Detection of Tick-Borne Pathogens in Ticks from Cattle in Western Highlands of Cameroon
Abstract
:1. Introduction
2. Materials and Methods
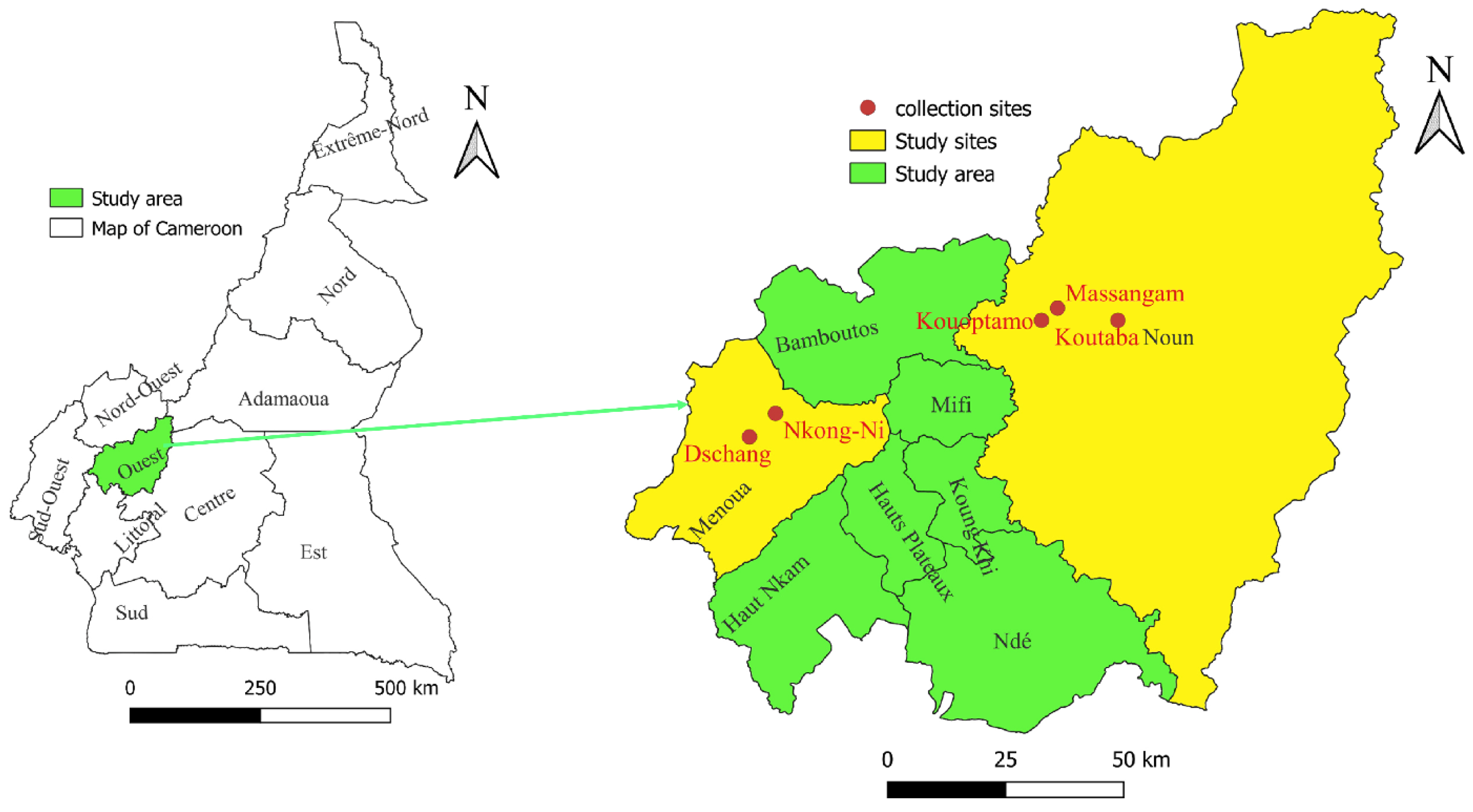
2.1. Study Area
2.2. Tick Collection and Morphological Identification
2.3. DNA Extraction and Molecular Detection of Microorganisms in Ticks Using Real-Time PCR
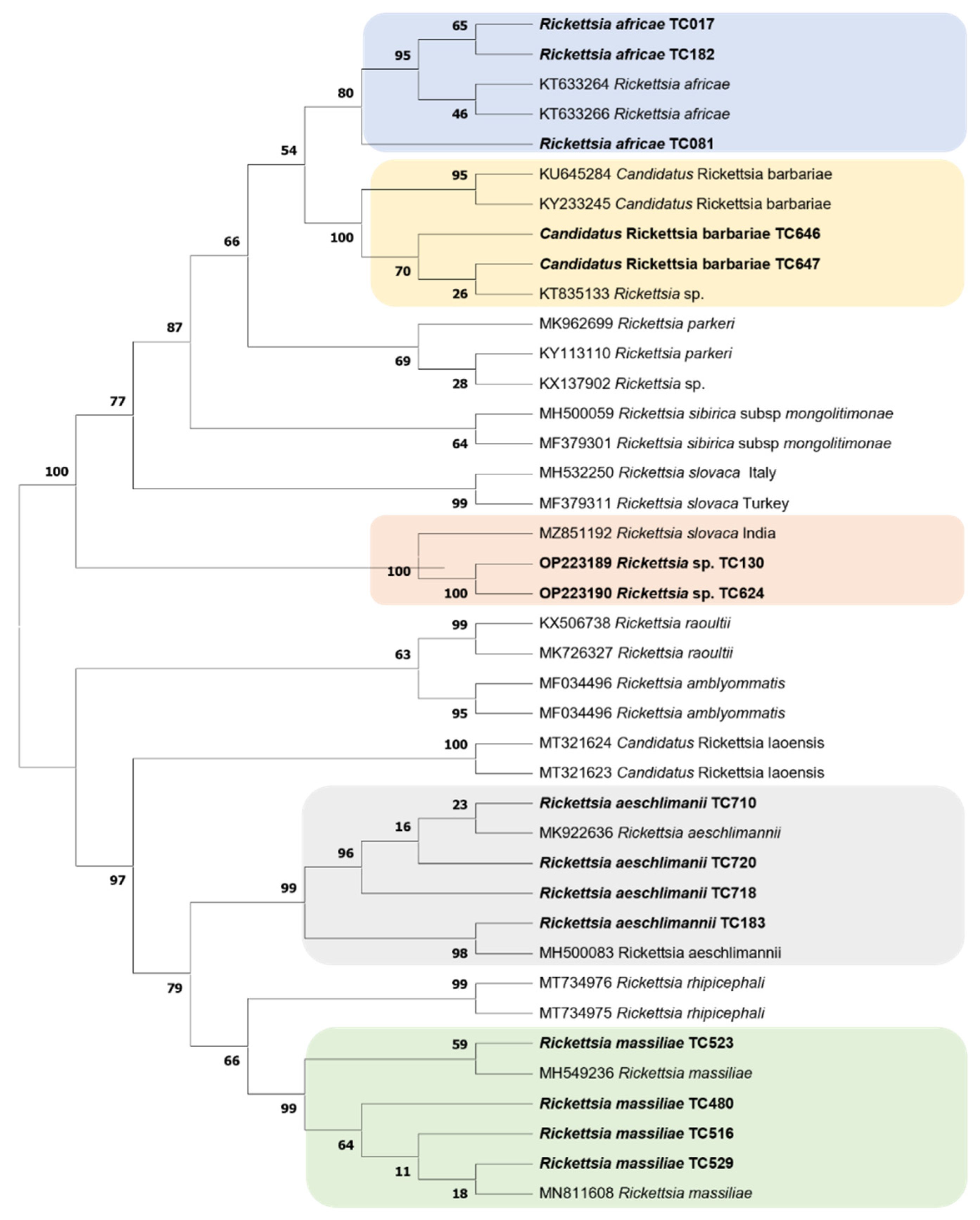
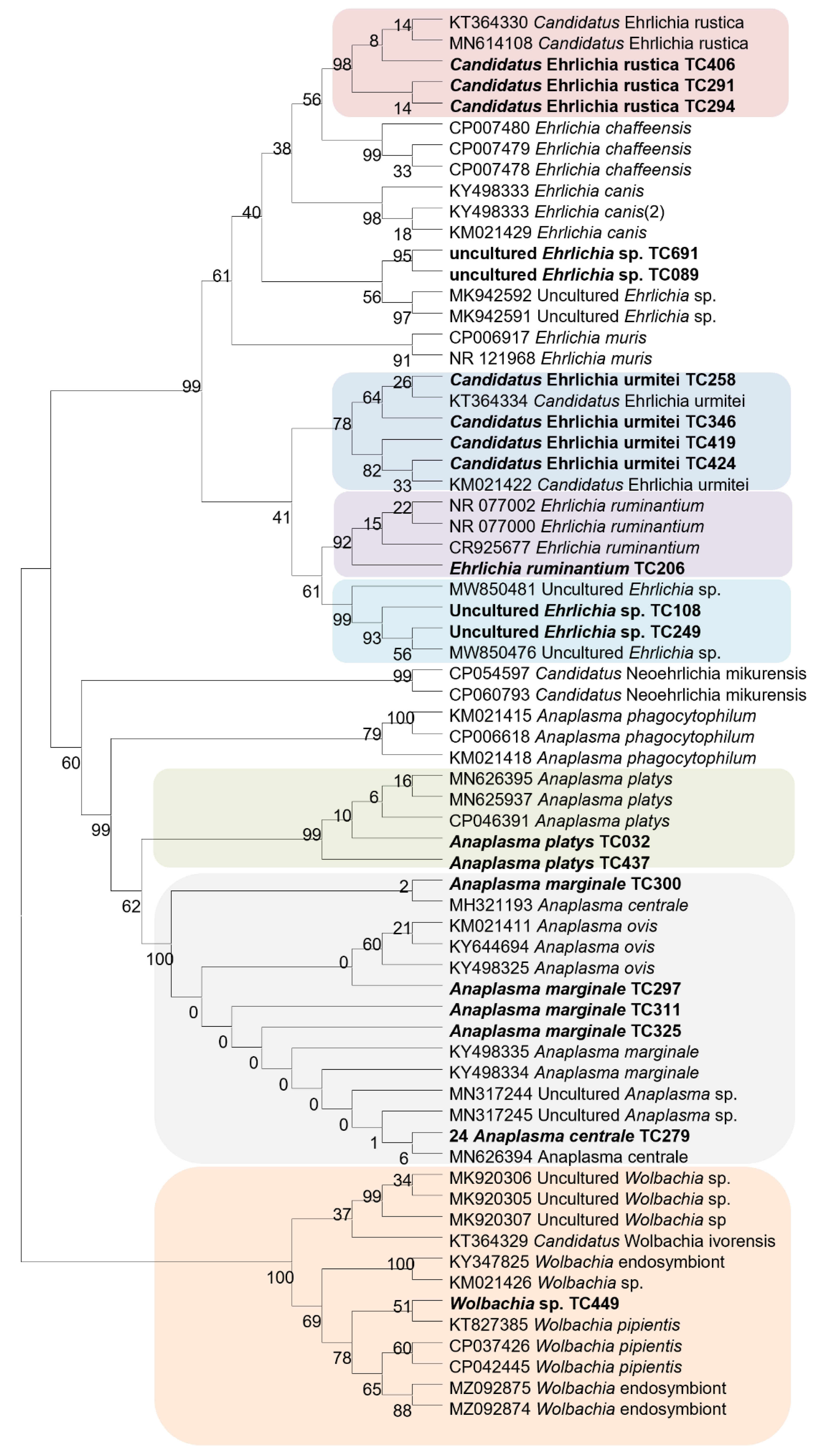
2.4. Standard PCR, Sequencing, and Phylogenetic Analysis
3. Results
3.1. Ticks
3.2. Detection of Microorganisms in Ticks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and Tick-Borne Diseases: A One Health Perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef]
- Parola, P.; Raoult, D. Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Jongejan, F.; Uilenberg, G. The Global Importance of Ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef]
- Kopp, N.; Diaz, D.; Amacker, M.; Odongo, D.O.; Beier, K.; Nitsch, C.; Bishop, R.P.; Daubenberger, C.A. Identification of a Synthetic Peptide Inducing Cross-Reactive Antibodies Binding to Rhipicephalus (Boophilus) decoloratus, Rhipicephalus (Boophilus) microplus, Hyalomma anatolicum anatolicum and Rhipicephalus appendiculatus BM86 Homologues. Vaccine 2009, 28, 261–269. [Google Scholar] [CrossRef]
- de Castro, J.J. Long-Term Studies on the Economic Impact of Ticks on Sanga Cattle in Zambia. Exp. Appl. Acarol. 1997, 21, 3–19. [Google Scholar] [CrossRef]
- INS, Elevage et Peche au Cameroun. Annuaire Statistique du Cameroun. 2017. Available online: https://books.google.fr/books?id=NLu9FwEXG90C (accessed on 24 April 2021).
- Raboloko, O.O.; Ramabu, S.S.; Guerrini, L.; Jori, F. Seroprevalence of Selected Tick Borne Pathogens and Diversity and Abundance of Ixodid Ticks (Acari: Ixodidae) at the Wildlife-Livestock Interface in Northern Botswana. Front. Vet. Sci. 2020, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Antunes, S.; Bonnet, S.; Cabezas-Cruz, A.; Domingos, A.G.; Estrada-Peña, A.; Johnson, N.; Kocan, K.M.; Mansfield, K.L.; Nijhof, A.M.; et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017, 7, 114. [Google Scholar] [CrossRef]
- Okello-Onen, J.; Hassan, S.M.; Essuman, S. Taxonomy of African Ticks: An Identification Manual; International Centre of Insect Physiology and Ecology (ICIPE): Nairobi, Kenya, 1999. [Google Scholar]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Peña, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks in Domestic Animals in Africa: A Guide to Identification of Species; Springer: New York, NY, USA, 2003. [Google Scholar]
- Laamari, A.; Kharrim, K.E.; Mrifag, R.; Boukbal, M.; Belghyti, D. Dynamique des populations de tiques parasites des bovins de la région du Gharb au Maroc. Rev. D’élevage Médecine Vét. Pays Trop. 2012, 65, 57–62. [Google Scholar] [CrossRef]
- Parola, P.; Inokuma, H.; Camicas, J.-L.; Brouqui, P.; Raoult, D. Detection and Identification of Spotted Fever Group Rickettsiae and Ehrlichiae in African Ticks. Emerg. Infect. Dis. 2001, 7, 1014–1017. [Google Scholar] [CrossRef]
- Estrada-Pena, A. Tick-Borne Pathogens, Transmission Rates and Climate Change. Front. Biosci. Landmark Ed. 2009, 14, 2674–2687. [Google Scholar] [CrossRef] [Green Version]
