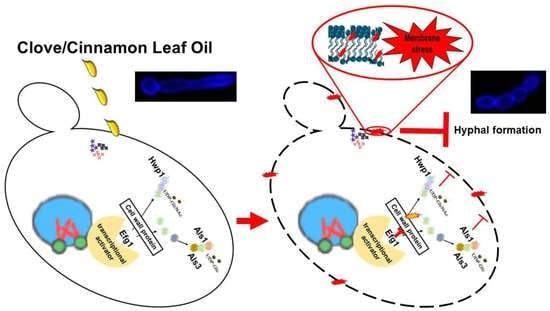
Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida albicans Virulence Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oils and Other Chemicals
2.2. Strains and Cultural Conditions
2.3. Gas Chromatography (GC)–Mass Spectrometry (MS) Analysis
2.4. Minimum Inhibitory Concentration (MIC) and Fractional Inhibitory Concentration Index (FICI)
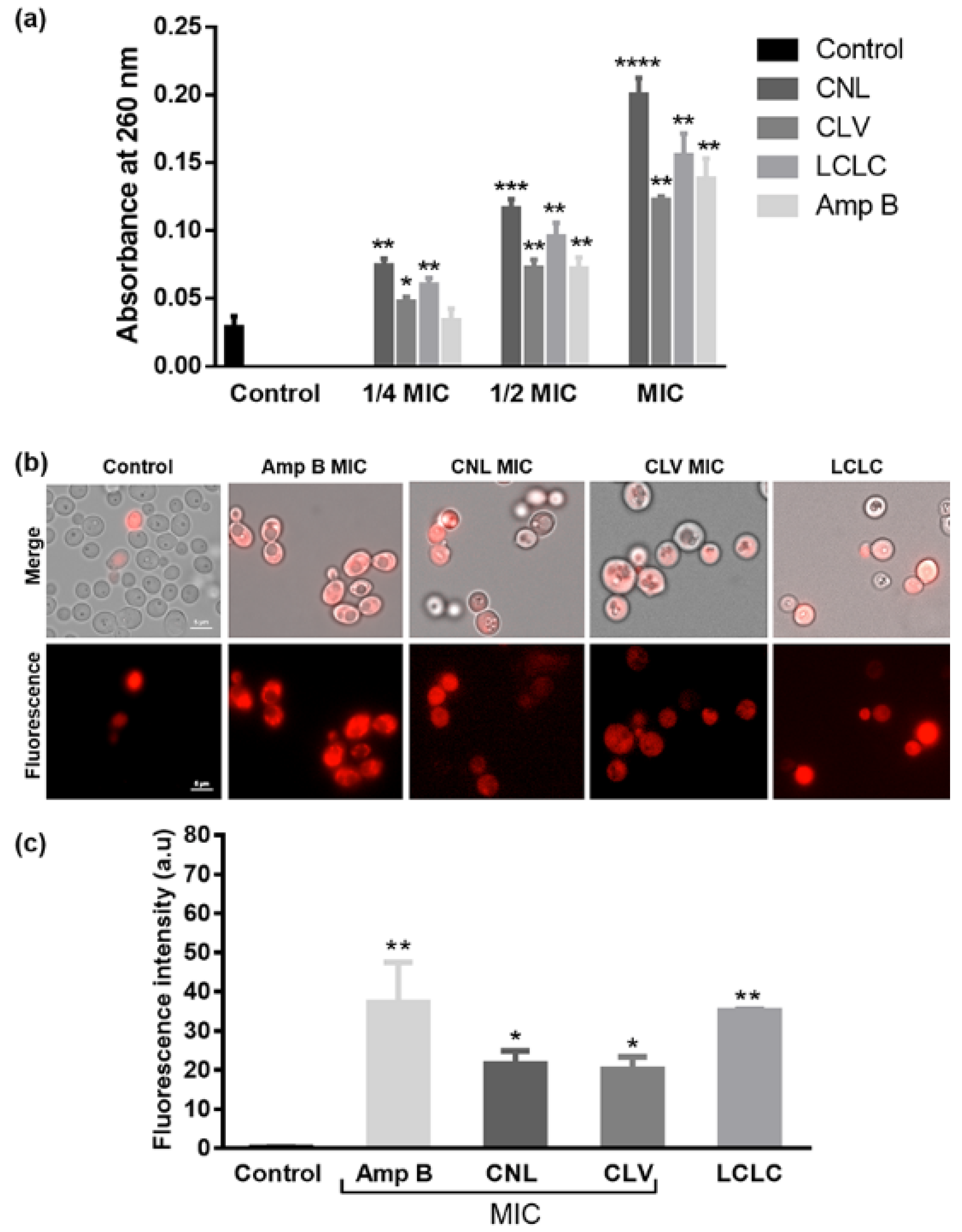
2.5. Membrane Leakage Assays
2.6. Propidium Iodide Uptake Assay
2.7. Yeast-to-Hyphal Transition Assay
2.8. Mycelial Growth Assay
2.9. Phospholipase and Proteinase Activity Assay
2.10. Statistical Analyses
3. Results
3.1. CNL and CLV Essential Oils Share Similar Major Components
3.2. CNL and CLV Are Additive and Synergistic against C. albicans
3.3. EOs Compromise Cell Membrane Integrity
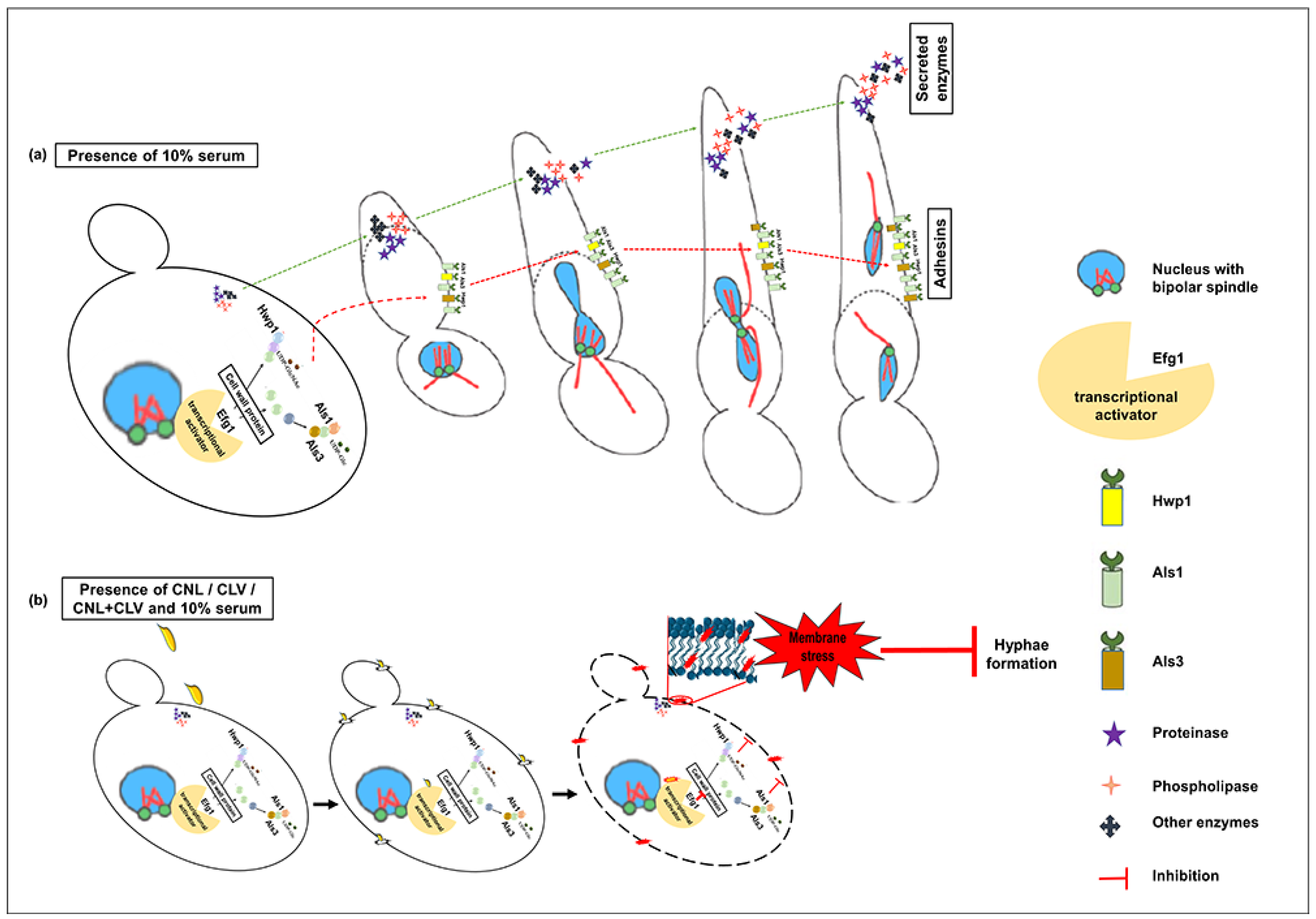
3.4. EOs Inhibit Germ Tube Formation in C. albicans RSY150
3.5. CNL with CLV Inhibits C. albicans Mycelial Growth
3.6. EO Exposure Impacts Exoenzyme Secretion
3.7. C. albicans Virulence Mutants have Differential Sensitivity to CNL and CLV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [Green Version]
- Sims, C.R.; Ostrosky-Zeichner, L.; Rex, J.H. Invasive Candidiasis in Immunocompromised Hospitalized Patients. Arch. Med. Res. 2005, 36, 660–671. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and Virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic Enzymes as Virulence Factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal Drug Resistance: Mechanisms, Epidemiology, and Consequences for Treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Vanden Bossche, H.; Dromer, F.; Improvisi, I.; Lozano-Chiu, M.; Rex, J.H.; Sanglard, D. Antifungal Drug Resistance in Pathogenic Fungi. Med. Mycol. 1998, 36 (Suppl. S1), 119–128. [Google Scholar] [PubMed]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting Virulence: A New Paradigm for Antifungals. Drug. Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting Virulence: A New Paradigm for Antimicrobial Therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A. In Vitro Anticandidal Potency of Syzygium Aromaticum (Clove) Extracts against Vaginal Candidiasis. BMC Complement. Med. Ther. 2020, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.A.; Ahmad, I. In Vitro Influence of Certain Essential Oils on Germ Tube Formation, Cell Surface Hydrophobicity, and Production of Proteinase and Hemolysin in Candida albicans. J. Nat. Pharm. 2012, 3, 110–117. [Google Scholar] [CrossRef]
