis. 2001, 24, 3–13. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Yu, Q.; Wei, S.; Liu, M.; Wei, J.; Qin, Q. Characterization of novel aptamers specifically directed to red-spotted grouper nervous necrosis virus (RGNNV)-infected cells for mediating targeted siRNA delivery. Front. Microbiol. 2020, 11, 660. [Google Scholar] [CrossRef]
- Ahmed, N.; Thompson, S.; Glaser, M. Global aquaculture productivity, environmental sustainability, and climate change adaptability. Environ. Manag. 2019, 63, 159–172. [Google Scholar] [CrossRef]
- Jin, Y.H.; Liao, B.; Migaud, H.; Davie, A. Physiological impact and comparison of mutant screening methods in piwil2 KO founder Nile tilapia produced by CRISPR/Cas9 system. Sci. Rep. 2020, 10, 12600. [Google Scholar] [CrossRef]
- Straume, A.H.; Kjærner-Semb, E.; Skaftnesmo, K.O.; Güralp, H.; Lillico, S.; Wargelius, A.; Edvardsen, R.B. A refinement to gene editing in Atlantic salmon using asymmetrical oligonucleotide donors. bioRxiv 2021. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.D.; Nissa, M.; Chen, J.; Zou, S.M. Disruption of mstna and mstnb gene through CRISPR/Cas9 leads to elevated muscle mass in blunt snout bream (Megalobrama amblycephala). Aquaculture 2020, 528, 735597. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Gao, J. Production of a mutant of large-scale loach Paramisgurnus dabryanus with skin pigmentation loss by genome editing with CRISPR/Cas9 system. Transgenic Res. 2019, 28, 341–356. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Du, J.; Si, Z.; Yang, H.; Xu, X.; Wang, C. ASIP disruption via CRISPR/Cas9 system induces black patches dispersion in Oujiang color common carp. Aquaculture 2019, 498, 230–235. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.Y.; Kim, J.W.; Kim, D.G.; Nam, B.H.; Kim, B.S.; Kim, W.J.; Kim, Y.O.; Cheong, J.; Kong, H.J. Molecular characterization of paralichthys olivaceus MAF1 and its potential role as an anti-viral hemorrhagic septicemia virus factor in hirame natural embryo cells. Int. J. Mol. Sci. 2021, 22, 1353. [Google Scholar] [CrossRef]
- Gratacap, R.L.; Regan, T.; Dehler, C.E.; Martin, S.A.; Boudinot, P.; Collet, B.; Houston, R.D. Efficient CRISPR/Cas9 genome editing in a salmonid fish cell line using a lentivirus delivery system. BMC Biotechnol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Datsomor, A.K.; Olsen, R.E.; Zic, N.; Madaro, A.; Bones, A.M.; Edvardsen, R.B.; Wargelius, A.; Winge, P. CRISPR/Cas9-mediated editing of Δ5 and Δ6 desaturases impairs Δ8-desaturation and docosahexaenoic acid synthesis in Atlantic salmon (Salmo salar L.). Sci. Rep. 2019, 9, 16888. [Google Scholar] [CrossRef] [Green Version]
- Datsomor, A.K.; Zic, N.; Li, K.; Olsen, R.E.; Jin, Y.; Vik, J.O.; Winge, P. CRISPR/Cas9-mediated ablation of elovl2 in Atlantic salmon (Salmo salar L.) inhibits elongation of polyunsaturated fatty acids and induces Srebp-1 and target genes. Sci. Rep. 2019, 9, 7533. [Google Scholar] [CrossRef] [Green Version]
- Blix, T.B.; Dalmo, R.A.; Wargelius, A.; Myhr, A.I. Genome editing on finfish: Current status and implications for sustainability. Rev. Aquac. 2021, 13, 2344–2363. [Google Scholar] [CrossRef]
- Elaswad, A.; Khalil, K.; Cline, D.; Page-McCaw, P.; Chen, W.; Michel, M.; Dunham, R. Microinjection of CRISPR/Cas9 protein into channel catfish, Ictalurus punctatus, embryos for gene editing. JoVE (J. Vis. Exp.) 2018, 131, e56275. [Google Scholar] [CrossRef]
- Elaswad, A.; Khalil, K.; Ye, Z.; Liu, Z.; Liu, S.; Peatman, E.; Odin, R.; Vo, K.; Drescher, D.; Gosh, K.; et al. Effects of CRISPR/Cas9 dosage on TICAM1 and RBL gene mutation rate, embryonic development, hatchability and fry survival in channel catfish. Sci. Rep. 2018, 8, 16499. [Google Scholar] [CrossRef]