- Gondard, M.; Cabezas-Cruz, A.; Charles, R.A.; Vayssier-Taussat, M.; Albina, E.; Moutailler, S. Ticks and Tick-Borne Pathogens of the Caribbean: Current Understanding and Future Directions for More Comprehensive Surveillance. Front. Cell. Infect. Microbiol. 2017, 7, 490. [Google Scholar] [CrossRef]
- Ndi, C.; Bayemi, P.H.; Ekue, F.N.; Tarounga, B. Preliminary Observations on Ticks and Tick-Borne Diseases in the North West Province of Cameroon. I. Babesiosis and Anaplasmosis. Rev. Elev. Med. Vet. Pays Trop. 1991, 44, 263–265. [Google Scholar] [CrossRef]
- Ndip, L.M.; Titanji, V.P.K.; Ndip, R.N.; Mcbride, J.W.; Bouyer, D.H.; Walker, D.H.; Fokam, E.B. Detection of Rickettsia africae in patients and ticks along the coastal region of Cameroon. Am. J. Trop. Med. Hyg. 2004, 71, 363–366. [Google Scholar] [CrossRef]
- Abanda, B.; Paguem, A.; Abdoulmoumini, M.; Kingsley, M.T.; Renz, A.; Eisenbarth, A. Molecular Identification and Prevalence of Tick-Borne Pathogens in Zebu and Taurine Cattle in North Cameroon. Parasit. Vectors 2019, 12, 448. [Google Scholar] [CrossRef]
- Lontsi-Demano, M.; Laroche, M.; Ngnindji, Y.C.; Djikolmbairangar, J.-E.; Mamoudou, A.; Tchuinkam, T. Breeder’s Knowledge on Ticks and Tick-Borne Diseases and Management Strategies in Menoua Division (Western Region of Cameroon). Int. J. Vet. Sci. Anim. Husb. 2021, 6, 12–21. [Google Scholar] [CrossRef]
- Achukwi, M.D.; Tanya, V.N.; Messiné, O.; Njongmeta, L.M. Etude comparative de l’infestation des bovins Namchi (Bos taurus) et Goudali de Ngaoundéré (Bos indicus) par la tique adulte Amblyomma variegatum. Rev. élev Med. Vet. Pays Trop. 2001, 54, 37. [Google Scholar] [CrossRef]
- Silatsa, B.A.; Kuiate, J.-R.; Njiokou, F.; Simo, G.; Feussom, J.-M.K.; Tunrayo, A.; Amzati, G.S.; Bett, B.; Bishop, R.; Githaka, N.; et al. A Countrywide Molecular Survey Leads to a Seminal Identification of the Invasive Cattle Tick Rhipicephalus (Boophilus) microplus in Cameroon, a Decade after It Was Reported in Cote d’Ivoire. Ticks Tick-Borne Dis. 2019, 10, 585–593. [Google Scholar] [CrossRef]
- Lontsi-Demano, M.; Ngnindji-Youdje, Y.; Laroche, M.; Bamou, R.; Talom, A.D.; Abah, S.; Fopa, F.; Mamoudou, A.; Tchuinkam, T. Cattle Trading Favors the Introduction and Establishment of the Invasive Tick Rhipicephalus (Boophilus) microplus in Menoua Division, West Region of Cameroon. J. Entomol. Zool. Stud. 2020, 8, 207–214. [Google Scholar]
- Guerrero, F.D.; Nene, V.M.; George, J.E.; Barker, S.C.; Willadsen, P. Sequencing a New Target Genome: The Boophilus microplus (Acari: Ixodidae) Genome Project. J. Med. Entomol. 2006, 43, 9–16. [Google Scholar] [CrossRef]
- Madder, M.; Adehan, S.; De Deken, R.; Adehan, R.; Lokossou, R. New Foci of Rhipicephalus microplus in West Africa. Exp. Appl. Acarol. 2012, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Adakal, H.; Biguezoton, A.; Zoungrana, S.; Courtin, F.; De Clercq, E.M.; Madder, M. Alarming Spread of the Asian Cattle Tick Rhipicephalus microplus in West Africa-Another Three Countries Are Affected: Burkina Faso, Mali and Togo. Exp. Appl. Acarol. 2013, 61, 383–386. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Pereira, D.F.S.; Melo-Junior, O.; Mariano, R.M.d.S.; Leite, J.C.; da Silva, A.V.; de Oliveira, D.S.; Gonçalves, A.A.M.; Lair, D.F.; Soares, I.d.S.; et al. Vaccine Approaches Applied to Controlling Dog Ticks. Ticks Tick-Borne Dis. 2021, 12, 101631. [Google Scholar] [CrossRef]
