Development and Optimization of Indirect ELISAs for the Detection of Anti-Capripoxvirus Antibodies in Cattle, Sheep, and Goat Sera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Sequence Selection, Cloning, and Expression of Proteins
2.2. Western Blot
2.3. Virus Neutralization Test
2.4. Serum Samples
2.4.1. Experimental and Field Sera
2.4.2. Specificity Control Sera
2.4.3. Longitudinal Sera
2.5. Development and Optimization of the CaPV iELISAs
2.5.1. Antigen Coating
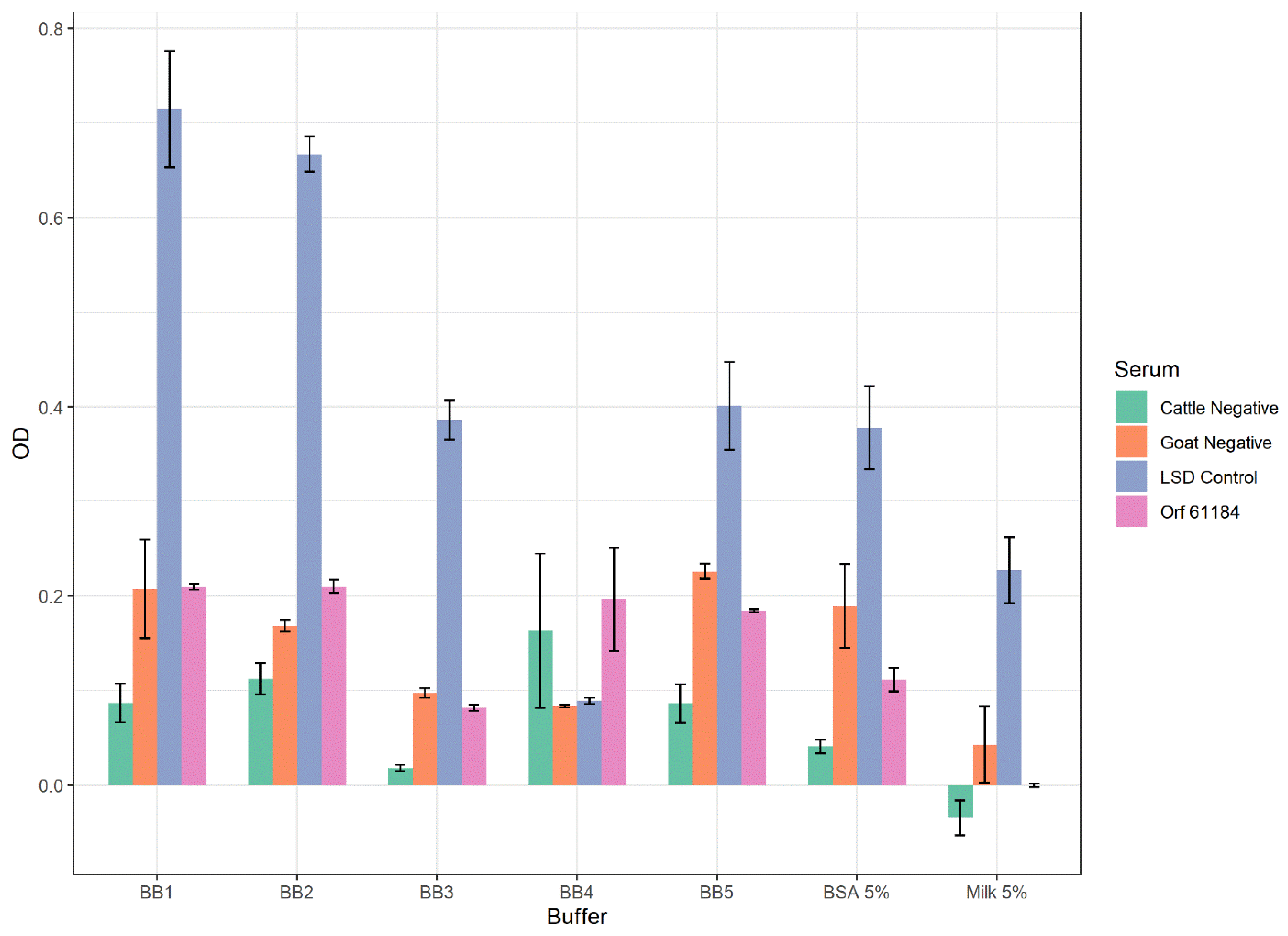
2.5.2. Effect of Blocking Buffers
2.5.3. Effect of Conjugates
2.5.4. LSD iELISA and SPP/GTP iELISA
2.6. Evaluation of Vaccinated Cattle Samples
2.7. Statistical Analysis
2.8. Institutional Review Board Statement
3. Results
3.1. Expression and Analysis of the Recombinant Truncated C-Type Lectin-Like Glycoprotein A34 and the EEV Glycoprotein A36
3.2. Optimization of the iELISAs
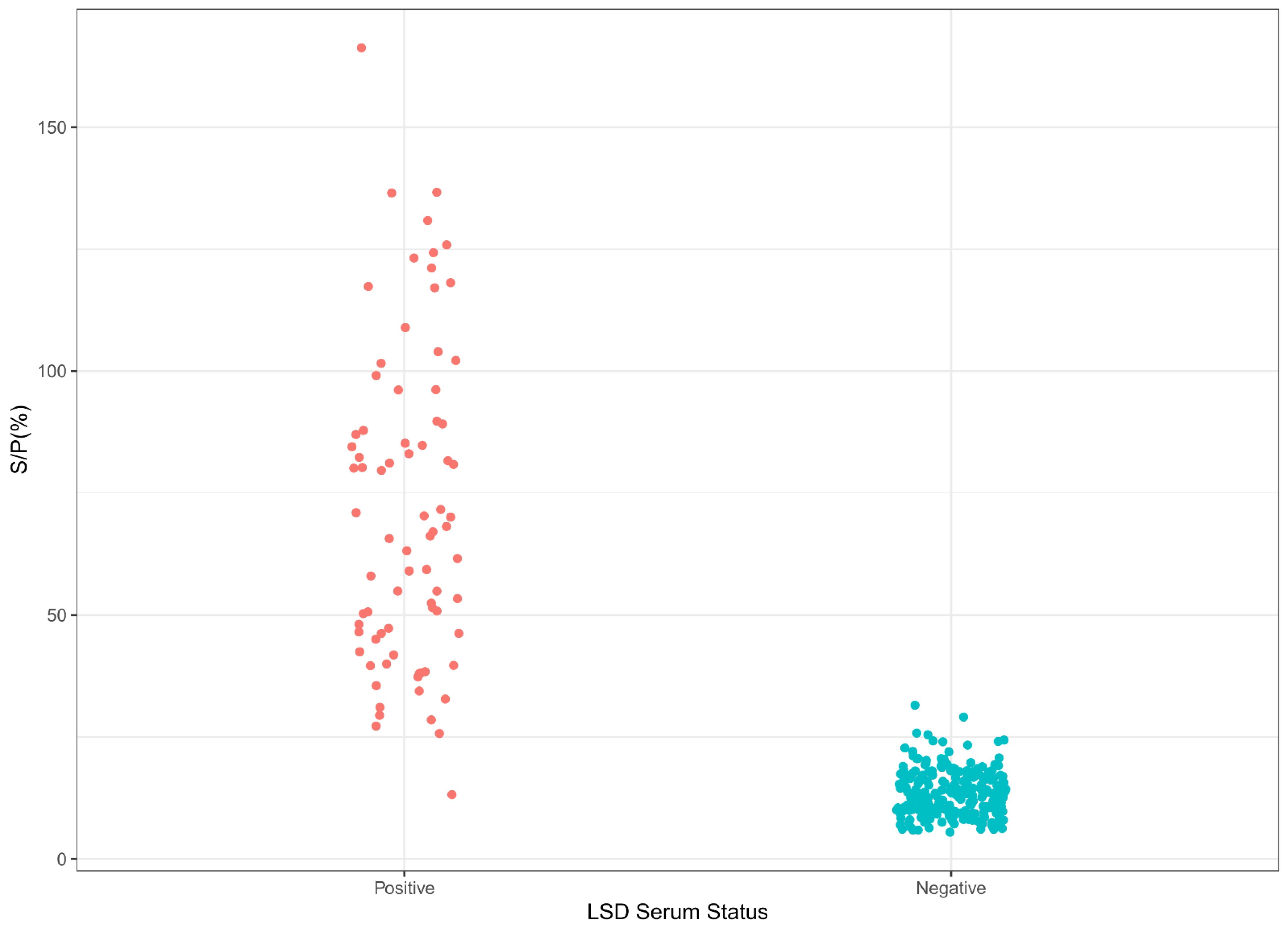
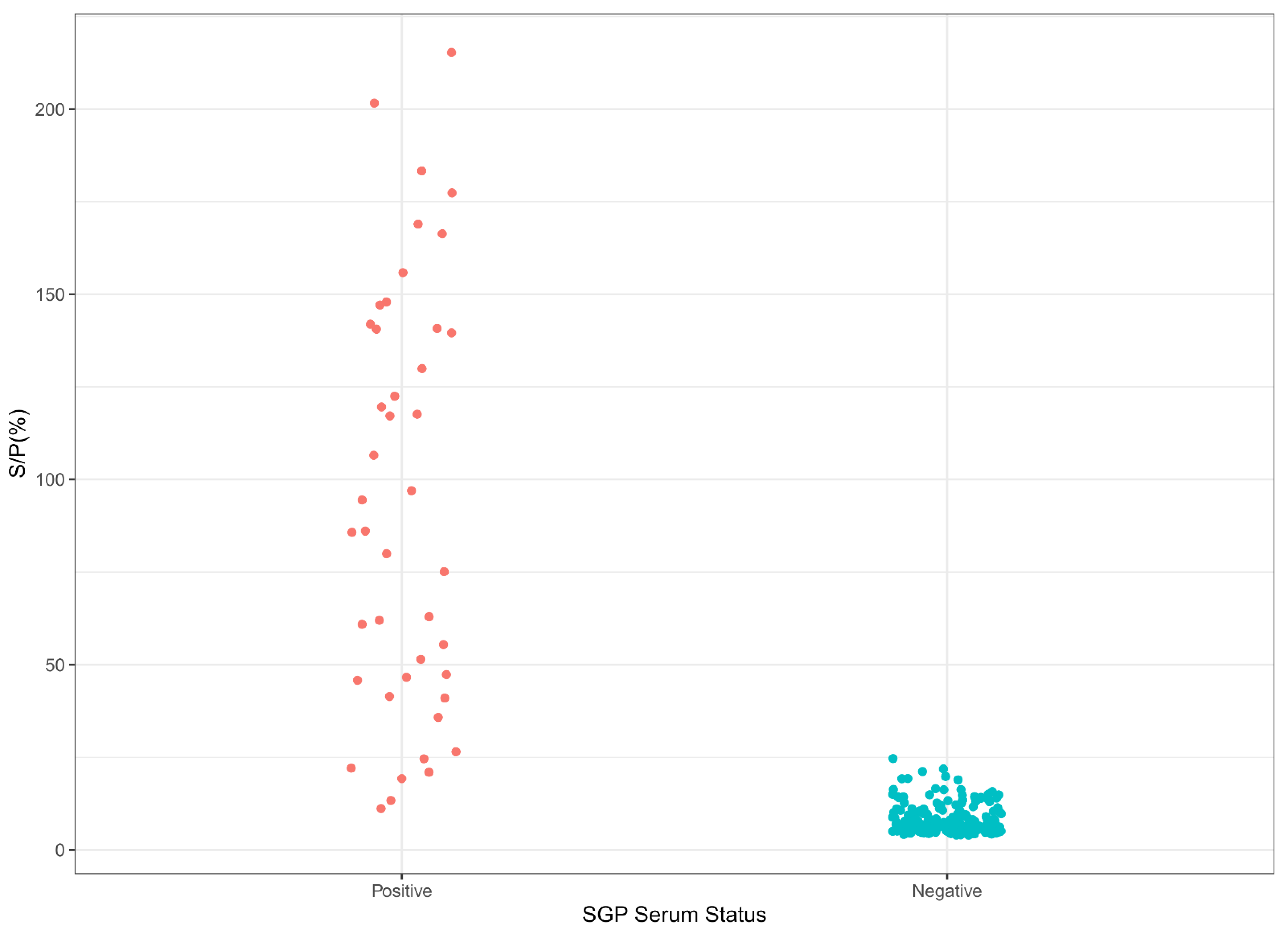
3.3. Cut-Off, Specificities, and Sensitivities of LSD iELISA and SPP/GTP iELISA
3.4. Analysis of the Cross-Reactivity to Anti-Parapoxvirus Antibodies
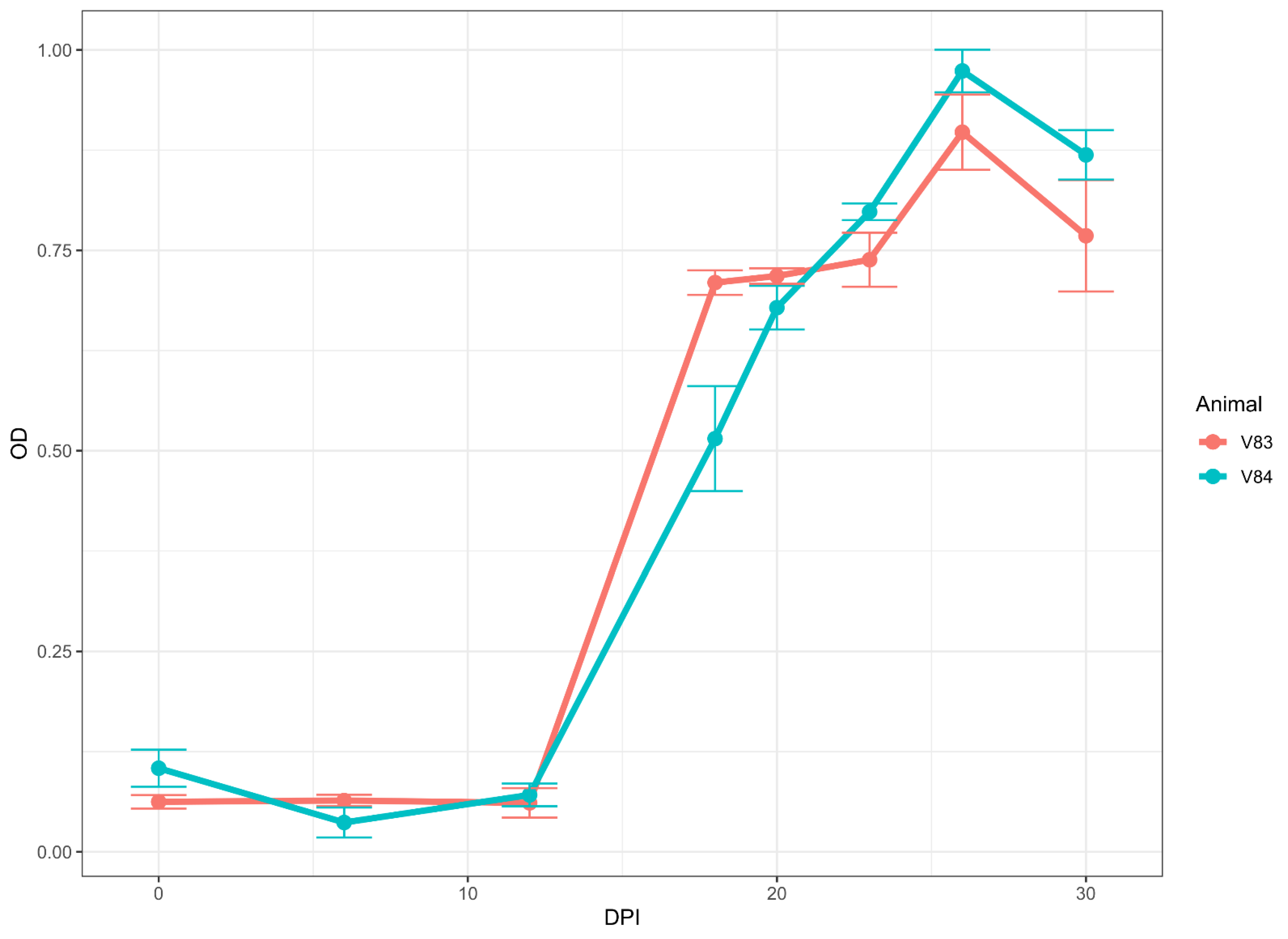
3.5. Antibody Detection in Sera from Longitudinal Studies on Experimentally Infected Animals
3.6. Performance of LSD iELISA on a Sample Panel of Serum from Vaccinated Cattle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV EC 52. International Committee on Taxonomy of Viruses (ICTV) Report on Virus Classification and Taxon Nomenclature; International Committee on Taxonomy of Viruses: Berlin, Germany, 2020. [Google Scholar]
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. Genome of Lumpy Skin Disease Virus. J. Virol. 2001, 75, 7122–7130. [Google Scholar] [CrossRef] [PubMed]