- Simora, R.M.C.; Xing, D.; Bangs, M.R.; Wang, W.; Ma, X.; Su, B.; Dunham, R.A. CRISPR/Cas9-mediated knock-in of alligator cathelicidin gene in a non-coding region of channel catfish genome. Sci. Rep. 2020, 10, 22271. [Google Scholar] [CrossRef]
- Dunham, R.A.; Warr, G.W.; Nichols, A.; Duncan, P.L.; Argue, B.; Middleton, D.; Kucuktas, H. Enhanced bacterial disease resistance of transgenic channel catfish Ictalurus punctatus possessing cecropin genes. Mar. Biotechnol. 2002, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, A.; Dunham, R. Disease reduction in aquaculture with genetic and genomic technology: Current and future approaches. Rev. Aquac. 2018, 10, 876–898. [Google Scholar] [CrossRef]
- Katayama, H.; Nagata, K.; Ohira, T.; Yumoto, F.; Tanokura, M.; Nagasawa, H. The solution structure of molt-inhibiting hormone from the Kuruma prawn Marsupenaeus japonicus. J. Biol. Chem. 2003, 278, 9620–9623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, H.; Ohira, T.; Nagata, S.; Nagasawa, H. Structure-activity relationship of crustacean molt-inhibiting hormone from the Kuruma prawn Marsupenaeus japonicus. Biochemistry 2004, 43, 9629–9635. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Lee, C.-Y.; Watson, R.D. Crustacean molt-inhibiting hormone: Structure, function, and cellular mode of action. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Treerattrakool, S.; Panyim, S.; Udomkit, A. Induction of ovarian maturation and spawning in Penaeus monodon broodstock by double-stranded RNA. Mar. Biotechnol. 2011, 13, 163–169. [Google Scholar] [CrossRef]
- Das, R.; Krishna, G.; Priyadarshi, H.; Gireesh-Babu, P.; Pavan-Kumar, A.; Rajendran, K.V.; Chaudhari, A. Captive maturation studies in Penaeus monodon by GIH silencing using constitutively expressed long hairpin RNA. Aquaculture 2015, 448, 512–520. [Google Scholar] [CrossRef]
- Lesk, A. Introduction to Bioinformatics; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Choudhary, S.; Ubale, A.; Padiya, J.; Mikkilineni, V. Application of Bioinformatics Tools in CRISPR/Cas, in CRISPR/Cas Genome Editing; Springer: Berlin/Heidelberg, Germany, 2020; pp. 31–52. [Google Scholar]
- Belhaj, K.; Chaparro-Garcia, A.; Kamoun, S.; Nekrasov, V. Plant genome editing made easy: Targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 2013, 9, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bortesi, L.; Fischer, R. The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol. Adv. 2015, 33, 41–52. [Google Scholar] [CrossRef]
- Jung, C.; Capistrano-Gossmann, G.; Braatz, J.; Sashidhar, N.; Melzer, S. Recent developments in genome editing and applications in plant breeding. Plant Breed. 2018, 137, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hwang, W.Y.; Fu, Y.; Reyon, D.; Maeder, M.L.; Tsai, S.Q.; Sander, J.D.; Peterson, R.T.; Yeh, J.R.; Joung, J.K. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 227–229. [Google Scholar] [CrossRef] [Green Version]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Bae, S.; Kim, J.-S. Cas-Designer: A web-based tool for the choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [Green Version]
- Ata, H.; Ekstrom, T.L.; Martínez-Gálvez, G.; Mann, C.M.; Dvornikov, A.V.; Schaefbauer, K.J.; Ma, A.C.; Dobbs, D.; Clark, K.J.; Ekker, S.C. Robust activation of microhomology-mediated end joining for precision gene editing applications. PLoS Genet. 2018, 14, e1007652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Wei, Z.; Dominguez, A.; Li, Y.; Wang, X.; Qi, L.S. CRISPR-ERA: A comprehensive design tool for CRISPR-mediated gene editing, repression, and activation. Bioinformatics 2015, 31, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Clement, K.; Rees, H.; Canver, M.C.; Gehrke, J.M.; Farouni, R.; Hsu, J.Y.; Cole, M.; Liu, D.R.; Joung, J.K.; Bauer, D.E.; et al. Analysis and comparison of genome editing using CRISPResso2. bioRxiv 2018, 392217. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef] [Green Version]