- Motta, P.; Porphyre, T.; Handel, I.; Hamman, S.M.; Ngu Ngwa, V.; Tanya, V.; Morgan, K.; Christley, R.; Bronsvoort, B.M. deC. Implications of the Cattle Trade Network in Cameroon for Regional Disease Prevention and Control. Sci. Rep. 2017, 7, 43932. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.; Walker, A. Tiques d’importance Médicale et Vétérinaire: Le Bassin Méditerranéen. ICTTD CD-ROM Mediterr. Ticks 2004, 12, 3–12. [Google Scholar]
- Diarra, A.Z.; Almeras, L.; Laroche, M.; Berenger, J.-M.; Koné, A.K.; Bocoum, Z.; Dabo, A.; Doumbo, O.; Raoult, D.; Parola, P. Molecular and MALDI-TOF Identification of Ticks and Tick-Associated Bacteria in Mali. PLoS Negl. Trop. Dis. 2017, 11, e0005762. [Google Scholar] [CrossRef]
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Normand, T.; Carette, O.; Demoncheaux, J.-P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M.; et al. Great Diversity of Piroplasmida in Equidae in Africa and Europe, Including Potential New Species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100332. [Google Scholar] [CrossRef]
- Kernif, T. Rickettsia africae in Hyalomma dromedarii Ticks from Sub-Saharan Algeria. Ticks Tick-Borne Dis. 2012, 3, 377–379. [Google Scholar] [CrossRef]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.-F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Coxiella burnetii in Humans and Ticks in Rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef]
- Dahmani, M.; Davoust, B.; Sambou, M.; Bassene, H.; Scandola, P.; Ameur, T.; Raoult, D.; Fenollar, F.; Mediannikov, O. Molecular Investigation and Phylogeny of Species of the Anaplasmataceae Infecting Animals and Ticks in Senegal. Parasit. Vectors 2019, 12, 495. [Google Scholar] [CrossRef]
- Djiba, M.L.; Mediannikov, O.; Mbengue, M.; Thiongane, Y.; Molez, J.-F.; Seck, M.T.; Fenollar, F.; Raoult, D.; Ndiaye, M. Survey of Anaplasmataceae Bacteria in Sheep from Senegal. Trop. Anim. Health Prod. 2013, 45, 1557–1561. [Google Scholar] [CrossRef]
- Mura, A.; Socolovschi, C.; Ginesta, J.; Lafrance, B.; Magnan, S.; Rolain, J.-M.; Davoust, B.; Raoult, D.; Parola, P. Molecular Detection of Spotted Fever Group Rickettsiae in Ticks from Ethiopia and Chad. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 945–949. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Stuhl, L.; Maurin, M.; Raoult, D. Evaluation of Antibiotic Susceptibilities of Three Rickettsial Species Including Rickettsia felis by a Quantitative PCR DNA Assay. Antimicrob. Agents Chemother. 2002, 46, 2747–2751. [Google Scholar] [CrossRef] [Green Version]
- Vanegas, A.; Keller, C.; Krüger, A.; Manchang, T.K.; Hagen, R.M.; Frickmann, H.; Veit, A.; Achukwi, M.D.; Krücken, J.; Poppert, S. Molecular Detection of Spotted Fever Group Rickettsiae in Ticks from Cameroon. Ticks Tick-Borne Dis. 2018, 9, 1049–1056. [Google Scholar] [CrossRef]