- Pires, R.H.; Montanari, L.B.; Martins, C.H.; Zaia, J.E.; Almeida, A.M.; Matsumoto, M.T.; Mendes-Giannini, M.J. Anticandidal Efficacy of Cinnamon Oil against Planktonic and Biofilm Cultures of Candida parapsilosis and Candida orthopsilosis. Mycopathologia 2011, 172, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Jantan, I.B.; Karim Moharam, B.A.; Santhanam, J.; Jamal, J.A. Correlation between Chemical Composition and Antifungal Activity of the Essential Oils of Eight Cinnamomum. Species. Pharm. Biol. 2008, 46, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical Composition, Antioxidant and Antifungal Potential of Clove (Syzygium Aromaticum) Essential Oil, Its Major Compound and Its Derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against E. Coli with an Improved Method. J. Food Sci. 2009, 74, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavy, M.H.; Basti, A.A.; Misaghi, A.; Salehi, T.Z.; Abbasifar, R.; Mousavi, H.A.E.; Alipour, M.; Razavi, N.E.; Gandomi, H.; Noori, N. Effect of Zataria Multiflora Boiss. Essential Oil and Nisin on Salmonella Typhimurium and Staphylococcus Aureus in a Food Model System and on the Bacterial Cell Membranes. Food Res. Int. 2008, 41, 1050–1057. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined Antimicrobial Effect of Essential Oils and Bacteriocins against Foodborne Pathogens and Food Spoilage Bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Jafri, H.; Banerjee, G.; Khan, M.S.A.; Ahmad, I.; Abulreesh, H.H.; Althubiani, A.S. Synergistic Interaction of Eugenol and Antimicrobial Drugs in Eradication of Single and Mixed Biofilms of Candida albicans and Streptococcus Mutans. AMB Express 2020, 10, 185. [Google Scholar] [CrossRef]
- Da Silva, I.C.G.; de Pontes Santos, H.B.; Cavalcanti, Y.W.; Nonaka, C.F.W.; de Sousa, S.A.; de Castro, R.D. Antifungal Activity of Eugenol and Its Association with Nystatin on Candida albicans. Pesqui. Bras. Odontopediatria Clin. Integr. 2017, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shahina, Z.; El-Ganiny, A.M.; Minion, J.; Whiteway, M.; Sultana, T.; Dahms, T.E.S. Cinnamomum Zeylanicum Bark Essential Oil Induces Cell Wall Remodelling and Spindle Defects in Candida albicans. Fungal Biol. Biotechnol. 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, K.H.; Lee, D.W.; Park, H.M.; Rhee, Y.H. Inhibition of Fungal Cell Wall Synthesizing Enzymes by Trans-Cinnamaldehyde. Biosci. Biotechnol. Biochem. 2000, 64, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; de, L.; Aquino, S.G.D.; Lima, J.M.D.; Castellano, L.R.; Castro, R.D.D. In Vitro Effect of Cinnamomum Zeylanicum Blume Essential Oil on Candida spp. Involved in Oral Infections. Evid.