- Güell, M.; Yang, L.; Church, G.M. Genome editing assessment using CRISPR Genome Analyzer (CRISPR-GA). Bioinformatics 2014, 30, 2968–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varshney, G.K.; Zhang, S.; Pei, W.; Adomako-Ankomah, A.; Fohtung, J.; Schaffer, K.; Carrington, B.; Maskeri, A.; Slevin, C.; Wolfsberg, T.; et al. CRISPRz: A database of zebrafish validated sgRNAs. Nucleic Acids Res. 2016, 44, D822–D826. [Google Scholar] [CrossRef]
- Shen, M.W.; Arbab, M.; Hsu, J.Y.; Worstell, D.; Culbertson, S.J.; Krabbe, O.; Cassa, C.A.; Liu, D.R.; Gifford, D.K.; Sherwood, R.I. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 2018, 563, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.; Crepaldi, L.; Alsinet, C.; Strong, A.J.; Kleshchevnikov, V.; De Angeli, P.; Páleníková, P.; Khodak, A.; Kiselev, V.; Kosicki, M.; et al. Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat. Biotechnol. 2019, 37, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA base-editing and prime-editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.H.; Park, J.; Lim, K.; Kim, S.; Yu, J.; Yu, E.; Kim, S.T.; Eils, R.; Kim, J.S.; Bae, S. Web-based design and analysis tools for CRISPR base editing. BMC Bioinform. 2018, 19, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, P.; Jakimo, N.; Jacobson, J.M. Minimal PAM specificity of a highly similar SpCas9 ortholog. Sci. Adv. 2018, 4, eaau0766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Kluesner, M.G.; Nedveck, D.A.; Lahr, W.S.; Garbe, J.R.; Abrahante, J.E.; Webber, B.R.; Moriarity, B.S. EditR: A method to quantify base editing from Sanger sequencing. CRISPR J. 2018, 1, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Billon, P.; Bryant, E.E.; Joseph, S.A.; Nambiar, T.S.; Hayward, S.B.; Rothstein, R.; Ciccia, A. CRISPR-mediated base editing enables efficient disruption of eukaryotic genes through induction of STOP codons. Mol. Cell 2017, 67, 1068–1079.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandage, R.; Després, P.C.; Yachie, N.; Landry, C.R. beditor: A computational workflow for designing libraries of guide RNAs for CRISPR-mediated base editing. Genetics 2019, 212, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Petree, C.; Requena, T.; Varshney, P.; Varshney, G.K. Expanding the CRISPR toolbox in zebrafish for studying development and disease. Front. Cell Dev. Biol. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Charo, R. The societal opportunities and challenges of genome editing. Genome Biol. 2015, 16, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoli, A.S.; Blix, T.; Myhr, A.I.; Xu, W.; Xu, X. Sustainable use of CRISPR/Cas in fish aquaculture: The biosafety perspective. Transgenic Res. 2021, 31, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.; Neuhauss, S.C. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther.-Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.; Martin, S.A.; Stevens, J.R.; Santos, E.M.; et al. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Rasool, M.; Idris, A.; Muzammil, S.; Alvi, R.F.; Khurshid, M.; Rasool, M.H.; Zhang, D.; Ma, Z.; Baloch, Z. CRISPR-Cas system: A potential alternative tool to cope antibiotic resistance. Antimicrob. Resist. Infect. Control. 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Ates, A.; Tastan, C.; Ermertcan, S. Gene Editing. Gene Ed. J. Young Open Free 2020, 1, 30–35. [Google Scholar]

