Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. M. hominis
2.2. Cell Line
2.3. Infectious Dose and Growth Curve
2.4. Clonogenic Assay
2.5. Sub-G0/G1 DNA Content and Cell Cycle Analyses Using Flow Cytometry
2.6. Protein Extraction and Immunoblotting
2.7. Cytokine Quantification
2.8. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analysis
3. Results
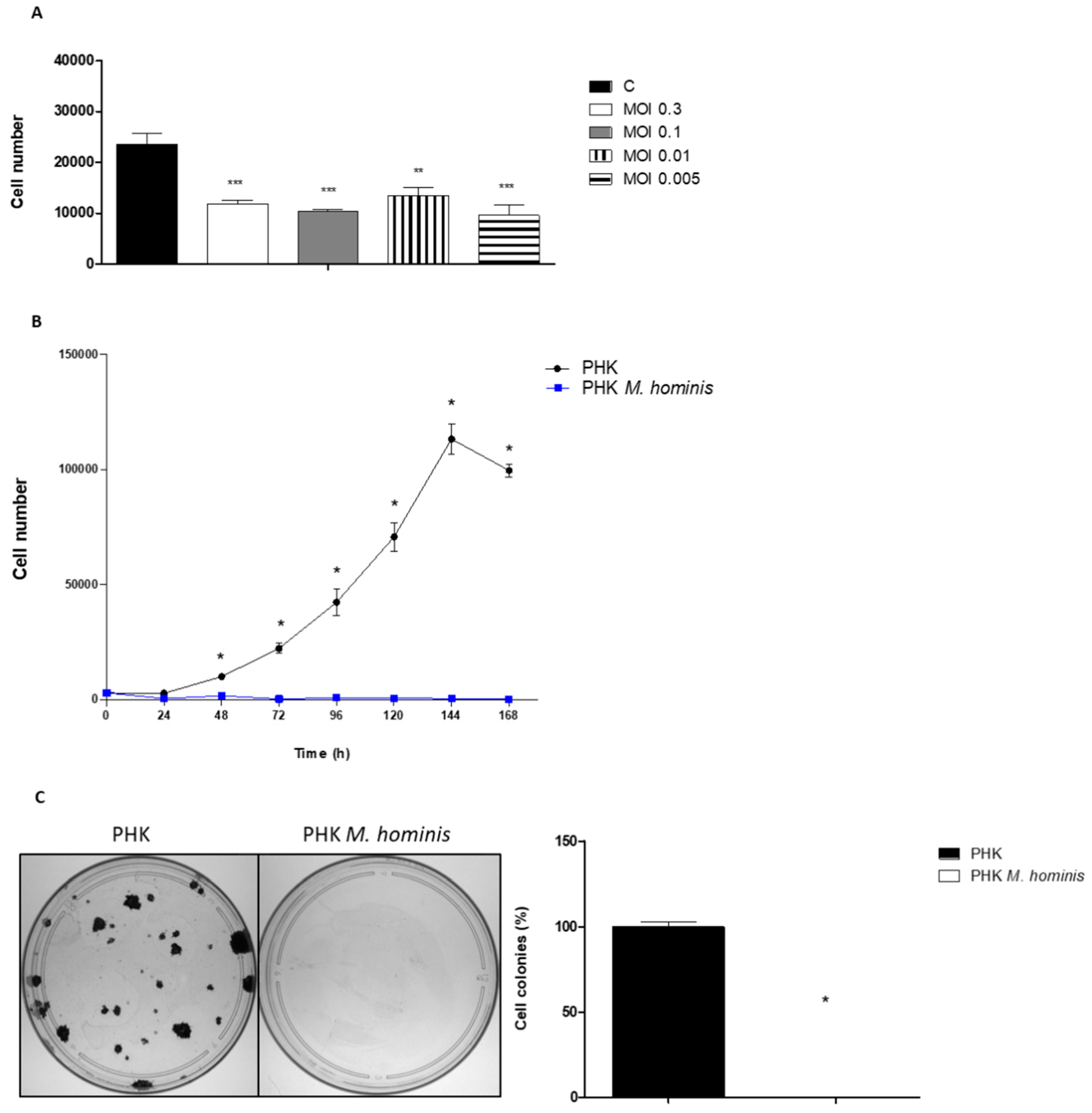
3.1. M. hominis Infection Alters the Growth of Keratinocytes Even at Low Doses
3.2. Infection by M. hominis Alters the Cell Cycle, Thus Promoting the Activation of DNA Damage and Oxidative Stress Signaling Pathways in Keratinocytes
3.3. M. hominis Infection Alters the Expression of Toll-like Receptors and Induces the Release of Pro-Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hopfe, M.; Deenen, R.; Degrandi, D.; Köhrer, K.; Henrich, B. Host Cell Responses to Persistent Mycoplasmas—Different Stages in Infection of HeLa Cells with Mycoplasma hominis. PLoS ONE 2013, 8, e54219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fang, D.-C.; Lan, C.-H.; Luo, Y.-H. Helicobacter pylori infection induces apoptosis in gastric cancer cells through the mitochondrial pathway. J. Gastroenterol. Hepatol. 2007, 22, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lo, S.-C. Effect of Mycoplasmas on Apoptosis of 32D Cells Is Species-Dependent. Curr. Microbiol. 2007, 54, 388–395. [Google Scholar] [CrossRef] [PubMed]
- A Keane, E.; Thomas, B.J.; Gilroy, C.B.; Renton, A.; Taylor-Robinson, D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: Observations on heterosexual women and their male partners. Int. J. STD AIDS 2000, 11, 356–360. [Google Scholar] [CrossRef]
- Campos, G.B.; Lobão, T.N.; Selis, N.N.; Amorim, A.T.; Martins, H.B.; Barbosa, D.S.D.; Oliveira, H.; Dos Santos, D.B.; Figueiredo, T.B.; Marques, L.M.; et al. Prevalence of Mycoplasma genitalium and Mycoplasma hominis in urogenital tract of Brazilian women. BMC Infect. Dis. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.M.; Fernandes, P.; Haddad, J.P.; Paiva, M.C.; Souza, M.D.C.M.; Andrade, T.C.A.; Fernandes, A.P. Frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma species in cervical samples. J. Obstet. Gynaecol. 2011, 31, 237–241. [Google Scholar] [CrossRef]
- Cox, C.; Watt, A.P.; McKenna, J.P.; Coyle, P.V. Mycoplasma hominis and Gardnerella vaginalis display a significant synergistic relationship in bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 481–487. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Fredlund, H.; Jensen, J.S.; Engstrand, L.; Unemo, M. Composition of the Vaginal Microbiota in Women of Reproductive Age—Sensitive and Specific Molecular Diagnosis of Bacterial Vaginosis Is Possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef] [Green Version]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host–Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Arya, O.P.; Tong, C.Y.W.; A Hart, C.; Pratt, B.C.; Hughes, S.; Roberts, P.; Kirby, P.; Howel, J.; McCormick, A.; Goddard, A.D. Is Mycoplasma hominis a vaginal pathogen? Sex. Transm. Infect. 2001, 77, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Garcia, E.K.; Contreras-Paredes, A.; Martinez-Abundis, E.; Garcia-Chan, D.; Lizano, M.; De La Cruz-Hernandez, E. Molecular epidemiology of bacterial vaginosis and its association with genital micro-organisms in asymptomatic women. J. Med Microbiol. 