-Based Complement. Altern. Med. 2018, 2018, 4045013. [Google Scholar] [CrossRef] [Green Version]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal Activity of the Clove Essential Oil from Syzygium Aromaticum on Candida, Aspergillus and Dermatophyte Species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Sasso, M.D.; Culici, M.; Alfieri, M. Eugenol and Thymol, Alone or in Combination, Induce Morphological Alterations in the Envelope of Candida albicans. Fitoterapia 2007, 78, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, E.; Omidi, M.; Bushehri, A.A.; Golshani, A.; Smith, M.L. The Antifungal Eugenol Perturbs Dual Aromatic and Branched-Chain Amino Acid Permeases in the Cytoplasmic Membrane of Yeast. PLoS ONE 2013, 8, e76028. [Google Scholar] [CrossRef] [Green Version]
- CLSI Clinical and Laboratory Standards Institute (CLSI). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Stringaro, A.; Vavala, E.; Colone, M.; Pepi, F.; Mignogna, G.; Garzoli, S.; Cecchetti, S.; Ragno, R.; Angiolella, L. Effects of Mentha Suaveolens Essential Oil Alone or in Combination with Other Drugs in Candida albicans. Evid.-Based Complement. Altern. Med. 2014, 2014, 125904. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.R.; Worthington, T.; Conway, B.R.; Lambert, P.A. Antimicrobial Efficacy of Eucalyptus Oil and 1,8-Cineole Alone and in Combination with Chlorhexidine Digluconate against Microorganisms Grown in Planktonic and Biofilm Cultures. J. Antimicrob. Chemother. 2009, 64, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal Effects of Melaleuca Alternifolia (Tea Tree) Oil and Its Components on Candida albicans, Candida Glabrata and Saccharomyces Cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Fiołka, M.J.; Czaplewska, P.; Macur, K.; Buchwald, T.; Kutkowska, J.; Paduch, R.; Kaczyński, Z.; Wydrych, J.; Urbanik-Sypniewska, T. Anti-Candida albicans Effect of the Protein-Carbohydrate Fraction Obtained from the Coelomic Fluid of Earthworm Dendrobaena Veneta. PLoS ONE 2019, 14, e0212869. [Google Scholar] [CrossRef] [Green Version]
- Setiawati, S.; Nuryastuti, T.; Ngatidjan, N.; Mustofa, M.; Jumina, J.; Fitriastuti, D. In Vitro Antifungal Activity of (1)-N-2-Methoxybenzyl-1,10-Phenanthrolinium Bromide against Candida albicans and Its Effects on Membrane Integrity. Mycobiology 2017, 45, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budzyńska, A.; Sadowska, B.; Więckowska-Szakiel, M.; Różalska, B. Enzymatic Profile, Adhesive and Invasive Properties of Candida albicans under the Influence of Selected Plant Essential Oils. Acta Biochim. Pol. 2014, 61, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Liao, K.; Wang, D. Effects of Magnolol and Honokiol on Adhesion, Yeast-Hyphal Transition, and Formation of Biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [Green Version]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate Method for Detection of Phospholipase Activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998; ISBN 9783930826483. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream 2005, 16, 65–120. [Google Scholar]
