Abstract
Leishmania parasites present astonishing adaptative abilities that represent a matter of life or death within disparate environments during the heteroxenous parasite life cycle. From an evolutionary perspective, organisms develop methods of overcoming such challenges. Strategies that extend beyond the genetic diversity have been discussed and include variability between parasite cells during the infections of their hosts. The occurrence of Leishmania subpopulation fluctuations with variable structural genomic contents demonstrates that a single strain might shelter the variability required to overcome inconsistent environments. Such intrastrain variability provides parasites with an extraordinary ability to adapt and thus survive and propagate. However, different perspectives on this evolution have been proposed. Strains or species living in the same environment can cooperate but also compete. These interactions might increase the replication rate of some parasites but cause the loss of more aggressive competitors for others. Adaptive responses to intra- and interspecific competition can evolve as a fixed strategy (replication is adapted to the average genetic complexity of infections) or an optional strategy (replication varies according to the genetic complexity of the current infection). This review highlights the complexity of interspecies and intrastrain interactions among Leishmania parasites as well as the different factors that influence this interplay.
Keywords:
Leishmania; coinfections; mixed infections; coculture; hybrid; intercellular communication 1. Introduction
Few reports have described infections by more than one Leishmania species, which is likely because of the lack of efficient diagnostic methods for cases of this nature. Therefore, the clinical and epidemiological impact of mixed infections remains to be explored, and the technical limitations for such studies must be overcome.
Direct parasitological examination by visualization of amastigotes in clinical specimens, which are used routinely in the diagnosis of leishmaniasis, does not allow for the identification of mixed infections because the number of morphological differences is insufficient to differentiate species. In turn, assays carried out after parasite isolation and cultivation do not always allow for the identification of mixed infections because in vitro maintenance may favor a particular species or even a specific strain. The introduction of molecular methods for the diagnosis of leishmaniasis and Leishmania typing applied directly to clinical specimens enables mixed infection detection. Nevertheless, the results will be affected by the sensitivity of the methodology employed and the parasite burden for each species.
In nature, mixed infections by Leishmania species are naturally present in vectors, reservoirs, and humans. Experimental coinfections have contributed to a better understanding of the mechanisms involved in these interactions and their consequences. For instance, mixed infections by different species of Leishmania can alter the transmission dynamics, as observed in gerbils infected by L. major and L. turanica [1]. Experimental mixed infections with these two species in Phlebotomus papatasi sand flies showed that although they coexist in the vector, the prevalence of each species may vary as a consequence of the shared environment [2]. In a human monocyte cell line previously infected with a Leishmania species, infection by another species is not impaired, even if the second species was added 3 h later [3]. A recent study of experimental coinfections using a hamster model (Mesocricetus auratus) infected with L. (L.) amazonensis and L. (L.) infantum performed clinical, histopathological, and immunological analyses and showed that mixed infections are associated with more severe clinical manifestations than single infections [4]. A case of human mucosal leishmaniasis (ML) involving coinfection by L. (L.) tropica and L. (L.) major was also reported [5], and mixed infections by different strains of L. infantum have been shown to influence the therapeutic response [6].
Inter- and intraspecies interactions occur among Leishmania parasites in both vertebrate and invertebrate hosts; genetic exchange has already been demonstrated for these parasites, and it mainly occurs during the development stage in sandflies. Putative hybrids and genetic recombination have been reported in different studies focused on Leishmania typing and genetic analyses of natural populations. The impact of this reproductive strategy is a current focus of debate [7]. Mosaic aneuploidy is an important feature in discussions of multiple infections in Leishmania, and in Leishmania parasites, this feature implies that each strain is already in a mixture, which represents a genetic adaptation strategy of this parasite [8]. Mosaic aneuploidy refers to a variation in the number of homologous chromosomes per cell within a subpopulation of cells. For example, 30% of cells presenting chromosome 2 in a diploid state, 20% as triploid and 50% as tetraploid
Thus, studies of mixed infections can ultimately contribute to elucidating the complex molecular basis of clinical and epidemiological features and the parasite-specific factors that might lead to the worsening or improvement of infections. The molecular mechanisms involved in the Leishmania–Leishmania interaction process have not been widely explored; however, they are essential for maintaining a microbial community [9] either by direct contact, media sharing or both. Moreover, the diversity of these communities has a profound impact on the biology of the parasite and, consequently, on the interaction of Leishmania with its vertebrate and invertebrate hosts. In vitro promastigote cultures have been investigated, and the in vitro growth of L. mexicana has been shown to be impaired when grown together with L. amazonensis [10]. The cocultivation of L. amazonensis strains with a distinct susceptibility profile to pentamidine demonstrated that in vitro growth depends on the interaction between strains that share the same environment [11]. The growth of L. donovani cocultured with Trypanosoma brucei was severely affected, and swelling and lysis were observed. In these cases, direct contact was necessary because T. brucei did not hinder growth when they were physically separated [12]. A species or strain may produce more than one agonist or antagonist element with different physicochemical and biological properties, which will depend on environmental conditions among other factors.
Studies with bacteria and fungi performed to characterize the biofilm secretome synthetized by a single or distinct species showed an increase in proteins secreted by mixed biofilms, thus reflecting the competition for iron by the microorganisms [13]. Leishmania promastigotes secrete proteins involved in immunomodulation, signal transduction and intracellular survival, such as HSP70, acid phosphatase, protein kinase C receptor (LACK), elongation factor 1, and triparedoxin peroxidase [14]. In addition, in vitro-secreted vesicles, exosomes and ectosomes carry various molecules, including GP63 surface metalloproteases, which is a critical parasite virulence factor. The characterization of exosomes produced by in vitro promastigotes indicates that they are similar to those observed during interactions with the vector insect [15]. Studies with trypanosomatids have shown the role of these vesicles in the process of parasite interactions, although their role in Leishmania–Leishmania interactions has not yet been investigated [16].
Some mechanisms allow a population of individuals to coordinate global behavior and act as a multicellular unit, which is a phenomenon known as “quorum sensing”, a process of cell–cell communication that allows microorganisms such as bacteria to share information, such as cell density, and respond by adjusting gene expression, consequently changing phenotypes. Quorum sensing is a poorly explored mechanism in Leishmania, which is a parasite with high genetic and phenotypic variability and with tremendous adaptive ability. An evaluation of T. congolense demonstrated that this parasite has a growth control mechanism based on density and that the interaction with other species of trypanosomes is dependent on quorum sensing [17].
This review article presents published data on natural and experimental mixed infections by distinct Leishmania species or strains and addresses the complexity of interspecies and intrastrain interactions in Leishmania parasites and the various factors influencing this relationship (Figure 1).
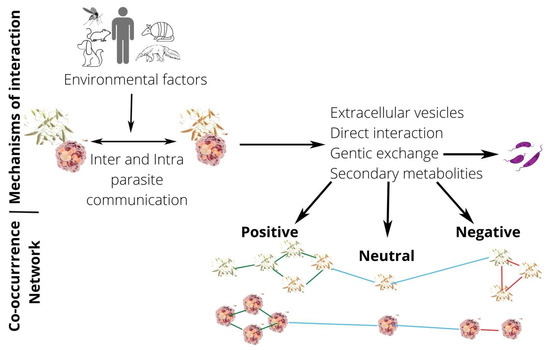
Figure 1.