- Mediannikov, O.; Diatta, G.; Fenollar, F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Tick-Borne Rickettsioses, Neglected Emerging Diseases in Rural Senegal. PLoS Negl. Trop. Dis. 2010, 4, e821. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Franc, M.; Davoust, B.; Raoult, D. Molecular Detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in Cat Fleas, France. Emerg. Infect. Dis. 2003, 9, 339–342. [Google Scholar] [CrossRef]
- Rolain, J.-M.; Raoult, D. Molecular Detection of Coxiella burnetii in Blood and Sera during Q Fever. QJM Int. J. Med. 2005, 98, 615–620. [Google Scholar] [CrossRef]
- Aubry, C.; Socolovschi, C.; Raoult, D.; Parola, P. Bacterial Agents in 248 Ticks Removed from People from 2002 to 2013. Ticks Tick-Borne Dis. 2016, 7, 475–481. [Google Scholar] [CrossRef]
- Ngoy, S.; Diarra, A.Z.; Laudisoit, A.; Gembu, G.-C.; Verheyen, E.; Mubenga, O.; Mbalitini, S.G.; Baelo, P.; Laroche, M.; Parola, P. Using MALDI-TOF Mass Spectrometry to Identify Ticks Collected on Domestic and Wild Animals from the Democratic Republic of the Congo. Exp. Appl. Acarol. 2021, 84, 637–657. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Raoult, D.; Fournier, P.E.; Fenollar, F.; Jensenius, M.; Prioe, T.; de Pina, J.J.; Caruso, G.; Jones, N.; Laferl, H.; Rosenblatt, J.E.; et al. Rickettsia africae, a Tick-Borne Pathogen in Travelers to Sub-Saharan Africa. N. Engl. J. Med. 2001, 344, 1504–1510. [Google Scholar] [CrossRef]
- Ehounoud, C.B.; Yao, K.P.; Dahmani, M.; Achi, Y.L.; Amanzougaghene, N.; N’Douba, A.K.; N’Guessan, J.D.; Raoult, D.; Fenollar, F.; Mediannikov, O. Multiple Pathogens Including Potential New Species in Tick Vectors in Cote d’Ivoire. PLoS Negl. Trop. Dis. 2016, 10, e0004367. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Raoult, D.; Parola, P. Update on Tick-Borne Bacterial Diseases in Europe. Parasite Paris Fr. 2009, 16, 259–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randolph, S.E. Transmission of Tick-Borne Pathogens between Co-Feeding Ticks: Milan Labuda’s Enduring Paradigm. Ticks Tick-Borne Dis. 2011, 2, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Belli, A.; Sarr, A.; Rais, O.; Rego, R.O.M.; Voordouw, M.J. Ticks Infected via Co-Feeding Transmission Can Transmit Lyme Borreliosis to Vertebrate Hosts. Sci. Rep. 2017, 7, 5006. [Google Scholar] [CrossRef]
- Kidd, L.; Maggi, R.; Diniz, P.P.V.P.; Hegarty, B.; Tucker, M.; Breitschwerdt, E. Evaluation of Conventional and Real-Time PCR Assays for Detection and Differentiation of Spotted Fever Group Rickettsia in Dog Blood. Vet. Microbiol. 2008, 129, 294–303. [Google Scholar] [CrossRef]
- Mura, A.; Masala, G.; Tola, S.; Satta, G.; Fois, F.; Piras, P.; Rolain, J.-M.; Raoult, D.; Parola, P. First Direct Detection of Rickettsial Pathogens and a New Rickettsia, “Candidatus Rickettsia Barbariae”, in Ticks from Sardinia, Italy. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008, 14, 1028–1033. [Google Scholar] [CrossRef]
- Sadeddine, R.; Diarra, A.Z.; Laroche, M.; Mediannikov, O.; Righi, S.; Benakhla, A.; Dahmana, H.; Raoult, D.; Parola, P. Molecular Identification of Protozoal and Bacterial Organisms in Domestic Animals and Their Infesting Ticks from North-Eastern Algeria. Ticks Tick-Borne Dis. 2020, 11, 101330. [Google Scholar] [CrossRef] [PubMed]