- Abdelwahab, S.I.; Mariod, A.A.; Taha, M.M.E.; Zaman, F.Q.; Abdelmageed, A.H.A.; Khamis, S.; Sivasothy, Y.; Awang, K. Chemical Composition and Antioxidant Properties of the Essential Oil of Cinnamomum Altissimum Kosterm. (Lauraceae). Arab. J. Chem. 2017, 10, 131–135. [Google Scholar] [CrossRef] [Green Version]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, L.; Jayawardena, B.; Abeywickrama, K. Fungicidal Activity of Essential Oils of Cinnamomum Zeylanicum (L.) and Syzygium Aromaticum (L.) Merr et L.M. Perry against Crown Rot and Anthracnose Pathogens Isolated from Banana. Lett. Appl. Microbiol. 2002, 35, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Faixová, Z.; Faix, S. Biological Effects of Rosemary Essential Oil (Review). Folia Vet. 2008, 52, 135–139. [Google Scholar]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczyńska, A. Effect of Clove and Thyme Essential Oils on Candida Biofilm Formation and the Oil Distribution in Yeast Cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, L.M.; Konopka, J.B. Plasma Membrane Organization Promotes Virulence of the Human Fungal Pathogen Candida albicans. J. Microbiol. 2016, 54, 178–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, H.J.; Köhler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous, C. albicans Mutants Are Avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef] [Green Version]
- Saville, S.P.; Lazzell, A.L.; Chaturvedi, A.K.; Monteagudo, C.; Lopez-Ribot, J.L. Efficacy of a Genetically Engineered Candida albicans Tet-NRG1 Strain as an Experimental Live Attenuated Vaccine against Hematogenously Disseminated Candidiasis. Clin. Vaccine Immunol. 2009, 16, 430–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musthafa, K.S.; Hmoteh, J.; Thamjarungwong, B.; Voravuthikunchai, S.P. Antifungal Potential of Eugenyl Acetate against Clinical Isolates of Candida Species. Microb. Pathog. 2016, 99, 19–29. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Brown, A.J.P.; Odds, F.C. Fungal Morphogenesis and Host Invasion. Curr. Opin. Microbiol. 2002, 5, 366–371. [Google Scholar] [CrossRef]
- Fan, Y.; He, H.; Dong, Y.; Pan, H. Hyphae-Specific Genes HGC1, ALS3, HWP1, and ECE1 and Relevant Signaling Pathways in Candida albicans. Mycopathologia 2013, 176, 329–335. [Google Scholar] [CrossRef]