Picture of Leishmania interactions through co-occurrence networks. Leishmania interactions are influenced by hosts’ factors (environmental factors) and can result in positive, neutral, and negative interactions types. In co-occurrence networks, nodes are represented by promastigotes (in invertebrate hosts) or amastigotes (in vertebrate hosts) of Leishmania spp., and edges are representing associations between parasites (nodes). Blue edges indicate neutral interactions, green edges indicate positive interactions, while red edges suggest negative interactions between Leishmania species, strains or genotypes.
Table 1 presents a summary of studies reporting inter- and intraspecies interactions among Leishmania parasites in their hosts or in experimental conditions in vitro and in vivo.

Table 1.
Summary of studies reporting natural or experimental interactions among Leishmania species or strains.
2. Natural Mixed Infections by Leishmania Species
Leishmaniasis is a complex disease that is endemic in large areas of the tropics, subtropics and Mediterranean basin, globally spanning more than 98 countries. The disease is caused by several Leishmania species, an obligate protistan parasite, and the transmission cycles involve different vertebrate and sandfly species. According to the World Health Organization [47], there are three main forms of this disease: visceral (also known as kala-azar), cutaneous (the most common), and mucocutaneous.
Despite some taxonomic controversies, there are more than 30 recognized Leishmania species found in mammals and reptiles, and one is found in sandflies only. At least 20 are pathogenic to humans across the world (Table 2). While some Leishmania species are geographically restricted to an endemic area, others are widespread. For example, in the Amazon region [48], a greater number of species coexist in sympatry, and some are only found there [49]. Importantly, although not yet well defined, different clones or strains may also be observed in this transmission environment. Such an intricate epidemiological scenario can contribute to the complexity of the disease assuming the possibility of mixed infections caused by different subpopulations, species or strains of Leishmania.

Table 2.
Classification of Leishmania species (Class Kinetoplastea; Order Trypanosomatida; Family Trypanosomatidae; Subfamily Leishmaniinae), excluding synonyms, nomen nudum and those not completely classified.
Mixed infections by Leishmania species are very likely to be underdiagnosed; nevertheless, coinfected human patients have been reported in the literature. Infection outcome is influenced by the Leishmania species involved, and multiple strains, genotypes or species infections are expected to impact host–parasite relationships. In mammalian hosts, the Leishmania infection profile is characterized by cytokine and chemokine production and may be related to the species of Leishmania as well as other factors [89]. Despite the host’s immunological competence, data from the literature indicate an essential role of the Leishmania species over the course of infection. In the Leishmania (Viannia) subgenera, for example, two species that cause tegumentary leishmaniasis may lead to different manifestations of the disease: L. naiffi, which is commonly associated with low virulence in cutaneous lesions [90,91] and the L. braziliensis, which is correlated with mucosal and atypical lesions frequently refractory to treatment [92,93,94,95]. In the Leishmania (Leishmania) subgenera, this polarization is also observed in infections caused by L. tropica and L. major, which lead to small or no lesions and severe lesions, respectively [96,97,98]. Determining how coinfecting Leishmania species interact with their hosts is not trivial. Within-species interactions can be direct (e.g., via resource competition) or indirect (e.g., via immunomodulation). Experimental infections in rhesus macaques indicate cross-reacting immune responses and possible cross-protection between taxonomically different Leishmania parasites [24]. Although existing data cannot be used to predict coinfection scenarios with different Leishmania species, such scenarios should be considered due to the potential complications in the course of the disease and response to the treatment.
A few reports have associated coinfection with therapeutic response or atypical clinical manifestations, although the differences relative to infection with the separate agents have not been presented. For example, a survey of Bolivian patients showed that 27.6% presented mixed infections by different species of Leishmania and 13.8% presented mixed infections by Leishmania species and T. cruzi [23], which significantly influenced the therapeutic outcomes. Treatment failure was linked to mixed infection by two L. infantum zymodemes, which present distinct biological behavior and different sensitivities to meglumine antimoniate [6]. Mixed infection by L. amazonensis and L. infantum was associated with diffuse cutaneous leishmaniasis (DCL) in a Bolivian patient. The lesion presents atypical characteristics, possibly due to coinfection, and abundant parasites and vacuolated histiocytes were observed, which is compatible with DCL diagnoses [22]. Atypical disseminated leishmaniasis was associated with mixed infection caused by L. guyanensis and L. amazonensis. Over the course of the infection, a mixed patterns of clinical, histopathological and immunological characteristics related to the two species were observed, such as the absence of cellular response and failure of therapy, which are consistent with L. amazonensis infection, and multiple concomitant lesions with a low antibody titer, which are consistent with L. guyanensis infection [40]. In Iran, a patient presenting mucosal leishmaniasis was reported to have nasal and oral lesions caused by L. major and L. tropica, respectively. The nasal lesions appeared before the oral lesions, suggesting that previous infection by L. major did not protect against L. tropica in this patient [5]. Conversely, a study using BALB/c mice reported that primary infection with L. tropica induces partial protection against L. major infection [26]. However, the further clinical progression of a subclinical infection associated with L. tropica in the Iranian patient after L. major infection cannot be excluded. Similarly, protection against L. braziliensis was not observed for a patient presenting coinfection with L. infantum (named L. donovani in that work) [19], and cutaneous lesions appeared after visceral leishmaniasis clinical signs; however, subclinical infection cannot be disregarded.
It is worth mentioning the mixed infections reports that do not address complications due to mixed infections. Typical cutaneous lesions caused by the coinfection of L. braziliensis and L. amazonensis were observed in a patient from the Brazilian Amazon region [18], and cutaneous leishmaniasis caused by a mixed natural infection by L. braziliensis and L. lainsoni has been demonstrated in a Peruvian patient, who showed a good response to treatment with sodium stibogluconate and no evidence of mucosal involvement [33].
The simultaneous presence of L. donovani and L. major in typical localized cutaneous ulcers of leishmaniasis patients from Sudan was reported, and no evidence of visceralization was observed [34]. The same coinfection was reported in a case from Iraq, although in this case, the patient presented concomitant visceral and cutaneous leishmaniasis [21]. Patients clinically diagnosed with visceral leishmaniasis presented mixed infection by L. donovani and L. major in the spleen, and the mixed cultures obtained from the tissue fragments were inoculated in laboratory animals, producing both visceral and cutaneous leishmaniasis [20].
Atypical clinical manifestations were observed in immunocompromised patients presenting infection by two Leishmania species. An atypical cutaneous lesion caused by L. donovani was observed in a Brazilian HIV-positive patient after long-term evidence was obtained of the visceral parasite L. infantum in the bone marrow [30]. A case of disseminated cutaneous leishmaniasis linked to mixed infection by L. infantum and L. major was reported for an Iranian HIV-positive patient who did not respond to a different therapeutic scheme [39]. Coinfection by two trypanosomatids, L. donovani and Leptomonas seymouri, was detected in immunocompromised PDKL patients [99]. A coinfection by L. infantum and a Crithidia-related parasite may also be associated with fatal visceral leishmaniasis [100,101].