- Abdelkadir, K.; Palomar, A.M.; Portillo, A.; Oteo, J.A.; Ait-Oudhia, K.; Khelef, D. Presence of Rickettsia aeschlimannii, “Candidatus Rickettsia barbariae” and Coxiella burnetii in Ticks from Livestock in Northwestern Algeria. Ticks Tick-Borne Dis. 2019, 10, 924–928. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Inokuma, H. Vectors and Reservoir Hosts of Anaplasmataceae. In Rickettsial Diseases; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-13200-1. [Google Scholar]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Inácio da Silva, L.M.; Dezordi, F.Z.; Paiva, M.H.S.; Wallau, G.L. Systematic Review of Wolbachia Symbiont Detection in Mosquitoes: An Entangled Topic about Methodological Power and True Symbiosis. Pathogens 2021, 10, 39. [Google Scholar] [CrossRef]
- Landmann, F. The Wolbachia Endosymbionts. Microbiol. Spectr. 2019, 7, BAI-0018-2019. [Google Scholar] [CrossRef]
- Bamou, R.; Diarra, A.Z.; Mayi, M.P.A.; Djiappi-Tchamen, B.; Antonio-Nkondjio, C.; Parola, P. Wolbachia Detection in Field-Collected Mosquitoes from Cameroon. Insects 2021, 12, 1133. [Google Scholar] [CrossRef]
- Walker, T.; Quek, S.; Jeffries, C.L.; Bandibabone, J.; Dhokiya, V.; Bamou, R.; Kristan, M.; Messenger, L.A.; Gidley, A.; Hornett, E.A.; et al. Stable High-Density and Maternally Inherited Wolbachia Infections in Anopheles moucheti and Anopheles demeilloni Mosquitoes. Curr. Biol. 2021, 31, 2310–2320.e5. [Google Scholar] [CrossRef]
- Boucheikhchoukh, M.; Laroche, M.; Aouadi, A.; Dib, L.; Benakhla, A.; Raoult, D.; Parola, P. MALDI-TOF MS Identification of Ticks of Domestic and Wild Animals in Algeria and Molecular Detection of Associated Microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 39–49. [Google Scholar] [CrossRef]
- Chao, L.-L.; Castillo, C.T.; Shih, C.-M. Molecular Detection and Genetic Identification of Wolbachia Endosymbiont in Rhipicephalus sanguineus (Acari: Ixodidae) Ticks of Taiwan. Exp. Appl. Acarol. 2021, 83, 115–130. [Google Scholar] [CrossRef]
- Plantard, O.; Bouju-Albert, A.; Malard, M.-A.; Hermouet, A.; Capron, G.; Verheyden, H. Detection of Wolbachia in the Tick Ixodes ricinus Is Due to the Presence of the Hymenoptera Endoparasitoid Ixodiphagus hookeri. PLoS ONE 2012, 7, e30692. [Google Scholar] [CrossRef]
- Cicculli, V.; DeCarreaux, D.; Ayhan, N.; Casabianca, F.; de Lamballerie, X.; Charrel, R.; Falchi, A. Molecular Screening of Anaplasmataceae in Ticks Collected from Cattle in Corsica, France. Exp. Appl. Acarol. 2020, 81, 561–574. [Google Scholar] [CrossRef]
- Frisch, J.E. Towards a Permanent Solution for Controlling Cattle Ticks. Int. J. Parasitol. 1999, 29, 57–71. [Google Scholar] [CrossRef]
- Lontsi-Demano, M.; Djikolbairangar, J.E.; Laroche, M.; Ngnindji-Youdje, Y.C.; Luogbou, N.D.D.; Abah, S.; Mamoudou, A.; Tchuinkam, T. Tick-Borne Haemoparasites of Veterinary Importance in Cattle in Menoua Division, Western Highlands of Cameroon. J. Fish. Livest. Prod. 2021, 9, 10. [Google Scholar]
- Abanda, B.; Paguem, A.; Achukwi, M.D.; Renz, A.; Eisenbarth, A. Development of a Low-Density DNA Microarray for Detecting Tick-Borne Bacterial and Piroplasmid Pathogens in African Cattle. Trop. Med. Infect. Dis. 2019, 4, 64. [Google Scholar] [CrossRef]