- Hoyer, L.L. The ALS Gene Family of Candida albicans. Trends Microbiol. 2001, 9, 176–180. [Google Scholar] [CrossRef]
- Li, F.; Palecek, S.P. EAP1, a Candida albicans Gene Involved in Binding Human Epithelial Cells. Eukaryot. Cell 2003, 2, 1266–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobile, C.J.; Schneider, H.A.; Nett, J.E.; Sheppard, D.C.; Filler, S.G.; Andes, D.R.; Mitchell, A.P. Complementary Adhesin Function in C. Albicans Biofilm Formation. Curr. Biol. 2008, 18, 1017–1024. [Google Scholar] [CrossRef]
- Almeida, R.S.; Brunke, S.; Albrecht, A.; Thewes, S.; Laue, M.; Edwards, J.E.; Filler, S.G.; Hube, B. The Hyphal-Associated Adhesin and Invasin Als3 of Candida albicans Mediates Iron Acquisition from Host Ferritin. PLoS Pathog. 2008, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans Biofilm Development Is Governed by Cooperative Attachment and Adhesion Maintenance Proteins. Npj Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising Polyphenols for the Clinical Management of Candida albicans Biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.C.; Lai, W.L.; Chuang, K.C.; Lee, M.H.; Tsai, Y.C. The Inhibitory Activity of Linalool against the Filamentous Growth and Biofilm Formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthamil, S.; Balasubramaniam, B.; Balamurugan, K.; Pandian, S.K. Synergistic Effect of Quinic Acid Derived From Syzygium Cumini and Undecanoic Acid Against Candida spp. Biofilm and Virulence. Front. Microbiol. 2018, 9, 2835. [Google Scholar] [CrossRef] [Green Version]
- Lone, S.A.; Ahmad, A. Inhibitory Effect of Novel Eugenol Tosylate Congeners on Pathogenicity of Candida albicans. BMC Complement. Med. Ther. 2020, 20, 131. [Google Scholar] [CrossRef]
- Bai, C.; Xu, X.L.; Chan, F.Y.; Lee, R.T.H.; Wang, Y. MNN5 Encodes an Iron-Regulated α-1,2-Mannosyltransferase Important for Protein Glycosylation, Cell Wall Integrity, Morphogenesis, and Virulence in Candida albicans. Eukaryot. Cell 2006, 5, 238–247. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.; MacCallum, D.M.; Bertram, G.; Munro, C.A.; Hughes, H.B.; Buurman, E.T.; Brown, A.J.P.; Odds, F.C.; Gow, N.A.R. Candida albicans Pmr1p, a Secretory Pathway P-Type Ca2+/Mn2+-ATPase, Is Required for Glycosylation and Virulence. J. Biol. Chem. 2005, 280, 23408–23415. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.; Hughes, H.B.; Munro, C.A.; Thomas, W.P.H.; MacCallum, D.M.; Bertram, G.; Atrih, A.; Ferguson, M.A.J.; Brown, A.J.P.; Odds, F.C.; et al. Outer Chain N-Glycans Are Required for Cell Wall Integrity and Virulence of Candida albicans. J. Biol. Chem. 2006, 281, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, L.L.; McNemar, M.D.; Saporito-Irwin, S.M.; Sypherd, P.S.; Fonzi, W.A. HWP1 Functions in the Morphological Development of Candida albicans Downstream of EFG1, TUP1, and RBF1. J. Bacteriol. 