Mixed Leishmania spp. infections have also been demonstrated in domestic hosts and reservoirs. Mixed infection by L. infantum and L. braziliensis has been reported in horses, dogs and synanthropic rodents. Nevertheless, whether such infections impact the epidemiology of leishmaniasis in urban areas has not been clarified [25,32,36,38]. Mixed infection with L. amazonensis and L. braziliensis in dogs has also been observed in an urban area endemic for visceral leishmaniasis [42] and mixed infection caused by other Leishmania species and a variety of Trypanosoma spp. [41,102]. Furthermore, naturally infected dogs are prone to multiple L. infantum genotype infections [45]. However, whether these infections could impact the course of the disease or other characteristics, such as parasite transmissibility, remains to be elucidated.
3. Coculture and Experimental Mixed Infections by Leishmania Species and Their Interactions
Microorganisms live in communities and thus present broad inter- and intraspecies interactions. These interactions can be beneficial or harmful and can influence the fitness of such microorganisms. Whether these interactions are neutral, competitive or cooperative will depend on several factors, including the genetic background of the interacting microorganisms. Cooperative behavior provides a direct or indirect benefit to organisms [103,104] and is likely to occur among closely related microorganisms. Competition is more expected among distantly related microorganisms [105] and may impact virulence [106]. For bacterial species, physical or chemical contact often changes the phenotype, thus allowing for competition, mutualism or commensalism, and these relationships may have influenced their evolution [107,108].
Several studies have addressed the interactions among coinfecting parasite species, strains, or genotypes; however, only a few have investigated intra- and interspecies interactions. Most previous studies have focused on describing the interactions, while few have reported on the underlying mechanisms and consequences. Interactions among coinfecting parasitic species represent a relevant mechanism to maintaining genetic variation [109].
In the late 1980s, a report suggested that factors excreted by L. amazonensis could inhibit the in vitro growth of L. mexicana promastigotas [10]. These species present similar growth patterns in the absence of metabolic competition. Differential abilities to overcome environmental conditions were observed in cocultures of L. donovani and Leptomonas seymouri obtained after parasite isolation from PDKL patients, and variations in the culture media conditions enabled L. seymouri elimination [110]. In a multiwell plate system, L. amazonensis strains resistant to pentamidine inhibited the in vitro growth of nonresistant lineages [11], suggesting that secreted factors present in the shared culture medium rather than physical contact led to such alterations.
Mixed cultures of L. donovani strains with different drug resistance levels demonstrated increased fitness in drug-resistant parasites compared to more susceptible parasites, and they also presented higher tolerance to stress conditions [111]. In Leishmania spp., many drug resistance mechanisms are concomitantly associated with higher virulence or superior redox resistance, which may result in independent phenotype selection not associated with drug pressure, thus leading to the emergence of resistant strains among parasitic populations [112].
Competition among L. major clones derived from the same strain has been demonstrated. Initially, a more virulent clone represented the dominant competitor in the mixed culture; however, after one month of culture, the more attenuated clone was the predominant clone. Culture and environmental conditions, such as pH, change over time and could lead to the superior growth of clones with greater tolerance to these conditions. Nutrient requirements could explain the differences between the two clones, and the more virulent clone could buffer the media, thereby creating appropriate conditions for adaptations in the more attenuated clone [113]. Mixed multiclonal infections by L. infantum show that the phenotype of the virulent clone was dominant relative to the phenotypes of the associated low-virulence clones. After a challenge with homologous or heterologous strains or clones, virulent phenotypes were conserved and expressed in naive mice independent of the preexisting parasite population.
Studies on experimental mixed infection by Leishmania species are scarce. The few studies on this topic point to an important impact when two Leishmania species are coinfecting vertebrate hosts. Experimental infection of gerbil (Rhombomys opimus) with L. major and L. turanica led to a persistent infection that can reach up to 18 months, while separate infection with these species remained in the skin of the gerbil, which is a natural host, for up to six months at most. Such synergy thus favors the maintenance of L. major from the transmission season until the next [1]. Experimental infections in the sandfly Phlebotomus papatasi showed that L. turanica and L. major are able to develop together and do not show signs of competition [2]. Thus, the ability of L. major and L. turanica to participate in mutualistic interactions in the insect vector would have an impact on the transmission of these parasites to the vertebrate host. Together, these characteristics have a relevant impact on the epidemiology of cutaneous leishmaniasis caused by L. major.
Concomitant experimental infections with Endotrypanum and L. guyanensis showed different patterns compared to single infections, and although the authors suggested that the presence of L. guyanensis would inhibit the development of Endotrypanum, all cultivated samples of the parasites recovered from infected flies were characterized as Endotrypanum, which is a parasite that grows faster than L. guyanensis in vitro [114]. Experimental coinfections and single infections by L. (L.) infantum and L. (V.) braziliensis were performed in Lutzomyia longipalpis and Lutzomyia migonei [44]. Infections by L. (L.) infantum reached higher rates and grew more vigorously than that of L. (V.) braziliensis. Typical suprapylarian and peripylarian development were observed for L. infantum and L. braziliensis, respectively, as expected. Both Leishmania species completed their life cycle and produced infective forms in both sand fly species studied. The same results were obtained in coinfection experiments, demonstrating that the two parasites conclude their development and do not compete.
A comparison of a single infection of macrophages (lineage U-937) showed that the infectivity of L. amazonensis was higher than that of L. infantum, and this result was maintained with the dominance of L. amazonensis in the coinfected macrophages; however, a small portion of macrophages presented more than four Leishmania, which were rarely from different species [3]. In golden hamsters, mixed infection with L. amazonensis and L. infantum was associated with more severe disease than single infections. This result suggests that mixed infections could better suppress host immunity, thus allowing the parasites to multiply and impair macrophage effector function [4]. This study showed an earlier increase in the spleen in mixed infections, which was probably as a result of L. amazonensis dissemination, although in later stages of infection, the authors detected L. infantum outcompeting L. amazonensis.
The difference in fitness among lineages of L. donovani with diverse drug resistance patterns was demonstrated using experimentally mixed cultures. Competition was not observed when experimentally resistant promastigotes of L. donovani were cocultivated with susceptible lines. However, resistant lineages were more tolerant when mixed cultures were subjected to diverse stress conditions [111]. These results indicate that resistant phenotypes in Leishmania may be associated with the greatest in vitro fitness rather than a fitness cost, as observed in other models [115].
Mixed infections in vertebrate hosts may occur within the same tissue or even in the same cell. In the case of Leishmania spp., a parasite in the parasitophorous vacuoles (PVs) of macrophages, there is also the possibility of mixed infection in the same vacuole [46]. It is known that L. amazonensis is able to enter Coxiella burnetti vacuoles and then survive, differentiate, and replicate therein [116,117,118]. Furthermore, it has been shown that the large adaptive vacuoles of L. amazonensis are permissive to T. cruzi survival and differentiation and that noninfective epimastigotes are saved from destruction within the chimeric PVs [116,117,118]. The large vacuole that houses the L. mexicana species complex can explain why this multiparasite interaction is not observed under other conditions. For example, a mixed infection of macrophages by L. infantum and Toxoplasma gondii showed that they share the same macrophage but not the same PV [116,117,118]. A study of mixed infection in macrophages by L. amazonensis and L. major found no fusion of PVs containing both amastigotes. Interestingly, PVs containing L. major promastigotes fused with preestablished L. amazonensis PVs. In these chimeric vacuoles, L. major promastigotes remained motile and multiplied but did not differentiate into amastigotes [28]. Considering the Leishmania–macrophage interaction, species-specific differences were demonstrated in the biogenesis of the PV. For example, amastigotes from the L. mexicana species complex use large vacuoles, which may contain many parasites, while a single L. major amastigote occupies a smaller tight PV [119]. The presence of both L. amazonensis and L. mexicana within the same communal vacuole has also been described [46].