2019, 68, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, A.; Gupta, S.; Mittal, A.; Chandra, P.; Gill, A.K. Correlation of mycoplasma with unexplained infertility. Arch. Gynecol. Obstet. 2009, 280, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Spiller, O.B. Emerging Pathogenic Respiratory Mycoplasma hominis Infections in Lung Transplant Patients: Time to Reassesses it’s Role as a Pathogen? eBioMedicine 2017, 19, 8–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor-Robinson, D.; Jensen, J.S. Mycoplasma genitalium: From Chrysalis to Multicolored Butterfly. Clin. Microbiol. Rev. 2011, 24, 498–514. [Google Scholar] [CrossRef] [Green Version]
- Krause, D.C.; Leith, D.K.; Wilson, R.M.; Baseman, J.B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect. Immun. 1982, 35, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Díaz-García, F.J.; Herrera-Mendoza, A.P.; Giono-Cerezo, S.; Guerra-Infante, F.M. Mycoplasma hominis attaches to and locates intracellularly in human spermatozoa. Hum. Reprod. 2006, 21, 1591–1598. [Google Scholar] [CrossRef] [Green Version]
- Henrich, B.; Kretzmer, F.; Deenen, R.; Köhrer, K. Validation of a novel Mho microarray for a comprehensive characterisation of the Mycoplasma hominis action in HeLa cell infection. PLoS ONE 2017, 12, e0181383. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.-H.; Tsai, S.; Rodriguez, J.; Lo, S.-C. Mycoplasmal Infections Prevent Apoptosis and Induce Malignant Transformation of Interleukin-3-Dependent 32D Hematopoietic Cells. Mol. Cell. Biol. 1999, 19, 7995–8002. [Google Scholar] [CrossRef] [Green Version]
- Barykova, Y.A.; Logunov, D.Y.; Shmarov, M.M.; Vinarov, A.Z.; Fiev, D.N.; Vinarova, N.A.; Rakovskaya, I.V.; Baker, P.S.; Shyshynova, I.; Stephenson, A.J.; et al. Association of Mycoplasma hominis infection with prostate cancer. Oncotarget 2011, 2, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Sfanos, K.S.; Isaacs, J.T. The “Infectious” Nature of Human Prostate Cancer: A Cautionary Note. Oncotarget 2011, 2, 281–283. [Google Scholar] [CrossRef] [Green Version]
- Rogers, M.B. Mycoplasma and cancer: In search of the link. Oncotarget 2011, 2, 271–273. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Zakariah, M.; Rolfo, C.; Robrecht, L.; Palaniappan, S. Prediction of mycoplasma hominis proteins targeting in mitochondria and cytoplasm of host cells and their implication in prostate cancer etiology. Oncotarget 2017, 8, 30830–30843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logunov, D.Y.; Scheblyakov, D.V.; Zubkova, O.V.; Shmarov, M.M.; Rakovskaya, I.V.; Gurova, K.V.; Tararova, N.D.; Burdelya, L.G.; Naroditsky, B.S.; Ginzburg, A.L.; et al. Mycoplasma infection suppresses p53, activates NF-κB and cooperates with oncogenic Ras in rodent fibroblast transformation. Oncogene 2008, 27, 4521–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caini, S.; Gandini, S.; Dudas, M.; Bremer, V.; Severi, E.; Gherasim, A. Sexually transmitted infections and prostate cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2014, 38, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Razin, S.; Tully, J.G. Molecular and Diagnostic Procedures in Mycoplasmology: Molecular characterization; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- You, X.; Wu, Y.; Zeng, Y.; Deng, Z.; Qiu, H.; Yu, M. Mycoplasma genitalium-derived lipid-associated membrane proteins induce activation of MAPKs, NF-κB and AP-1 in THP-1 cells. FEMS Immunol. Med. Microbiol. 