1999, 181, 5273–5279. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; VandeWalle, K.; López-Ribot, J.L.; Wickes, B.L. The Filamentation Pathway Controlled by the Efg1 Regulator Protein Is Required for Normal Biofilm Formation and Development in Candida albicans. FEMS Microbiol. Lett. 2002, 214, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.W.L.; Pang, L.M.; Wang, Y. From Jekyll to Hyde: The Yeast–Hyphal Transition of Candida albicans. Pathogens 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, R.K.; Bennett, R.J. Microtubule Motor Protein Kar3 Is Required for Normal Mitotic Division and Morphogenesis in Candida albicans. Eukaryot. Cell 2008, 7, 1460–1474. [Google Scholar] [CrossRef] [Green Version]
- Gillum, A.M.; Tsay, E.Y.H.; Kirsch, D.R. Isolation of the Candida albicans Gene for Orotidine-5′-Phosphate Decarboxylase by Complementation of S. Cerevisiae Ura3 and E. Coli PyrF Mutations. MGG Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef]
- Roemer, T.; Jiang, B.; Davison, J.; Ketela, T.; Veillette, K.; Breton, A.; Tandia, F.; Linteau, A.; Sillaots, S.; Marta, C.; et al. Large-Scale Essential Gene Identification in Candida albicans and Applications to Antifungal Drug Discovery. Mol. Microbiol. 2003, 50, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Oh, S.H.; Cheng, G.; Green, C.B.; Nuessen, J.A.; Yeater, K.; Leng, R.P.; Brown, A.J.P.; Hoyer, L.L. ALS3 and ALS8 Represent a Single Locus That Encodes a Candida albicans Adhesin; Functional Comparisons between Als3p and Als1p. Microbiology 2004, 150, 2415–2428. [Google Scholar] [CrossRef]


| (a) CNL | ||||
|---|---|---|---|---|
| Compound | % Yield | Exp RI a | Lit RI a | ID Method b |
| α-pinene | 1 | 935 | 932 | MS |
| camphene | 0.4 | 950 | 946 | MS |
| benzaldehyde | 0.3 | 961 | 952 | RI, MS |
| β-pinene/sabinene * | 0.3 | 979 | 974 | MS |
| α-phellandrene | 1.2 | 1007 | 1002 | RI, MS |
| α-terpinene | 0.1 | 1018 | 1014 | RI, MS |
| p-cymene | 0.9 | 1026 | 1020 | RI, MS |
| limonene | 0.7 | 1031 | 1025 | MS |
| terpinolene | 0.1 | 1090 | 1086 | RI, MS |
| linalool | 2.5 | 1102 | 1095 | RI, MS |
| α-terpineol | 0.2 | 1193 | 1186 | RI, MS |
| E-cinnamaldehyde | 1.3 | 1277 | 1267 | MS |
| cinnamyl alcohol | 0.2 | 1314 | 1259 | MS |
| eugenol | 73 | 1376 | 1361 | MS |
| α-copaene | 2.4 | 1383 | 1345 | MS |
| β-caryophyllene | 4.8 | 1428 | 1412 | MS |
| E-cinnamyl acetate | 2.3 | 1450 | 1443 | RI, MS |