- Hailemariam, Z.; Krücken, J.; Baumann, M.; Ahmed, J.S.; Clausen, P.-H.; Nijhof, A.M. Molecular Detection of Tick-Borne Pathogens in Cattle from Southwestern Ethiopia. PLoS ONE 2017, 12, e0188248. [Google Scholar] [CrossRef] [PubMed]
- Chiuya, T.; Masiga, D.K.; Falzon, L.C.; Bastos, A.D.S.; Fèvre, E.M.; Villinger, J. Tick-Borne Pathogens, Including Crimean-Congo Haemorrhagic Fever Virus, at Livestock Markets and Slaughterhouses in Western Kenya. Transbound. Emerg. Dis. 2021, 68, 2429–2445. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-Infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a Veterinarian. Parasit. Vectors 2013, 6, 103. [Google Scholar] [CrossRef]
- Elelu, N.; Ferrolho, J.; Couto, J.; Domingos, A.; Eisler, M.C. Molecular Diagnosis of the Tick-Borne Pathogen Anaplasma marginale in Cattle Blood Samples from Nigeria Using QPCR. Exp. Appl. Acarol. 2016, 70, 501–510. [Google Scholar] [CrossRef]
- Peter, T.F.; Burridge, M.J.; Mahan, S.M. Ehrlichia ruminantium Infection (Heartwater) in Wild Animals. Trends Parasitol. 2002, 18, 214–218. [Google Scholar] [CrossRef]
- Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Detection of Ehrlichia ruminantium Infection in Cattle in Cameroon. BMC Res. Notes 2018, 11, 388. [Google Scholar] [CrossRef]
- Awa, D.N. Serological Survey of Heartwater Relative to the Distribution of the Vector Amblyomma variegatum and Other Tick Species in North Cameroon. Vet. Parasitol. 1997, 68, 165–173. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Bouattour, A.; Camicas, J.-L.; Guglielmone, A.; Horak, I.; Jongejan, F.; Latif, A.; Pegram, R.; Walker, A.R. The Known Distribution and Ecological Preferences of the Tick Subgenus Boophilus (Acari: Ixodidae) in Africa and Latin America. Exp. Appl. Acarol. 2006, 38, 219–235. [Google Scholar] [CrossRef]
- McCoy, B.N.; Maïga, O.; Schwan, T.G. Detection of Borrelia theileri in Rhipicephalus geigyi from Mali. Ticks Tick-Borne Dis. 2014, 5, 401–403. [Google Scholar] [CrossRef]
- Sharma, S.P.; Amanfu, W.; Losho, T.C. Bovine Borreliosis in Botswana. Onderstepoort J. Vet. Res. 2000, 67, 221–223. [Google Scholar]
- Silatsa, B.A.; Simo, G.; Githaka, N.; Kamga, R.; Oumarou, F.; Keambou Tiambo, C.; Machuka, E.; Domelevo, J.; Odongo, D.; Bishop, R.; et al. First Detection of Theileria parva in Cattle from Cameroon in the Absence of the Main Tick Vector Rhipicephalus appendiculatus. Transbound. Emerg. Dis. 2020, 67, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Vanderburg, S.; Rubach, M.P.; Halliday, J.E.B.; Cleaveland, S.; Reddy, E.A.; Crump, J.A. Epidemiology of Coxiella burnetii Infection in Africa: A OneHealth Systematic Review. PLoS Negl. Trop. Dis. 2014, 8, e2787. [Google Scholar] [CrossRef]
- Duron, O.; Sidi-Boumedine, K.; Rousset, E.; Moutailler, S.; Jourdain, E. The Importance of Ticks in Q Fever Transmission: What Has (and Has Not) Been Demonstrated? Trends Parasitol. 2015, 31, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Reye, A.L.; Arinola, O.G.; Hübschen, J.M.; Muller, C.P. Pathogen Prevalence in Ticks Collected from the Vegetation and Livestock in Nigeria. Appl. Environ. Microbiol. 2012, 78, 2562–2568. [Google Scholar] [CrossRef]
- Levin, M.L.; Fish, D. Acquisition of Coinfection and Simultaneous Transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis Ticks. Infect. Immun. 2000, 68, 2183–2186. [Google Scholar] [CrossRef] [Green Version]