4. Do Coinfections Promote Hybrid Formation?
Genotype, strain, and species interactions among Leishmania parasites can occur in both vertebrate and invertebrate hosts. Nevertheless, genetic exchange has been mainly demonstrated to occur in sand fly vectors, with experimental evidence of intraspecific hybrids of L. major, L. tropica, and L. donovani [29,31,43], cross-species hybrids between L. major and L. infantum [37], and intraclonal or selfing hybrids of L. infantum [35]. Although few studies have investigated hybrid formation during vertebrate infection [27], a study that performed DNA quantification showed that infected macrophages could harbor 4N amastigotes, suggesting that genetic exchange is possible in mammalian host cells [120]. Recently, the possibility of intraclonal and interspecific genetic exchange among parasites of the L. mexicana complex was explored, and unlike other Leishmania species, the species of this complex replicate in spacious communal vacuoles that may provide an environment favorable to genetic exchange, although the resulting products of those putative genetic events were unstable [46].
Cell fusion in L. infantum and L. tropica was observed following promastigote axenic in vitro culture. Fusion began with an attachment of the posterior extremities of two ovoid flagellates, which was followed by complete fusion, with the disappearance of adjacent cell membranes and the appearance of a larger and shorter flagella [121]. Evidence of sexual reproduction in Leishmania was also quantitatively demonstrated through microspectrophotometric analyses of nuclear fusion in the intracellular form, i.e., the amastigote, within the mammalian host [120]. Furthermore, fluorescence microscopy showed that L. donovani hybrids occurred in experimentally infected sandflies, although the hybrids were not viable in vitro [29].
Broad agreement has been reached that Leishmania possesses the machinery for genetic exchange, and the debate regarding reproductive strategies pertains mainly to the frequency of sexual recombination and its impact on population structure. To date, the most accepted environment for generating Leishmania hybrids is inside the invertebrate host among promastigotes. Double-drug-resistant clones could be generated by coinfecting sand flies with various pairwise combinations of parental lines expressing distinct drug-resistant markers. In almost every case, these clones appeared to be full genomic hybrids based on their biparental inheritance of allelic markers distributed across the nuclear genome [27,31,43]. The parental chromosome contributions were consistent with a meiotic process, and deep sequencing of backcross progeny clones revealed genome-wide recombination patterns, indicating that classic crossovers occur at meiosis [43].
Several studies have isolated strains characterized as putative hybrids between different Leishmania species. They are most common hybrids between closely related species, such as some dispersed on the American continent, namely, L. braziliensis and L. panamensis [122], L. braziliensis and L. peruviana [123], L. braziliensis and L. guyanensis [124], L. naiffi and L. lainsoni [125], and recently natural L. guyanensis/L. shawi hybrids were isolated from patients with American Cutaneous Leishmaniasis in the Amazon region of Brazil [126]. Hybrids between closely related species from the Old World were also described, namely, L. major and L. arabica [127,128]. Putative hybrids between L. major and L. infantum, which are phylogenetically distant species with different vectors and reservoir hosts, have also been described [129] but less frequently. The fitness of L. major/L. infantum hybrids was increased when compared with that of L. infantum. Genetic exchange appears to have conferred a certain level of L. major lipophosphoglycan (LPG) to the mentioned hybrids, thus enabling them to survive in the specific vector P. papatasi, which is permissive to L. major but not to L. infantum [130]. In addition to altered transmission capabilities or the production of a more aggressive infection [131], the consequences of genetic exchange may have many kinds of epidemiological significance. Of note is the outbreak of CL in Peru in the 1990s, which was associated with the F1 hybrids of L. braziliensis and L. peruviana [132]. Indeed, genetic exchange might facilitate the emergence and spread of new phenotypic traits [133].
Beyond the observations of hybrids between Leishmania species and strains, recent studies have reported mito-nuclear discordance among Leishmania strains, but it is not clear how this occurs and if it is the same mechanism of formation of nuclear DNA hybrids [134]. Analysis of complete genome sequences of a large sample of L. braziliensis and L. peruviana strains from Peru showed evidence of meiotic-like recombination between Leishmania species, resulting in full-genome hybrids. Analysis of the mitochondrial genome of hybrid strains indicated that they consisted of homogeneous uniparental maxicircles but minicircles derived from both parental species [132].
5. The Occurrence of a Subpopulation of Parasites within One Strain—Aneuploid Mosaicism and Haplotype Selection/Fluctuation: Already a Mixed Content?
Various studies have reported in recent years that one Leishmania isolate is composed of cells presenting different homologous chromosome contents and variable gene copy numbers. This feature was better characterized by FISH in 2011 and is referred to as aneuploidy mosaicism [135]. Further studies demonstrated that the aneuploidy profile of an isolate might change as a consequence of environmental conditions, which is a reflection of this strategic adaptations harbored by Leishmania parasites [136]. Such plasticity of genes and whole chromosome copy numbers directly affects the parasite transcriptome. Thus, same-strain phenotypic variances are likely to occur depending on the fluctuation of these subpopulations of cells carrying distinct genome contents.
Based on the above statements, we assume that aneuploidy mosaicism introduces complexity to discussions of multiple infections in Leishmania. This mosaic feature implies that each strain is already a mixture in a sense, which represents a strategy of the parasite to balance short-term and long-term adaptation [137]. Selection of haplotypes that results in allelic frequency modification (haplotype fluctuation:) and karyotype fluctuation (implies a preexistence of karyotypic mosaicism of the population of parasites in a given condition, e.g., population of parasites maintained in culture, parasites in the infection of their hosts) that may be of benefit to the parasite will ultimately maintain the variability and potentially promote phenotypic variance in the Leishmania isolate. Therefore, a given isolate might find different solutions for environmental challenges, such as drug exposition and in vitro culture. Considering the present discussion in this review, we believe that new single-cell-based techniques will be able to reveal the effects of a subpopulation of cells based on tracking and determining the genome content individually [138]; moreover, such techniques will contribute to mapping the interaction between parasite cells, either from different species/strains or within a given isolate.
Another critical point regarding plasticity is the genetic fluctuation in the leishmanial strain. This parasite is unique in its ability to increase its genetic diversity, in which both the karyotype and the number of haplotypes are changed. L. donovani promastigote strains isolated from golden hamsters were compared among early and late passages. Fluctuations in the allele frequency were observed along the passages, indicating mosaic aneuploidy in different combinations. Only 10% of the 204 observed karyotypes showed a high frequency. In vivo analyses demonstrated different localizations of the aneuploid profile subpopulations in the liver and spleen. These results suggest that different alleles could be related to specific localizations in the host and represent the fitness of diverse subpopulations. Altogether, these observations may indicate that Leishmania spp. are able to change their genetic repertoire, thereby magnifying their ability to adapt to stressful and divergent environmental conditions, improving their survival, and increasing the diversity within the populations [137].