- Tulman, E.; Afonso, C.; Lu, Z.; Zsak, L.; Sur, J.-H.; Sandybaev, N.; Kerembekova, U.; Zaitsev, V.; Kutish, G.; Rock, D. The Genomes of Sheeppox and Goatpox Viruses. J. Virol. 2002, 76, 6054–6061. [Google Scholar] [CrossRef]
- Le Goff, C.; Lamien, C.E.; Fakhfakh, E.; Chadeyras, A.; Aba-Adulugba, E.; Libeau, G.; Tuppurainen, E.; Wallace, D.B.; Adam, T.; Silber, R.; et al. Capripoxvirus G-Protein-Coupled Chemokine Receptor: A Host-Range Gene Suitable for Virus Animal Origin Discrimination. J. Gen. Virol. 2009, 90, 1967–1977. [Google Scholar] [CrossRef]
- Lamien, C.E.; Le Goff, C.; Silber, R.; Wallace, D.B.; Gulyaz, V.; Tuppurainen, E.; Madani, H.; Caufour, P.; Adam, T.; El Harrak, M. Use of the Capripoxvirus Homologue of Vaccinia Virus 30 KDa RNA Polymerase Subunit (RPO30) Gene as a Novel Diagnostic and Genotyping Target: Development of a Classical PCR Method to Differentiate Goat Poxvirus from Sheep Poxvirus. Vet. Microbiol. 2011, 149, 30–39. [Google Scholar] [CrossRef] [PubMed]
- OIE. Sheep Pox and Goat Pox. In OIE Terrestrial Manual; OIE: Paris, France, 2017; pp. 1–12. [Google Scholar]
- Dutta, T.K.; Roychoudhury, P.; Kawlni, L.; Lalmuanpuia, J.; Dey, A.; Muthuchelvan, D.; Mandakini, R.; Sarkar, A.; Ramakrishnan, M.A.; Subudhi, P.K. An Outbreak of Goatpox Virus Infection in Wild Red Serow (Capricornis Rubidus) in Mizoram, India. Transbound. Emerg. Dis. 2019, 66, 181–185. [Google Scholar] [CrossRef]
- Bora, D.P.; Ahmed, J.; Tadap, S.; Pariat, A.O.; Mech, P.; Panda, S.P.; Tashi, T.; Kakati, P.; Ingtipi, S.; Qayum, A.; et al. Evidence of Transmission of Goatpox between Domestic Goats and Wild Himalayan Goral (Naemorhedus Goral) in Arunachal Pradesh, India. J. Wildl. Dis. 2021, 57, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Tuppurainen, E.S.M.; Oura, C.A.L. Review: Lumpy Skin Disease: An Emerging Threat to Europe, the Middle East and Asia. Transbound. Emerg. Dis. 2012, 59, 40–48. [Google Scholar] [CrossRef]