2008, 52, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Wang, S.; Zeng, Y.; You, X.; Ma, X.; Wu, N.; Wu, Y. Binding of CD14 to Mycoplasma genitalium-Derived Lipid-Associated Membrane Proteins Upregulates TNF-α. Inflammation 2014, 37, 322–330. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Quinet, A.; Vessoni, A.T.; Rocha, C.R.; Gottifredi, V.; Biard, D.; Sarasin, A.; Menck, C.F.; Stary, A. Gap-filling and bypass at the replication fork are both active mechanisms for tolerance of low-dose ultraviolet-induced DNA damage in the human genome. DNA Repair 2014, 14, 27–38. [Google Scholar] [CrossRef]
- Jiang, W.; Yan, Y.; Ji, W.; Wang, Y.; Chen, Z. Clinical significance of different bacterial load of Mycoplasma pneumoniae in patients with Mycoplasma pneumoniae pneumonia. Braz. J. Infect. Dis. 2014, 18, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Horner, P.; Donders, G.; Cusini, M.; Gomberg, M.; Jensen, J.; Unemo, M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticumin men and women?—A position statement from the EuropeanSTIGuidelines Editorial Board. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1845–1851. [Google Scholar] [CrossRef] [Green Version]
- Foschi, C.; Salvo, M.; D’Antuono, A.; Gaspari, V.; Banzola, N.; Cevenini, R.; Marangoni, A. Distribution of genital Mollicutes in the vaginal ecosystem of women with different clinical conditions. New Microbiol. 2018, 41, 225–229. [Google Scholar] [PubMed]
- Taylor-Robinson, D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium. Res. Microbiol. 2017, 168, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.L.; Danielewski, J.; Bodiyabadu, K.; Machalek, D.A.; Bradshaw, C.S.; Costa, A.-M.; Birnie, J.; Garland, S.M. Analysis of Infection Loads in Mycoplasma genitalium Clinical Specimens by Use of a Commercial Diagnostic Test. J. Clin. Microbiol. 2019, 57, e00344-19. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Chicas, A.; Olivier, M.; Taya, Y.; Tyagi, S.; Kramer, F.R.; Bargonetti, J. A DNA damage signal is required for p53 to activate gadd45. Cancer Res. 2000, 60, 1711–1719. [Google Scholar]
- Smith, M.L.; Chen, I.T.; Zhan, Q.; Bae, I.; Chen, C.Y.; Gilmer, T.M.; Kastan, M.B.; O’Connor, P.M.; Fornace, A.J., Jr. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 1994, 266, 1376–1380. [Google Scholar] [CrossRef] [Green Version]
- Taylor, W.R.; Stark, G.R. Regulation of the G2/M transition by p53. Oncogene 2001, 20, 1803–1815. [Google Scholar] [CrossRef] [Green Version]