| Name | Function | URL | Reference |
|---|---|---|---|
| CRISPRScan |
| https://www.crisprscan.org accessed on 1 September 2022 | [68] |
| CHOPCHOP |
| http://chopchop.cbu.uib.no accessed on 1 September 2022 | [69] |
| ccTop |
| https://crispr.cos.uni-heidelberg.de accessed on 1 September 2022 | [70] |
| Cas-Designer |
| http://www.rgenome.net accessed on 1 September 2022 | [71] |
| MENTHU |
| http://genesculpt.org/menthu accessed on 1 September 2022 | [72] |
| CRISPR-ERA |
| http://crispr-era.stanford.edu accessed on 1 September 2022 | [73] |
| CRISPResso 2 |
| http://crispresso.pinellolab.partners.org accessed on 1 September 2022 | [74] |
| Cas-Analyzer |
| http://www.rgenome.net/Cas-analyzer accessed on 2 September 2022 | [75] |
| CRISPR-GA |
| http://crispr-ga.net accessed on 2 September 2022 | [76] |
| CRISPRz |
| https://research.nhgri.nih.gov/CRISPRz accessed on 2 September 2022 | [77] |
| inDelphi |
| https://indelphi.giffordlab.mit.edu accessed on 2 September 2022 | [78] |
| FORECasT |
| https://partslab.sanger.ac.uk/FORECasT accessed on 2 September 2022 | [79] |
| Resources | Function | URL | References |
|---|---|---|---|
| BE-Analyzer | Used as a rapid evaluation tool for CRISPR-base edited cells of NGS data. | http://www.rgenome.net/be-analyzer accessed on 2 September 2022 | [80] |
| BE-Designer | Used for CRISPR base editing, a designer of guide RNA. | http://www.rgenome.net/be-designer accessed on 2 September 2022 | [80] |
| BEEP | Used for analysis of Sanger sequencing ab1 files for CRISPR-mediated base editing effectiveness. | https://github.com/mitmedialab/BEEP accessed on 3 September 2022 | [81] |
| CRISPR-SKIP | Used to select the exons that can be skipped by modifying the flanking G nucleotide. | https://knoweng-0.igb.illinois.edu/crispr-skip accessed on 3 September 2022 | [82] |
| CRISPResso 2 | Used as a tool for next-generation sequencing data. | http://crispresso.pinellolab.partners.org accessed on 3 September 2022 | [74] |
| EditR | A single Sanger sequencing run can be used to predict possible editing in a guide RNA region. | http://baseeditr.com accessed on 3 September 2022 | [83] |
| iSTOP | A database of sgRNAs for CRISPR-dependent base editing of STOP codons (sgSTOPs). | https://www.ciccialab-database.com accessed on 3 September 2022 | [84] |
| Beditor | Designing Guide RNA Libraries for CRISPR-Mediated Base Editing | https://github.com/rraadd88/beditor accessed on 3 September 2022 | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdous, M.A.; Islam, S.I.; Habib, N.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Shafie, A. CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective. Microorganisms 2022, 10, 2012. https://doi.org/10.3390/microorganisms10102012
Ferdous MA, Islam SI, Habib N, Almehmadi M, Allahyani M, Alsaiari AA, Shafie A. CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective. Microorganisms. 2022; 10(10):2012. https://doi.org/10.3390/microorganisms10102012
Chicago/Turabian StyleFerdous, Md. Akib, Sk Injamamul Islam, Nasim Habib, Mazen Almehmadi, Mamdouh Allahyani, Ahad Amer Alsaiari, and Alaa Shafie. 2022. "CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective" Microorganisms 10, no. 10: 2012. https://doi.org/10.3390/microorganisms10102012
APA StyleFerdous, M. A., Islam, S. I., Habib, N., Almehmadi, M., Allahyani, M., Alsaiari, A. A., & Shafie, A. (2022). CRISPR-Cas Genome Editing Technique for Fish Disease Management: Current Study and Future Perspective. Microorganisms, 10(10), 2012. https://doi.org/10.3390/microorganisms10102012