- OIE. OIE Listed Diseases. 2020. Available online: https://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2020/ (accessed on 18 January 2020).
- Tony McNulty. United Kingdom Statutory Instruments Prevention and Suppression of Terrorism No. 926 (2007) Part 7 of the Anti-Terrorism, Crime and Security Act 2001 (Extension to Animal Pathogens); UK, 2007. Available online: https://www.legislation.gov.uk/uksi/2007/926/pdfs/uksi_20070926_en.pdf (accessed on 18 January 2020).
- United States Animal and Plant Health Inspection Service. International Sanitary and Phytosanitary Standards; Docket No. APHIS-2016-0060: USA, 2016; p. 6. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/international/sa_phytostandards/ct_adopted_standards (accessed on 18 January 2020).
- Tuppurainen, E.S.M.; Venter, E.H.; Shisler, J.L.; Gari, G.; Mekonnen, G.A.; Juleff, N.; Lyons, N.A.; De Clercq, K.; Upton, C.; Bowden, T.R.; et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017, 64, 729–745. [Google Scholar] [CrossRef]
- Gelaye, E.; Lamien, C.E. Sheep and Goat Pox. In Transboundary Animal Diseases in Sahelian Africa and Connected Regions; Springer: Berlin/Heidelberg, Germany, 2019; pp. 289–303. [Google Scholar]
- Elhaig, M.M.; Selim, A.; Mahmoud, M. Lumpy Skin Disease in Cattle: Frequency of Occurrence in a Dairy Farm and a Preliminary Assessment of Its Possible Impact on Egyptian Buffaloes. Onderstepoort J. Vet. Res. 2017, 84, 1–6. [Google Scholar] [CrossRef]