- Fornace, A.J.; Nebert, D.W.; Hollander, M.C.; Luethy, J.D.; Papathanasiou, M.; Fargnoli, J.; Holbrook, N.J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 1989, 9, 4196–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbastabar, M.; Kheyrollah, M.; Azizian, K.; Bagherlou, N.; Tehrani, S.S.; Maniati, M.; Karimian, A. Multiple functions of p27 in cell cycle, apoptosis, epigenetic modification and transcriptional regulation for the control of cell growth: A double-edged sword protein. DNA Repair 2018, 69, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Tanaka, Y.; Oshino, R.; Okado, S.; Hori, M.; Isobe, K.-I. GADD34 suppresses lipopolysaccharide-induced sepsis and tissue injury through the regulation of macrophage activation. Cell Death Dis. 2016, 7, e2219. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Q.; A Lord, K.; Alamo, I.; Hollander, M.C.; Carrier, F.; Ron, D.; Kohn, K.W.; Hoffman, B.; A Liebermann, D.; Fornace, A. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 1994, 14, 2361–2371. [Google Scholar] [CrossRef] [Green Version]
- Hollander, M.C.; Zhan, Q.; Bae, I.; Fornace, A.J. Mammalian GADD34, an Apoptosis- and DNA Damage-inducible Gene. J. Biol. Chem. 1997, 272, 13731–13737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, M.C.; Poola-Kella, S.; Fornace, A.J. Gadd34 functional domains involved in growth suppression and apoptosis. Oncogene 2003, 22, 3827–3832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetti, F.; Curreli, S.; Zella, D. Mycoplasmas–Host Interaction: Mechanisms of Inflammation and Association with Cellular Transformation. Microorganisms 2020, 8, 1351. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Gueven, N. The complexity of p53 stabilization and activation. Cell Death Differ. 2006, 13, 941–950. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle 2010, 9, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Kruse, J.-P.; Gu, W. Modes of p53 Regulation. Cell 2009, 137, 609–622. [Google Scholar] [CrossRef] [Green Version]
- Haronikova, L.; Olivares-Illana, V.; Wang, L.; Karakostis, K.; Chen, S.; Fåhraeus, R. The p53 mRNA: An integral part of the cellular stress response. Nucleic Acids Res. 2019, 47, 3257–3271. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Lin, Y.; Wu, X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002, 16, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Ben-Menachem, G.; Mousa, A.; Brenner, T.; Pinto, F.; Zã¤Hringer, U.; Rottem, S. Choline deficiency induced by Mycoplasma fermentans enhances apoptosis of rat astrocytes. FEMS Microbiol. Lett. 2001, 201, 157–162. [Google Scholar] [CrossRef]
- Wang, K.; Li, L.; Zhang, Y.; Gao, D. Crosstalk between signaling pathways and DNA damage response. Genome Instab. Dis. 2019, 1, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Buzańska, L.; Zabłocka, B.; Dybel, A.; Domańska-Janik, K.; Albrecht, J. Delayed induction of apoptosis by ammonia in C6 glioma cells. Neurochem. Int. 2000, 37, 287–297. [Google Scholar] [CrossRef]
- Thatte, H.S.; Rhee, J.-H.; E Zagarins, S.; Treanor, P.R.; Birjiniuk, V.; Crittenden, M.D.; Khuri, S.F. Acidosis-induced apoptosis in human and porcine heart. Ann. Thorac. Surg. 2004, 77, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-K.; Chen, S.-E.; Chang, L.-C. The Role of HO-1 and Its Crosstalk with Oxidative Stress in Cancer Cell Survival. Cells 2021, 10, 2401. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-S.; Zhou, Y.-N.; Li, L.; Li, S.-F.; Long, D.; Chen, X.-L.; Zhang, J.-B.; Feng, L.; Li, Y.-P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, 25, 101109. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, Z.; Zhang, H.; Xie, B. Analysis of the Oxidative Stress Status in Nonspecific Vaginitis and Its Role in Vaginal Epithelial Cells Apoptosis. BioMed Res. Int. 2015, 2015, 795656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almagor, M.; Kahane, I.; Yatziv, S. Role of superoxide anion in host cell injury induced by mycoplasma pneumoniae infection. A study in normal and trisomy 21 cells. J. Clin. Investig. 1984, 73, 842–847. [Google Scholar] [CrossRef]