6. Intercellular Communication
Leishmania spp. are heteroxenous unicellular parasites. The survival achieved thus far by different species of this parasite is based on their successful morphological–biochemical–physiological adaptations, environmental sensing ability, molecular and genetic organization to optimize responses and interactions, and communication within contiguous populations (intra/interstrain and intra/interspecies). Many functions and molecules have been studied and are related to environmental sensing and adaptative responses, such as cAMP (cyclic adenosine monophosphate), inositol phosphatases, kinases, phosphoproteins and heat shock proteins in kinetoplastids. Related genes are usually involved in the influence of signal transduction on infectivity, cell growth, and differentiation. Approximately 6% of the genes localized on chromosomes 1 and 3 are associated with these processes [139].
In response to environmental challenges, single and multicellular organisms exhibit a conserved signaling pathway composed of surface receptors that transduce signals to kinases and phosphatases. Downstream, these cascades result in variations in gene expression and protein abundance, thus providing phenotypic variations [140]. In parasitic protozoa, the relationship between signaling pathway components and regulators is not fully understood [141]. Parasites have developed a range of mechanisms for communicating with each other, which sometimes occurs directly from parasite to parasite or is driven by the infected host cell—or components derived from it—as an intermediary. By emitting signals that can be dispersed within the host, parasites can also have wide-ranging effects on the course of an infection and its pathology. Intercellular communication mechanisms may rely on direct cell–cell contact or extracellular vesicles (EVs) for the transfer of secreted molecules [142]. Exosomes represent the smallest type of EV and may contain lipids, proteins, mRNAs and microRNAs [143]. Exosomes from Leishmania spp. contain chaperones (e.g., Hsp70), biogenesis (e.g., clathrin) and cytoskeletal proteins (e.g., actin, tubulin), toxins, virulence factors (e.g., GP63) and RNAs [144]. Secreted exosomes can be incorporated during parasite cell interactions, further inducing differentiation, changes in infectivity, etc. Therefore, EVs constitute a system of signal transference among cells [145].
In T. brucei, exosomes influence social motility by inhibiting parasitic growth under stressful conditions, thus leading to stress signal secretion for contiguous parasites [146]. It was demonstrated that purified exosomes derived from drug-resistant L. infantum strains (resistant to antimony, miltefosine or amphotericin B) differed in the content composition, size, distribution and morphology. These mechanisms might be shared within the parasitic population, possibly resulting in increased survival and resistance to other stressful conditions [147]. Moreover, the endosymbiont Leishmania RNA virus (LRV), which has been related to worsening disease prognosis [148], exploits the exosome pathway to transmit the viral particle from one parasite to another [15].
There are mechanisms that enable a population of individual cells to coordinate global behavior and act as a multicellular unit, which is a phenomenon known as quorum sensing (QS). Microorganisms may coexist in narrow associations, where they interact and communicate with each other to better adapt to the environment and coordinate each other’s functions within their respective niches. Intercellular communication may occur by genetic or biochemical transfer that may be mediated by vesicles [149]. QS in trypanosomatids has been studied among inter- and intraspecies. T. congolense was able to promote differentiation to the stumpy forms of T. brucei in vitro, while in vivo coinfection accelerated the stumpy form of T. brucei differentiation, resulting in lower parasitemia. This effect was lost when the QS pathway was compromised by the silencing of TbHYP2. TbHYP2 was previously identified as part of the T. brucei QS pathway [17,150].
QS in T. brucei is associated with different factors, such as small secreted molecules, stump induction factor (SIF), flagellar motility and some specific genes. SIF and flagellar signaling are associated with the cAMP cascade in different forms directly associated with social motility, thus influencing parasitemia. cAMP is produced by receptors of adenylate cyclase (AC), and at the parasitemia peak, the levels of cAMP increase approximately three times. In contrast, cAMP decreases significantly during the transition to the stumpy form [150,151,152]. Flagellar phosphodiesterase PDEB1 is related to restricted and local cAMP production by AC [153]. Recently, it was demonstrated that PDEB1 is necessary for in vitro signals for social motility. Parasites lacking PDEB1 displayed increased levels of cAMP in the flagellum and cell, and they could not produce localized cAMP and respond to the signals associated with peritrophic matrix crossing, which would result in impaired vector colonization.
Another signaling mechanism was associated with the atypical kinase DYRK, which has been identified and described as an important component of the QS cascade in T. brucei and perhaps in trypanosomatids since Leishmania spp. orthologs were also identified [154]. The DYRK family in Leishmania spp. consists of eight members. DYRK1 was implicated in stationary-phase survival and infectivity and localized in the flagellar pocket area (strongly associated with QS in trypanosomatids). Knockout of DYRK1 in L. infantum led to an increased proliferation rate in the logarithmic phase compared to the wild type, and the overexpression of this gene resulted in decreased proliferation. During the stationary phase, knockout was morphologically and biochemically distinct from that of the wild type, exhibiting a rounded shape and a cytoplasm with intense vacuolization, lipid body accumulation and a switch in the ratio of saturated/polyunsaturated lipids. Finally, DYRK1 knockout influenced metacyclogenesis and dramatically reduced the performance in in vitro infection [155].
Noncoding RNA is a group of ribonucleic acid molecules comprising small nuclear RNA, small interfering RNA, long noncoding RNA and microRNA [156]. MicroRNA may be transferred by exosomes influencing host cells [157]. Bacterial noncoding RNAs are classified as small RNAs, and their role in QS has recently been suggested, especially for bacterial survival in harsh environments [158]. Noncoding RNA are among a small group of Trypanosoma brucei genes showing transiently increased transcript levels across the slender to stumpy transition point [159], but so far, researchers have not demonstrated the role of noncoding RNA in QS in any trypanosomatid.
The majority of microRNA studies in Leishmania spp. are focused on host interactions and the immune response. Many studies have demonstrated that infection by Leishmania spp. influences the microRNA profile in the host (including macrophages and dendritic cells from humans, dogs and mice) in association with virulence factors [160]. The inhibition of some microRNAs reduced L. braziliensis growth earlier after in vitro macrophage infection [161]. The role of microRNAs in cross-Leishmania species (or strains or genotypes) communication is an open avenue to be explored, which might contribute to a better understanding of many biological processes occurring in the dynamic interaction among Leishmania parasites during vertebrate and invertebrate infections.
Intercellular communication includes the interplay of features according to a very intricate orchestra. Many factors may interact, resulting in a beneficial or negative relationship.
7. Conclusions
The change in Leishmania spp. fitness and behavior might be a result of the interplay among diverse factors. The parasitic genetic background is a source of phenotypic variability, which might be selected as an environmental change and challenge response. Host immunity may represent an important source of these challenges. The possibility of interactions within inter/intraspecies, genetic variability and intercellular communication might provide sources for enrichment in parasitic plasticity. In addition, amplified genetic polymorphisms, hybrid generation and phenotypic adaptations (e.g., behavior, fitness) may arise. Moreover, the complex balance of these multiple factors and features could lead to diverse disease outcomes. Multiple infections in either invertebrate or vertebrate hosts may correspond to diverse prognoses. Finally, deeper studies and a better understanding of these interactions are mandatory. In association with molecular tools, they may afford valuable methods of improving disease prognosis and drive better treatment designs.