- Sprygin, A.; Artyuchova, E.; Babin, Y.U.; Prutnikov, P.; Kostrova, E.; Byadovskaya, O.; Kononov, A. Epidemiological Characterization of Lumpy Skin Disease Outbreaks in Russia in 2016. Transbound. Emerg. Dis. 2018, 65, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Roche, X.; Rozstalnyy, A.; TagoPacheco, D.; Pittiglio, C.; Kamata, A.; Beltran Alcrudo, D.; Bisht, K.; Karki, S.; Kayamori, J.; Larfaoui, F. Introduction and Spread of Lumpy Skin Disease in South, East and Southeast Asia: Qualitative Risk Assessment and Management; Food & Agriculture Org.: Rome, Italy, 2021; ISBN 92-5-133563-X. [Google Scholar]
- Tuppurainen, E.S.M.; Venter, E.H.; Coetzer, J.A.W. The Detection of Lumpy Skin Disease Virus in Samples of Experimentally Infected Cattle Using Different Diagnostic Techniques. Onderstepoort J. Vet. Res. 2005, 72, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Balinsky, C.A.; Delhon, G.; Smoliga, G.; Prarat, M.; French, R.A.; Geary, S.J.; Rock, D.L.; Rodriguez, L.L. Rapid Preclinical Detection of Sheeppox Virus by a Real-Time PCR Assay. J. Clin. Microbiol. 2008, 46, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Lamien, C.E.; Lelenta, M.; Goger, W.; Silber, R.; Tuppurainen, E.; Matijevic, M.; Luckins, A.G.; Diallo, A. Real Time PCR Method for Simultaneous Detection, Quantitation and Differentiation of Capripoxviruses. J. Virol. Methods 2011, 171, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chibssa, T.R.; Settypalli, T.B.K.; Berguido, F.J.; Grabherr, R.; Loitsch, A.; Tuppurainen, E.; Nwankpa, N.; Tounkara, K.; Madani, H.; Omani, A. An HRM Assay to Differentiate Sheeppox Virus Vaccine Strains from Sheeppox Virus Field Isolates and Other Capripoxvirus Species. Sci. Rep. 2019, 9, 6646. [Google Scholar] [CrossRef] [PubMed]