- Miles, R.J.; Taylor, R.R.; Varsani, H. Oxygen uptake and H2O2 production by fermentative Mycoplasma spp. J. Med Microbiol. 1991, 34, 219–223. [Google Scholar] [CrossRef]
- Sun, G.; Xu, X.; Wang, Y.; Shen, X.; Chen, Z.; Yang, J. Mycoplasma pneumoniae Infection Induces Reactive Oxygen Species and DNA Damage in A549 Human Lung Carcinoma Cells. Infect. Immun. 2008, 76, 4405–4413. [Google Scholar] [CrossRef] [Green Version]
- Vitula, F.; Peckova, L.; Bandouchova, H.; Pohanka, M.; Novotny, L.; Jira, D.; Kral, J.; Ondracek, K.; Osickova, J.; Zendulkova, D.; et al. Mycoplasma gallisepticum infection in the grey partridge Perdix perdix: Outbreak description, histopathology, biochemistry and antioxidant parameters. BMC Vet. Res. 2011, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Nunoya, T.; Tajima, M.; Yagihashi, T. Decrease in catalase activity of cultured cells by mycoplasma gallisepticum infection. Vet. Microbiol. 1987, 13, 343–351. [Google Scholar] [CrossRef]
- Almagor, M.; Yatziv, S.; Kahane, I. Inhibition of host cell catalase by Mycoplasma pneumoniae: A possible mechanism for cell injury. Infect. Immun. 1983, 41, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, H.; Freitas, C.; Alves, P.; Moreira, J.; Carrondo, M. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzym. Microb. Technol. 2000, 27, 43–52. [Google Scholar] [CrossRef]
- Halliwell, B. Phagocyte-derived reactive species: Salvation or suicide? Trends Biochem. Sci. 2006, 31, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.d.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Bobermin, L.D.; Wartchow, K.M.; Flores, M.P.; Leite, M.C.; Quincozes-Santos, A.; Gonçalves, C.-A. Ammonia-induced oxidative damage in neurons is prevented by resveratrol and lipoic acid with participation of heme oxygenase. NeuroToxicology 2015, 49, 28–35. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Jiang, Y.; Zhao, Y.; Sun, L.; Zheng, B.; Chen, L.; Liu, Z.; Zheng, X.; Yi, K.; et al. Effects of ammonia on apoptosis and oxidative stress in bovine mammary epithelial cells. Mutagenesis 2018, 33, 291–299. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Jacquier-Sarlin, M.R.; Fuller, K.; Dinh-Xuan, A.T.; Richard, M.J.; Polla, B.S. Protective effects of hsp70 in inflammation. Experientia 1994, 50, 1031–1038. [Google Scholar] [CrossRef]
- van Eden, W.; Broere, F.; van der Zee, R. Heat Shock Proteins; Wiley Online Library: New York, NY, USA, 2017; pp. 813–830. [Google Scholar] [CrossRef]