| Microorganisms | Targeted Sequence | Primers (5′-3′) and Probes (Used for qPCR Screening or Sequencing) | References |
|---|---|---|---|
| Anaplasmataceae | 23S (TtAna) | f_TGACAGCGTACCTTTTGCAT r_GTAACAGGTTCGGTCCTCCA p_6FAM-GGATTAGACCCGAAACCAAG | [32,33] |
| 23S (520-bp) | f_ATAAGCTGCGGGGAATTGTC r_TGCAAAAGGTACGCTGTCAC | ||
| Piroplasmida | 5.8S | f_AYYKTYAGCGRTGGATGTC r_TCGCAGRAGTCTKCAAGTC p_FAM-TTYGCTGCGTCCTTCATCGTTGT-MGB | [32] |
| 18S (969-bp) | f1_GCGAATGGCTCATTAIAACA f4_CACATCTAAGGAAGGCAGCA f3_GTAGGGTATTGGCCTACCG * r4_AGGACTACGACGGTATCTGA * | ||
| Rickettsia | gltA (RKND03) | f_GTGAATGAAAGATTACACTATTTAT r_GTATCTTAGCAATCATTCTAATAGC p_6FAM-CTATTATGCTTGCGGCTGTCGGTTC | [34,35] |
| ITS (Rafricae) | f_TGCAACACGAAGCACAAAAC r_CCTCTTGCGAAACTCTACTTTTGA 6FAM-CGTGTGGATTCGAGCACCGGA | [30] | |
| OmpA (630-bp) | 70_ATGGCGAATATTTCTCCAAAA 701_GTTCCGTTAATGGCAGCATCT 180_GCAGCGATAATGCTGAGTA * | [12,36] | |
| gltA (400-bp) | f_ATGACCAATGAAAATAATAAT r_CTTATACTCTCTATGTACA | ||
| Borrelia | ITS4 | f_GGCTTCGGGTCTACCACATCTA r_CCGGGAGGGGAGTGAAATAG p_6FAM-TGCAAAAGGCACGCCATCACC | [37] |
| flaB (344-bp) | f_TGGTATGGGAGTTTCTGG r_TAAGCTGACTAATACTAATTACCC | ||
| Bartonella | ITS2 | f_GATGCCGGGGAAGGTTTTC r_GCCTGGGAGGACTTGAACCT p_GCGCGCGCTTGATAAGCGTG | [38] |
| Correlia burnetii | IS30A | f_CGCTGACCTACAGAAATATGTCC r_GGGGTAAGTAAATAATACCTTCTGG p_CATGAAGCGATTTATCAATACGTGTATG | [39] |
| IS1111A | f_CAAGAAACGTATCGCTGTGGC r_CACAGAGCCACCGTATGAATC 6FAM-CCGAGTTCGAAACAATGAGGGCTG | [31] |
| Tick Genus | Tick Species | No of Ticks Collected | Sex | Menoua Division | Noun Division | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Nkong-Ni | Dschang | Kouoptamo | Massangam | Koutaba | |||
| Amblyomma | Am. variegatum | 353 | 245 | 108 | 80 (30) | 87 (42) | 35 (6) | 67 (16) | 84 (14) |
| Rhipicephalus | Rh. microplus | 552 | 168 | 384 | 207 (153) | 101 (62) | 104 (83) | 88 (56) | 52 (30) |
| Rh. annulatus | 6 | 6 | 0 | 0 | 6 | 0 | 0 | 0 | |
| Rh. decoloratus | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | |
| Rh. lunulatus | 387 | 239 | 148 | 70 (20) | 105 (50) | 45 (15) | 92 (41) | 75 (22) | |
| Rh. sanguineus | 48 | 40 | 8 | 38 (8) | 10 (0) | 0 | 0 | 0 | |
| Rh. muhsamae | 10 | 10 | 0 | 7 | 3 | 0 | 0 | 0 | |
| Rhipicephalus spp. | 35 | 0 | 35 | 5 (5) | 26 (26) | 0 | 4 (4) | 0 | |
| Haemaphysalis | Ha. leachi | 45 | 35 | 10 | 35 (10) | 10 (0) | 0 | 0 | 0 |
| Hyalomma | Hy. rufipes | 16 | 10 | 6 | 16 (6) | 0 | 0 | 0 | 0 |
| Hy. truncatum | 25 | 14 | 11 | 25 (11) | 0 | 0 | 0 | 0 | |
| Ixodes | Ix. rasus | 3 | 0 | 3 | 3 | 0 | 0 | 0 | |
| Total | 1483 | 770 | 713 | 489 | 348 | 184 | 251 | 211 | |
| Tick Species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism | Target Sequence | Am. variegatum | Rh. microplus | Rh. sanguineus | Ha. leachi | Rh. lunulatus | Rh. muhsamae | Hy. rufipes | Hy. truncatum | (%) Pos/Total |
| Rickettsia spp. | gltA (RKND03) | 78.8% (171/217) | 0.6% (2/308) | 4.3% (2/46) | - | 8.8% (24/272) | 35% (3/12) | 39% (16/41) | 23% (218/944) | |
| R. africae | poT15-dam2 | 77.4% (168/217) | 0.6% (2/308) | - | - | - | - | - | 8% (2/25) | 18.2% (172/944) |