- Settypalli, T.B.K.; Lamien, C.E.; Spergser, J.; Lelenta, M.; Wade, A.; Gelaye, E.; Loitsch, A.; Minoungou, G.; Thiaucourt, F.; Diallo, A. One-Step Multiplex RT-QPCR Assay for the Detection of Peste Des Petits Ruminants Virus, Capripoxvirus, Pasteurella Multocida and Mycoplasma Capricolum Subspecies (Ssp.) Capripneumoniae. PLoS ONE 2016, 11, e0153688. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, E.; Lamien, C.E.; Silber, R.; Tuppurainen, E.S.M.; Grabherr, R.; Diallo, A. Development of a Cost-Effective Method for Capripoxvirus Genotyping Using Snapback Primer and DsDNA Intercalating Dye. PLoS ONE 2013, 8, e75971. [Google Scholar] [CrossRef] [PubMed]
- Stram, Y.; Kuznetzova, L.; Friedgut, O.; Gelman, B.; Yadin, H.; Rubinstein-Guini, M. The Use of Lumpy Skin Disease Virus Genome Termini for Detection and Phylogenetic Analysis. J. Virol. Methods 2008, 151, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.; Edwards, L.; Tuppurainen, E.S.M.; Bachanek-Bankowska, K.; Oura, C.A.L.; Mioulet, V.; King, D.P. Detection of Capripoxvirus DNA Using a Novel Loop-Mediated Isothermal Amplification Assay. BMC Vet. Res. 2013, 9, 90. [Google Scholar] [CrossRef]
- Gelaye, E.; Lamien, C.E. Lumpy Skin Disease and Vectors of LSDV. In Transboundary Animal Diseases in Sahelian Africa and Connected Regions; Springer: Berlin/Heidelberg, Germany, 2019; pp. 267–288. [Google Scholar]
- Lumpy Skin Disease. In OIE Terrestrial Manual; OIE: Paris, France, 2018.
- Commission, I.O. of Epizootics.B.S.; Committee, I.O. of Epizootics.I. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees; Office International des Épizooties: Paris, France, 2017; Volume 2. [Google Scholar]