Author Contributions
Conceptualization: B.D.d.C., T.M.P. and E.C.; B.D.d.C., T.M.P., L.M.C., G.P.d.S., M.C.B., L.d.O.R.P. and E.C. contributed writing, reviewing and editing this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Elisa Cupolillo: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001; CNPq (Research Fellow, 302622/2017-9), FAPERJ (CNE, E26-202.569/2019; Temáticos, E26-210.038/2020).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Strelkova, M.V.; Eliseev, L.N.; Ponirovsky, E.N.; Dergacheva, T.I.; Annacharyeva, D.K.; Erokhin, P.I.; Evans, D.A. Mixed Leishmanial Infections in Rhombomys Opimus: A Key to the Persistence of Leishmania Major from One Transmission Season to the Next. Ann. Trop. Med. Parasitol. 2001, 95, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Chajbullinova, A.; Votypka, J.; Sadlova, J.; Kvapilova, K.; Seblova, V.; Kreisinger, J.; Jirku, M.; Sanjoba, C.; Gantuya, S.; Matsumoto, Y.; et al. The Development of Leishmania turanica in Sand Flies and Competition with L. major. Parasites Vectors 2012, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.M.; Flath, B.; Presber, W. Mixed Infection of Human U-937 Cells by Two Different Species of Leishmania. Am. J. Trop. Med. Hyg. 1998, 59, 182–188. [Google Scholar] [CrossRef] [PubMed]
- DE Lima Celeste, J.L.; Venuto Moura, A.P.; França-Silva, J.C.; Matos DE Sousa, G.; Oliveira Silva, S.; Norma Melo, M.; Luiz Tafuri, W.; Carvalho Souza, C.; Monteiro DE Andrade, H. Experimental Mixed Infection of Leishmania (Leishmania) Amazonensis and Leishmania (L.) Infantum in Hamsters (Mesocricetus auratus). Parasitology 2017, 144, 1191–1202. [Google Scholar] [CrossRef]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Daneshbod, Y. Mixed Mucosal Leishmaniasis Infection Caused by Leishmania Tropica and Leishmania Major. J. Clin. Microbiol. 2012, 50, 3805–3808. [Google Scholar] [CrossRef]
- Antoniou, M.; Doulgerakis, C.; Pratlong, F.; Dedet, J.P.; Tselentis, Y. Short Report: Treatment Failure Due to Mixed Infection by Different Strains of the Parasite Leishmania Infantum. Am. J. Trop. Med. Hyg. 2004, 71, 71–72. [Google Scholar] [CrossRef]
- Gibson, W. The Sexual Side of Parasitic Protists. Mol. Biochem. Parasitol. 2021, 243, 111371. [Google Scholar] [CrossRef]
- Grünebast, J.; Clos, J. Leishmania: Responding to Environmental Signals and Challenges without Regulated Transcription. Comput. Struct. Biotechnol. J. 2020, 18, 4016–4023. [Google Scholar] [CrossRef]
- Zuñiga, C.; Zaramela, L.; Zengler, K. Elucidation of Complexity and Prediction of Interactions in Microbial Communities. Microb. Biotechnol. 2017, 10, 1500–1522. [Google Scholar] [CrossRef]
- Pacheco, R.S.; Grimaldi Júnior, G.; Morel, C.M. Inhibition of Growth of Leishmania Mexicana Mexicana by Leishmania Mexicana Amazonensis during “in Vitro” Co-Cultivation. Mem. Inst. Oswaldo Cruz. 1987, 82, 537–542. [Google Scholar] [CrossRef][Green Version]
- Agnew, P.; Holzmuller, P.; Michalakis, Y.; Sereno, D.; Lemesre, J.L.; Renaud, F. In Vitro Growth of Leishmania Amazonensis Promastigotes Resistant to Pentamidine Is Dependent on Interactions among Strains. Antimicrob. Agents Chemother. 2001, 45, 1928–1929. [Google Scholar] [CrossRef] [PubMed]
- Coppens, I.; Ter Kuile, B.H.; Opperdoes, F.R. Impairment of Growth of Leishmania Donovani by Trypanosoma Brucei during Co-Culture. Parasitology 1992, 105 Pt 3, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Purschke, F.G.; Hiller, E.; Trick, I.; Rupp, S. Flexible Survival Strategies of Pseudomonas Aeruginosa in Biofilms Result in Increased Fitness Compared with Candida Albicans. Mol. Cell Proteom. 2012, 11, 1652–1669. [Google Scholar] [CrossRef]
- Cuervo, P.; De Jesus, J.B.; Saboia-Vahia, L.; Mendonça-Lima, L.; Domont, G.B.; Cupolillo, E. Proteomic Characterization of the Released/Secreted Proteins of Leishmania (Viannia) Braziliensis Promastigotes. J. Proteom. 2009, 73, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Atayde, V.D.; Aslan, H.; Townsend, S.; Hassani, K.; Kamhawi, S.; Olivier, M. Exosome Secretion by the Parasitic Protozoan Leishmania within the Sand Fly Midgut. Cell Rep. 2015, 13, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Szempruch, A.J.; Dennison, L.; Kieft, R.; Harrington, J.M.; Hajduk, S.L. Sending a Message: Extracellular Vesicles of Pathogenic Protozoan Parasites. Nat. Rev. Microbiol. 2016, 14, 669–675. [Google Scholar] [CrossRef]
- Silvester, E.; Young, J.; Ivens, A.; Matthews, K.R. Interspecies Quorum-Sensing in Co-Infections Can Manipulate Trypanosome Transmission Potential. Nat. Microbiol. 2017, 2, 1471–1479. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; Shaw, J.J.; Ribeiro, R.d.S. Cutaneous leishmaniasis in Amazonia. Report of the 1st human case of mixed infection, determined by 2 different Leishmania species: Leishmania brasiliensis and Leishmania mexicana amazonensis. Rev. Inst. Med. Trop. Sao Paulo 1984, 26, 272–275. [Google Scholar] [CrossRef]
- Oliveira Neto, M.P.; Marzochi, M.C.; Grimaldi Júnior, G.; Pacheco, R.S.; Toledo, L.M.; Momen, H. Concurrent Human Infection with Leishmania Donovani and Leishmania Braziliensis Braziliensis. Ann. Trop. Med. Parasitol. 1986, 80, 587–592. [Google Scholar] [CrossRef]
- Mebrahtu, Y.B.; Lawyer, P.G.; Hendricks, L.D.; Muigai, R.; Oster, C.N.; Perkins, P.V.; Koech, D.K.; Pamba, H.; Roberts, C.R. Concurrent Infection with Leishmania Donovani and Leishmania Major in a Kenyan Patient: Clinical Description and Parasite Characterization. Am. J. Trop. Med. Hyg. 1991, 45, 290–296. [Google Scholar] [CrossRef]