- Spierings, J.; Van Eden, W. Heat shock proteins and their immunomodulatory role in inflammatory arthritis. Rheumatology 2017, 56, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, H.M. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD14. BMB Rep. 2017, 50, 55–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zariffard, M.R.; Novak, R.M.; Lurain, N.; Sha, B.E.; Graham, P.; Spear, G.T. Induction of Tumor Necrosis Factor–α Secretion and Toll-Like Receptor 2 and 4 mRNA Expression by Genital Mucosal Fluids from Women with Bacterial Vaginosis. J. Infect. Dis. 2005, 191, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Santos-Junior, M.N.; Rezende, I.S.; Souza, C.L.S.; Barbosa, M.S.; Campos, G.B.; Brito, L.F.; Queiroz, C.; Barbosa, E.N.; Teixeira, M.M.; Da Silva, L.O.; et al. Ureaplasma diversum and Its Membrane-Associated Lipoproteins Activate Inflammatory Genes Through the NF-κB Pathway via Toll-Like Receptor 4. Front. Microbiol. 2018, 9, 1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltier, M.R.; Freeman, A.J.; Mu, H.H.; Cole, B.C. Characterization of the Macrophage-Stimulating Activity from Ureaplasma urealyticum. Am. J. Reprod. Immunol. 2007, 57, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kimura, Y.; Kida, Y.; Kuwano, K.; Tachibana, M.; Hashino, M.; Watarai, M. Cytadherence of Mycoplasma pneumoniae Induces Inflammatory Responses through Autophagy and Toll-Like Receptor 4. Infect. Immun. 2014, 82, 3076–3086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahams, V.M.; Potter, J.A.; Bhat, G.; Peltier, M.R.; Saade, G.; Menon, R. Bacterial Modulation of Human Fetal Membrane Toll-like Receptor Expression. Am. J. Reprod. Immunol. 2013, 69, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Yu, J.; Zhou, X.; Li, Z.; Xia, Y.; Luo, Z.; Wu, Y. Synergism between upregulation of Rab7 and inhibition of autophagic degradation caused by mycoplasma facilitates intracellular mycoplasma infection. Mol. Med. Rep. 2014, 9, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Liu, X.; Li, D.; Chen, T.; Cai, Z.; Cao, X. Late Endosome/Lysosome-Localized Rab7b Suppresses TLR9-Initiated Proinflammatory Cytokine and Type I IFN Production in Macrophages. J. Immunol. 2009, 183, 1751–1758. [Google Scholar] [CrossRef] [Green Version]
- Schultz, T.; Blumenthal, A. The RP105/MD-1 complex: Molecular signaling mechanisms and pathophysiological implications. J. Leukoc. Biol. 2017, 101, 183–192. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, A.T.; Lino, V.d.S.; Marques, L.M.; Martins, D.J.; Braga Junior, A.C.R.; Campos, G.B.; Oliveira, C.N.T.; Boccardo, E.; Timenetsky, J. Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes. Microorganisms 2022, 10, 1962. https://doi.org/10.3390/microorganisms10101962
Amorim AT, Lino VdS, Marques LM, Martins DJ, Braga Junior ACR, Campos GB, Oliveira CNT, Boccardo E, Timenetsky J. Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes. Microorganisms. 2022; 10(10):1962. https://doi.org/10.3390/microorganisms10101962
Chicago/Turabian StyleAmorim, Aline Teixeira, Vanesca de Souza Lino, Lucas Miranda Marques, Davi Jardim Martins, Antonio Carlos Ricardo Braga Junior, Guilherme Barreto Campos, Caline Novais Teixeira Oliveira, Enrique Boccardo, and Jorge Timenetsky. 2022. "Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes" Microorganisms 10, no. 10: 1962. https://doi.org/10.3390/microorganisms10101962
APA StyleAmorim, A. T., Lino, V. d. S., Marques, L. M., Martins, D. J., Braga Junior, A. C. R., Campos, G. B., Oliveira, C. N. T., Boccardo, E., & Timenetsky, J. (2022). Mycoplasma hominis Causes DNA Damage and Cell Death in Primary Human Keratinocytes. Microorganisms, 10(10), 1962. https://doi.org/10.3390/microorganisms10101962