- Carn, V.M.; Kitching, R.P.; Hammond, J.M.; Chand, P.; Anderson, J.; Black, D.N. Use of a Recombinant Antigen in an Indirect ELISA for Detecting Bovine Antibody to Capripoxvirus. J. Virol. Methods 1995, 53, 273. [Google Scholar] [CrossRef]
- Heine, H.G.; Stevens, M.P.; Foord, A.J.; Boyle, D.B. A Capripoxvirus Detection PCR and Antibody ELISA Based on the Major Antigen P32, the Homolog of the Vaccinia Virus H3L Gene. J. Immunol. Methods 1999, 227, 187–196. [Google Scholar] [CrossRef]
- Babiuk, S.; Wallace, D.B.; Smith, S.J.; Bowden, T.R.; Dalman, B.; Parkyn, G.; Copps, J.; Boyle, D.B. Detection of Antibodies against Capripoxviruses Using an Inactivated Sheeppox Virus ELISA. Transbound. Emerg. Dis. 2009, 56, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Chen, Y.; Wu, J.; Shang, Y.; Liu, X. Serodiagnosis of Sheeppox and Goatpox Using an Indirect ELISA Based on Synthetic Peptide Targeting for the Major Antigen P32. Virol. J. 2010, 7, 245. [Google Scholar] [CrossRef] [PubMed]
- Bowden, T.R.; Coupar, B.E.; Babiuk, S.L.; White, J.R.; Boyd, V.; Duch, C.J.; Shiell, B.J.; Ueda, N.; Parkyn, G.R.; Copps, J.S.; et al. Detection of Antibodies Specific for Sheeppox and Goatpox Viruses Using Recombinant Capripoxvirus Antigens in an Indirect Enzyme-Linked Immunosorbent Assay. J. Virol. Methods 2009, 161, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, E. Genetic Characterization of Poxviruses Infecting Ruminants and Camels in Ethiopia and the Development of Differential Diagnostic and Disease Surveillance Tools. Ph.D. Thesis, BOKU-Universität für Bodenkultur, Vienna, Austria, 2016. [Google Scholar]