| α-humulene | 0.8 | 1461 | 1452 | MS |
| viridiflorene | 0.2 | 1501 | 1471 | MS |
| δ−χαδινενε | 0.2 | 1529 | 1501 | MS |
| eugenyl acetate | 2 | 1534 | 1521 | RI, MS |
| caryophyllene oxide | 0.4 | 1591 | 1582 | MS |
| benzyl benzoate | 2.4 | 1772 | 1759 | RI, MS |
| Total | 97.7 | |||
| (b) CLV | ||||
| Compound | % Yield | Exp RI a | Lit RI a | ID Method b |
| eugenol | 75 | 1378 | 1361 | RI, MS |
| methyl eugenol | 0.2 | 1406 | 1403 | MS |
| β-caryophyllene | 11.7 | 1416 | 1412 | RI, MS |
| α-humulene | 2 | 1451 | 1446 | RI, MS |
| δ−χαδινενε | 0.2 | 1522 | 1518 | RI, MS |
| eugenyl acetate | 9.9 | 1529 | 1526 | RI, MS |
| caryophyllene oxide | 0.4 | 1580 | 1573 | RI, MS |
| Total | 99.4 | |||
| MIC Range (µg/mL) | ||
|---|---|---|
| C. albicans Strains | CNL | CLV |
| RSY150 | 500–600 | 469–600 |
| ATCC 10231 | 1000–1250 | 750–938 |
| Genital Specimen | 625–1000 | 750–938 |
| Blood Culture | 625–1000 | 750–938 |
| Fluconazole Resistant Genital Specimen | 500–625 | 469–750 |
| Enzyme Activity | Control | CNL 1/2 MIC | CLV 1/2 MIC | CNL+CLV(1/2) ‡ |
|---|---|---|---|---|
| C. albicans RSY150 | ||||
| Phospholipase (Pz) | 0.86 ± 0.02 | 0.89 ± 0.04 | 1.00 ± 0.01 * | 1.00 ± 0.02 * |
| Proteinase (Prz) | 0.32 ± 0.05 | 1.00 ± 0.01 * | 1.00 ± 0.03 * | 1.00 ± 0.05 * |
| C. albicans ATCC 10231 | ||||
| Phospholipase (Pz) | 0.65 ± 0.05 | 0.76 ± 0.00 * | 0.70 ± 0.03 | 0.73 ± 0.01 * |
| Proteinase (Prz) | 0.56 ± 0.05 | 1.00 ± 0.01 * | 0.83 ± 0.03 * | 1.00 ± 0.02 * |
| C. albicans Strains | CNL | CLV | Amp B |
|---|---|---|---|
| als1Δ/Δ (1467) | NC | + | NC |
| als3Δ/Δ (1843) | + | NC | NC |
| hwp1Δ/HWP1+ | ++ | ++ | NC |
| HLC67(efg1Δ/Δ) | +++ | ++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahina, Z.; Molaeitabari, A.; Sultana, T.; Dahms, T.E.S. Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida albicans Virulence Traits. Microorganisms 2022, 10, 1989. https://doi.org/10.3390/microorganisms10101989
Shahina Z, Molaeitabari A, Sultana T, Dahms TES. Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida albicans Virulence Traits. Microorganisms. 2022; 10(10):1989. https://doi.org/10.3390/microorganisms10101989
Chicago/Turabian StyleShahina, Zinnat, Ali Molaeitabari, Taranum Sultana, and Tanya Elizabeth Susan Dahms. 2022. "Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida albicans Virulence Traits" Microorganisms 10, no. 10: 1989. https://doi.org/10.3390/microorganisms10101989
APA StyleShahina, Z., Molaeitabari, A., Sultana, T., & Dahms, T. E. S. (2022). Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida albicans Virulence Traits. Microorganisms, 10(10), 1989. https://doi.org/10.3390/microorganisms10101989