| Anaplasmatacae | 23SrRNA(TtAna) | 7.4% (16/217) | 25% (77/308) | 8.3% (4/48) | 14% (6/43) | 14% (38/272) | - | - | - | 14.9% (141/944) |
| Piroplasmida | 5.8S/Piro 18S | 1.4% (3/217) | 5.5% (17/308) | - | 2.3% (1/43) | 2.6% (7/272) | - | 6.3% (1/16) | - | 3% (29/944) |
| Bartonela spp. | (Barto ITS2)/gltA | - | 0.6% (2/308) | - | - | - | - | - | - | 0.2% (2/944) |
| Borrelia spp. | (Bor ITS4) | 0.5% (1/217) | 0.6% (2/308) | - | 2.3% (1/43) | - | - | - | - | 0.4% (4/944) |
| C. burnetii | (IS1111)/ITS30A | - | - | - | - | 0.3% (1/272) | - | 6.3% (1/16) | 12% (3/25) | 0.5% (5/944) |
| Tick Species | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism | Target Sequence | Per. Ident (%) | Am. variegatum | Rh. microplus | Rh. sanguineus | Ha. leachi | Rh. lunulatus | Rh. muhsamae | Hy. rufipes | Hy. truncatum | (%) Sequences Obtained/Pos qPCR |
| Rickettsia aeschlimannii | ompA | 99.49–100 | - | - | 50% (1/2) | - | - | - | 75% (12/16) | 4% (1/25) | 6.4% (14/218) |
| Rickettsia massiliae | 99.83–100 | - | - | - | - | 59.2% (16/27) | 10% (1/10) | - | 7.8% (17/218) | ||
| Candidatus Rickettsia barbariae | 99.49 | - | - | - | - | - | 20% (2/10) | - | 0.9% (2/218) | ||
| Rickettsia sp. | 97.88–97.92 | - | - | - | - | 7.4% (2/27) | - | - | 0.9% (2/218) | ||
| Anaplasma centrale | 23S Ana | 100 | - | 1.3% (1/77) | - | - | - | - | - | 0.7% (1/141) | |
| Ehrlichia ruminantium | 100 | 6.2% (1/16) | - | - | - | - | - | - | 0.7% (1/141) | ||
| uncultured Ehrlichia sp. | 98.32–100 | - | 3.9% (3/77) | 25% (1/4) | 16.7% (1/6) | 2.6% (1/38) | - | - | - | 4.2% (6/141) | |
| Candidatus Ehrlichia urmitei | 99.16–100 | - | 13% (10/77) | - | - | - | - | - | 7% (10/141) | ||
| Anaplasma marginale | 99.79–100 | - | 6.5% (5/77) | - | 16.7% (1/6) | - | - | - | 4.3% (6/141) | ||
| Candidatus Ehrlichia rustica | 98.6–100 | - | 9% (7/77) | - | 16.7% (1/6) | 28.9% (11/38) | - | - | - | 13.5% (19/141) | |
| Anaplasma platys | 98.72–98.74 | - | 1.3% (1/77) | 25% (1/4) | - | - | - | - | - | 1.4% (2/141) | |
| Wolbachia pipientis | 99.78 | - | - | - | 16.7% (1/6) | - | - | - | - | 0.7% (1/141) | |
| Borrelia theileri | flaB | 100 | 1.3% (1/77) | 25% (1/4) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngnindji-Youdje, Y.; Diarra, A.Z.; Lontsi-Demano, M.; Tchuinkam, T.; Parola, P. Detection of Tick-Borne Pathogens in Ticks from Cattle in Western Highlands of Cameroon. Microorganisms 2022, 10, 1957. https://doi.org/10.3390/microorganisms10101957
Ngnindji-Youdje Y, Diarra AZ, Lontsi-Demano M, Tchuinkam T, Parola P. Detection of Tick-Borne Pathogens in Ticks from Cattle in Western Highlands of Cameroon. Microorganisms. 2022; 10(10):1957. https://doi.org/10.3390/microorganisms10101957
Chicago/Turabian StyleNgnindji-Youdje, Yannick, Adama Zan Diarra, Michel Lontsi-Demano, Timoléon Tchuinkam, and Philippe Parola. 2022. "Detection of Tick-Borne Pathogens in Ticks from Cattle in Western Highlands of Cameroon" Microorganisms 10, no. 10: 1957. https://doi.org/10.3390/microorganisms10101957
APA StyleNgnindji-Youdje, Y., Diarra, A. Z., Lontsi-Demano, M., Tchuinkam, T., & Parola, P. (2022). Detection of Tick-Borne Pathogens in Ticks from Cattle in Western Highlands of Cameroon. Microorganisms, 10(10), 1957. https://doi.org/10.3390/microorganisms10101957