- Wolffe, E.J.; Katz, E.; Weisberg, A.; Moss, B. The A34R Glycoprotein Gene Is Required for Induction of Specialized Actin-Containing Microvilli and Efficient Cell-to-Cell Transmission of Vaccinia Virus. J. Virol. 1997, 71, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.A.; Smith, G.L. Identification and Characterization of an Extracellular Envelope Glycoprotein Affecting Vaccinia Virus Egress. J. Virol. 1992, 66, 1610–1621. [Google Scholar] [CrossRef]
- Galmiche, M.C.; Goenaga, J.; Wittek, R.; Rindisbacher, L. Neutralizing and Protective Antibodies Directed against Vaccinia Virus Envelope Antigens. Virology 1999, 254, 71–80. [Google Scholar] [CrossRef]
- Xiao, Y.; Isaacs, S.N. Enzyme-Linked Immunosorbent Assay (ELISA) and Blocking with Bovine Serum Albumin (BSA)—Not All BSAs Are Alike. J. Immunol. Methods 2012, 384, 148–151. [Google Scholar] [CrossRef]
- Chart, H.; Evans, J.; Chalmers, R.M.; Salmon, R.L. Escherichia Coli O157 Serology: False-positive ELISA Results Caused by Human Antibodies Binding to Bovine Serum Albumin. Lett. Appl. Microbiol. 1998, 27, 76–78. [Google Scholar] [CrossRef]
- Tuppurainen, E.S.; Lamien, C.E.; Diallo, A. Capripox (Lumpy Skin Disease, Sheep Pox, and Goat Pox). Vet. Vaccines Princ. Appl. 2021, 383–397. [Google Scholar]
- EFSA. Urgent Advice on Lumpy Skin Disease- European Food Safety Authority; EFSA: Parma, Italy, 2016. [Google Scholar]
- Venkatesan, G.; Kumar Teli, M.; Sankar, M.; Kumar, A.; Dashprakash, M.; Arya, S.; Madhavan, A.; Ramakrisnan, M.A.; Pandey, A.B. Expression and Evaluation of Recombinant P32 Protein Based ELISA for Sero-Diagnostic Potential of Capripox in Sheep and Goats. Mol. Cell. Probes 2018, 37, 48–54. [Google Scholar] [CrossRef]
- Innovative Diagnostics. Validation Report ID Screen® Capripox Double Antigen Multi-Species; 2017; p. 16. Available online: https://www.id-vet.com/produit/id-screen-capripox-double-antigen-multi-species/ (accessed on 18 January 2020).