- Al-Diwany, L.J.; Al-Awkati, N.A.; Atia, M.; Rassam, M.B. Concomitant Natural Infection with L. donovani and L. major: A Case Report from Iraq. Soz. Praventivmed. 1995, 40, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Mollinedo, S.; Torrez, M.; Muñoz, M.; Bañuls, A.L.; Le Pont, F. Co-Infection by Leishmania Amazonensis and L. infantum/L. chagasi in a Case of Diffuse Cutaneous Leishmaniasis in Bolivia. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 529–532. [Google Scholar] [CrossRef]
- Bastrenta, B.; Mita, N.; Buitrago, R.; Vargas, F.; Flores, M.; Machane, M.; Yacsik, N.; Torrez, M.; Le Pont, F.; Brenière, F. Human Mixed Infections of Leishmania spp. and Leishmania-Trypanosoma Cruzi in a Sub Andean Bolivian Area: Identification by Polymerase Chain Reaction/Hybridization and Isoenzyme. Mem. Inst. Oswaldo Cruz 2003, 98, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Porrozzi, R.; Teva, A.; Amaral, V.F.; Santos da Costa, M.V.; Grimaldi, G. Cross-Immunity Experiments between Different Species or Strains of Leishmania in Rhesus Macaques (Macaca Mulatta). Am. J. Trop. Med. Hyg. 2004, 71, 297–305. [Google Scholar] [CrossRef][Green Version]
- Madeira, M.F.; Schubach, A.; Schubach, T.M.P.; Pacheco, R.S.; Oliveira, F.S.; Pereira, S.A.; Figueiredo, F.B.; Baptista, C.; Marzochi, M.C.A. Mixed Infection with Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi in a Naturally Infected Dog from Rio de Janeiro, Brazil. Trans. R.Soc. Trop. Med. Hyg. 2006, 100, 442–445. [Google Scholar] [CrossRef]
- Mahmoudzadeh-Niknam, H.; Kiaei, S.S.; Iravani, D. Leishmania Tropica Infection, in Comparison to Leishmania Major, Induces Lower Delayed Type Hypersensitivity in BALB/c Mice. Korean J. Parasitol. 2007, 45, 103–109. [Google Scholar] [CrossRef][Green Version]
- Akopyants, N.S.; Kimblin, N.; Secundino, N.; Patrick, R.; Peters, N.; Lawyer, P.; Dobson, D.E.; Beverley, S.M.; Sacks, D.L. Demonstration of Genetic Exchange during Cyclical Development of Leishmania in the Sand Fly Vector. Science 2009, 324, 265–268. [Google Scholar] [CrossRef]
- Real, F.; Mortara, R.A.; Rabinovitch, M. Fusion between Leishmania Amazonensis and Leishmania Major Parasitophorous Vacuoles: Live Imaging of Coinfected Macrophages. PLoS Negl. Trop. Dis. 2010, 4, e905. [Google Scholar] [CrossRef]
- Sadlova, J.; Yeo, M.; Seblova, V.; Lewis, M.D.; Mauricio, I.; Volf, P.; Miles, M.A. Visualisation of Leishmania Donovani Fluorescent Hybrids during Early Stage Development in the Sand Fly Vector. PLoS ONE 2011, 6, e19851. [Google Scholar] [CrossRef]
- Santos-Oliveira, J.R.; Da-Cruz, A.M.; Pires, L.H.S.; Cupolillo, E.; Kuhls, K.; Giacoia-Gripp, C.B.W.; Oliveira-Neto, M.P. Atypical Lesions as a Sign of Cutaneous Dissemination of Visceral Leishmaniasis in a Human Immunodeficiency Virus-Positive Patient Simultaneously Infected by Two Viscerotropic Leishmania Species. Am. J. Trop. Med. Hyg. 2011, 85, 55–59. [Google Scholar] [CrossRef]
- Inbar, E.; Akopyants, N.S.; Charmoy, M.; Romano, A.; Lawyer, P.; Elnaiem, D.-E.A.; Kauffmann, F.; Barhoumi, M.; Grigg, M.; Owens, K.; et al. The Mating Competence of Geographically Diverse Leishmania Major Strains in Their Natural and Unnatural Sand Fly Vectors. PLoS Genet. 2013, 9, e1003672. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.R.; Silva, S.O.; Moreira, F.M.; Prado, L.G.; Fantini, P.; Maranhão, R.d.P.A.; da Silva Filho, J.M.; Melo, M.N.; Palhares, M.S. First Evidence of Autochthonous Cases of Leishmania (Leishmania) Infantum in Horse (Equus Caballus) in the Americas and Mixed Infection of Leishmania Infantum and Leishmania (Viannia) Braziliensis. Vet. Parasitol. 2013, 197, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Veland, N.; Valencia, B.M.; Alba, M.; Adaui, V.; Llanos-Cuentas, A.; Arevalo, J.; Boggild, A.K. Simultaneous Infection with Leishmania (Viannia) Braziliensis and L. (V.) Lainsoni in a Peruvian Patient with Cutaneous Leishmaniasis. Am. J. Trop. Med. Hyg. 2013, 88, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.M.; Ravagnan, S.; Fusaro, A.; Hassan, M.M.; Bakheit, S.M.; Mukhtar, M.M.; Cattoli, G.; Capelli, G. Concomitant Infection with Leishmania Donovani and L. Major in Single Ulcers of Cutaneous Leishmaniasis Patients from Sudan. J. Trop. Med. 2014, 2014, 170859. [Google Scholar] [CrossRef]
- Calvo-Álvarez, E.; Álvarez-Velilla, R.; Jiménez, M.; Molina, R.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. First Evidence of Intraclonal Genetic Exchange in Trypanosomatids Using Two Leishmania Infantum Fluorescent Transgenic Clones. PLoS Negl. Trop. Dis. 2014, 8, e3075. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.Q.; Madeira, M.d.F.; Bittencourt, V.R.E.P.; Pacheco, R.d.S. Cutaneous and Visceral Leishmaniasis Co-Infection in Dogs from Rio de Janeiro, Brazil: Evaluation by Specific PCR and RFLP-PCR Assays. Rev. Soc. Bras. Med. Trop. 2014, 47, 243–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Romano, A.; Inbar, E.; Debrabant, A.; Charmoy, M.; Lawyer, P.; Ribeiro-Gomes, F.; Barhoumi, M.; Grigg, M.; Shaik, J.; Dobson, D.; et al. Cross-Species Genetic Exchange between Visceral and Cutaneous Strains of Leishmania in the Sand Fly Vector. Proc. Natl. Acad. Sci. USA 2014, 111, 16808–16813. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.d.C.; Cruz, I.; Cañavate, C.; de Melo, L.A.; Pereira, A.A.S.; Madeira, F.A.M.; Valério, S.A.N.; Cunha, H.M.; Paglia, A.P.; Gontijo, C.M.F. Mixed Infection of Leishmania Infantum and Leishmania Braziliensis in Rodents from Endemic Urban Area of the New World. BMC Vet. Res. 2015, 11, 71. [Google Scholar] [CrossRef][Green Version]
- Badirzadeh, A.; Mohebali, M.; Sabzevari, S.; Ghafoori, M.; Arzamani, K.; Seyyedin, M.; Hashemi, S.A. Case Report: First Coinfection Report of Mixed Leishmania Infantum/Leishmania Major and Human Immunodeficiency Virus-Acquired Immune Deficiency Syndrome: Report of a Case of Disseminated Cutaneous Leishmaniasis in Iran. Am. J. Trop. Med. Hyg. 2018, 98, 122–125. [Google Scholar] [CrossRef]