- Wolff, J.; King, J.; Moritz, T.; Pohlmann, A.; Hoffmann, D.; Beer, M.; Hoffmann, B. Experimental Infection and Genetic Characterization of Two Different Capripox Virus Isolates in Small Ruminants. Viruses 2020, 12, 1098. [Google Scholar] [CrossRef]
- Adedeji, A.J.; Ijoma, S.I.; Atai, R.B.; Dogonyaro, B.B.; Adole, J.A.; Maurice, N.A.; Osemeke, O.H.; Waziri, I.A.; Atuman, Y.J.; Lyons, N.A.; et al. Household and Animal Factors Associated with Sheeppox and Goatpox Sero-Prevalence and Identification of High-Risk Areas in Selected States of Northern Nigeria. Prev. Vet. Med. 2021, 196, 105473. [Google Scholar] [CrossRef]
- Calistri, P.; DeClercq, K.; De Vleeschauwer, A.; Gubbins, S.; Klement, E.; Stegeman, A.; Cortiñas Abrahantes, J.; Antoniou, S.E.; Broglia, A.; Gogin, A. Lumpy Skin Disease: Scientific and Technical Assistance on Control and Surveillance Activities. EFSA J. 2018, 16, e05452. [Google Scholar] [CrossRef]
- Chibssa, T.R.; Sombo, M.; Lichoti, J.K.; Adam, T.I.B.; Liu, Y.; Elraouf, Y.A.; Grabherr, R.; Settypalli, T.B.K.; Berguido, F.J.; Loitsch, A. Molecular Analysis of East African Lumpy Skin Disease Viruses Reveals a Mixed Isolate with Features of Both Vaccine and Field Isolates. Microorganisms 2021, 9, 1142. [Google Scholar] [CrossRef]
- Carn, V.; Kitching, R. An Investigation of Possible Routes of Transmission of Lumpy Skin Disease Virus (Neethling). Epidemiol. Infect. 1995, 114, 219–226. [Google Scholar] [CrossRef]
- Caufour, P.; Rufael, T.; Lamien, C.E.; Lancelot, R.; Kidane, M.; Awel, D.; Sertse, T.; Kwiatek, O.; Libeau, G.; Sahle, M.; et al. Protective Efficacy of a Single Immunization with Capripoxvirus-Vectored Recombinant Peste Des Petits Ruminants Vaccines in Presence of Pre-Existing Immunity. Vaccine 2014, 32, 3772–3779. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319242776. [Google Scholar]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Perkins, N.J.; Schisterman, E.F. The Inconsistency of “Optimal” Cutpoints Obtained Using Two Criteria Based on the Receiver Operating Characteristic Curve. Am. J. Epidemiol. 2006, 163, 670–675. [Google Scholar] [CrossRef]
- Liu, X. Classification Accuracy and Cut Point Selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
| Primer’s Name | Sequence (5′–3′) |
|---|---|
| C-type-GW-For | GGGGACAAGTTTGTACAAAAAAGCAGGCTTAATACGATACAA AGATGAACTATTTCCTAATGTATGTAATAAAGGATGGG |
| C-type-GW-Rev | GGGGACCACTTTGTACAAGAAAGCTGGGTATCAATTATATAA CTTTTAACACAGATTAT |
| EEVGp-GW-For | GGGGACAAGTTTGTACAAAAAAGCAGGCTTAGAATACAAAAAT GTTATTAAAAAAATGTTATTTAAA |
| EEVGp-GW-Rev | GGGGACCACTTTGTACAAGAAAGCTGGTATTAACAACAA TTATAATAGTTTGACTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berguido, F.J.; Gelaye, E.; Liu, Y.; Davaasuren, B.; Krstevski, K.; Djadjovski, I.; Ivanova, E.; Goujgoulova, G.; Loitsch, A.; Tuppurainen, E.; et al. Development and Optimization of Indirect ELISAs for the Detection of Anti-Capripoxvirus Antibodies in Cattle, Sheep, and Goat Sera. Microorganisms 2022, 10, 1956. https://doi.org/10.3390/microorganisms10101956
Berguido FJ, Gelaye E, Liu Y, Davaasuren B, Krstevski K, Djadjovski I, Ivanova E, Goujgoulova G, Loitsch A, Tuppurainen E, et al. Development and Optimization of Indirect ELISAs for the Detection of Anti-Capripoxvirus Antibodies in Cattle, Sheep, and Goat Sera. Microorganisms. 2022; 10(10):1956. https://doi.org/10.3390/microorganisms10101956
Chicago/Turabian StyleBerguido, Francisco J., Esayas Gelaye, Yang Liu, Batdorj Davaasuren, Kiril Krstevski, Igor Djadjovski, Emiliya Ivanova, Gabriela Goujgoulova, Angelika Loitsch, Eeva Tuppurainen, and et al. 2022. "Development and Optimization of Indirect ELISAs for the Detection of Anti-Capripoxvirus Antibodies in Cattle, Sheep, and Goat Sera" Microorganisms 10, no. 10: 1956. https://doi.org/10.3390/microorganisms10101956
APA StyleBerguido, F. J., Gelaye, E., Liu, Y., Davaasuren, B., Krstevski, K., Djadjovski, I., Ivanova, E., Goujgoulova, G., Loitsch, A., Tuppurainen, E., Chibssa, T. R., Caufour, P., Samojlović, M., Lazić, S., Petrović, T., Vidanović, D., Bertagnoli, S., Grabherr, R., Diallo, A., ... Lamien, C. E. (2022). Development and Optimization of Indirect ELISAs for the Detection of Anti-Capripoxvirus Antibodies in Cattle, Sheep, and Goat Sera. Microorganisms, 10(10), 1956. https://doi.org/10.3390/microorganisms10101956