- Gosch, C.S.; Resende, B.S.; Amorim, C.B.; Marques, C.P.; Pereira, L.I.d.A.; Pinto, S.A.; Uliana, S.R.B.; Coelho, A.C.; Ribeiro-Dias, F.; Dorta, M.L. Case Report: Atypical Cutaneous Leishmaniasis in a Patient with Mixed Leishmania Guyanensis and Leishmania Amazonensis Infection. Am. J. Trop. Med. Hyg. 2018, 99, 1165–1169. [Google Scholar] [CrossRef]
- Villagrán Herrera, M.E.; Valdez, F.C.; Moreno, M.S.; Martínez Ibarra, J.A.; Cabrera, J.A.D.D. Coinfection of and Leishmania Spp. in Synanthropic Reservoirs (Canis Familiaris)in an Endemic Area of The State of Querétaro, Use of FeSODe as an Antigenic Tool. J. Prev. Med. 2018, 3, 10. [Google Scholar] [CrossRef]
- Alves Souza, N.; Souza Leite, R.; de Oliveira Silva, S.; Groenner Penna, M.; Figueiredo Felicori Vilela, L.; Melo, M.N.; de Andrade, A.S.R. Detection of Mixed Leishmania Infections in Dogs from an Endemic Area in Southeastern Brazil. Acta Trop 2019, 193, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Inbar, E.; Shaik, J.; Iantorno, S.A.; Romano, A.; Nzelu, C.O.; Owens, K.; Sanders, M.J.; Dobson, D.; Cotton, J.A.; Grigg, M.E.; et al. Whole Genome Sequencing of Experimental Hybrids Supports Meiosis-like Sexual Recombination in Leishmania. PLoS Genet. 2019, 15, e1008042. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Sadlova, J.; Lestinova, T.; Vojtkova, B.; Jancarova, M.; Podesvova, L.; Yurchenko, V.; Dantas-Torres, F.; Brandão-Filho, S.P.; Volf, P. Experimental Infections and Co-Infections with Leishmania Braziliensis and Leishmania Infantum in Two Sand Fly Species, Lutzomyia Migonei and Lutzomyia Longipalpis. Sci. Rep. 2020, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Cupolillo, E.; Cavalcanti, A.S.; Ferreira, G.E.M.; Boité, M.C.; Morgado, F.N.; Porrozzi, R. Occurrence of Multiple Genotype Infection Caused by Leishmania Infantum in Naturally Infected Dogs. PLoS Negl. Trop. Dis. 2020, 14, e0007986. [Google Scholar] [CrossRef]
- Telittchenko, R.; Descoteaux, A. Study on the Occurrence of Genetic Exchange Among Parasites of the Leishmania Mexicana Complex. Front. Cell. Infect. Microbiol. 2020, 10, 607253. [Google Scholar] [CrossRef]
- Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 12 September 2022).
- de Almeida, J.V.; de Souza, C.F.; Fuzari, A.A.; Joya, C.A.; Valdivia, H.O.; Bartholomeu, D.C.; Brazil, R.P. Diagnosis and Identification of Leishmania Species in Patients with Cutaneous Leishmaniasis in the State of Roraima, Brazil’s Amazon Region. Parasit. Vectors 2021, 14, 32. [Google Scholar] [CrossRef]
- Leishmania General Information. Available online: https://leishmania.ird.fr/# (accessed on 12 September 2022).
- Laveran, A.; Mesnil, F. Sur Un Protzaire Nouveau (Piroplasma Donovani Lav. et Mesn.). Parasite d’une Fievre de l’Inde. C.R. Acad. Sci. 1903, 137, 957–961. [Google Scholar]
- Wright, J.H. Protozoa in a Case of Tropical Ulcer (“Delhi Sore”). J. Med. Res. 1903, 10, 472–482.7. [Google Scholar]
- Nicolle, C. Sur Trois Cas d’infection Splénique Infantile à Corps de Leishman Observés En Tunisie. Arch. Inst. Pasteur. Tunis. 1908, 3, 1–26. [Google Scholar]
- Yakimoff, W.L.; Schokhor, N.I. Recherches Sur Les Maladies Tropicales Humaines et Animales Au Turkestan. II. La Leishmaniose Cutanée (Bouton d’Orient) Spontanée Du Chien Turkestan. Bull. Soc. Pathol. Exot. 1914, 7, 186–187. [Google Scholar]
- Castellani, A.; Chalmers, A.J. Manual of Tropical Medicine, 3rd ed.; Baillière, Tindall and Cox: London, UK, 1919; pp. 1–2510. [Google Scholar]
- Biagi, F. Some comments on leishmaniasis and its agents: Leishmania tropica mexicana, new subspecies. Medicina (Mex) 1953, 33, 401–406. [Google Scholar] [PubMed]
- Wang, J.; Qu, J.; Guan, L. A Study of Leishmania Parasite of Big Gerbil in Northwest China. Acta Parasitol. Sin. 1964, 1, 105–117. [Google Scholar]
- Lainson, R.; Shaw, J.J. Leishmaniasis of the New World: Taxonomic Problems. Br. Med. Bull. 1972, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Bray, R.S.; Ashford, R.W.; Bray, M.A. The Parasite Causing Cutaneous Leishmaniasis in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1973, 67, 345–348. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J. The Role of Animals in the Epidemiology of South American Leishmaniasis. In Biology of the Kinetoplastida; Lumsden, W.H.R., Evans, D.A., Eds.; Academic Press: London, UK, 1979; pp. 1–116. [Google Scholar]
- Bonfante-Garrido, R. New Subspecies of Leishmania Isolated in Venezuela. In Proceedings of the X International Congress on Tropical Medicine and Malaria, Manila, Philippines, 9–15 November 1980. [Google Scholar]
- Rioux, J.; Lanotte, G.; Pratlong, F. Leishmania Killicki n. Sp. (Kinetoplastida-Trypanosomatidae). In Leishmania: Taxonomie et Phylogénèse: Applications Éco-Épidémiologiques; IMEEE: Montpellier, France, 1986; pp. 139–142. [Google Scholar]
- Peters, W.; Elbihari, S.; Evans, D.A. Leishmania Infecting Man and Wild Animals in Saudi Arabia. 2. Leishmania Arabica n. Sp. Trans. R Soc. Trop. Med. Hyg. 1986, 80, 497–502. [Google Scholar] [CrossRef]
- Strelkova, M.V.; Shurkhal, A.V.; Kellina, O.I.; Eliseev, L.N.; Evans, D.A.; Peters, W.; Chapman, C.J.; Le Blancq, S.M.; van Eys, G.J. A New Species of Leishmania Isolated from the Great Gerbil Rhombomys Opimus. Parasitology 1990, 101 Pt 3, 327–335. [Google Scholar] [CrossRef]
- Yoshida, E.L.A.; Cuba, C.A.C.; Pacheco, R.d.S.; Cupolillo, E.; Tavares, C.C.; Machado, G.M.C.; Momen, H.; Grimaldi Junior, G. Description of Leishmania (Leishmania) Forattinii Sp. n., a New Parasite Infecting Opossums and Rodents in Brazil. Mem. Inst. Oswaldo Cruz 1993, 88, 397–406. [Google Scholar] [CrossRef]
- Shaw, J.; Pratlong, F.; Floeter-Winter, L.; Ishikawa, E.; El Baidouri, F.; Ravel, C.; Dedet, J.-P. Characterization of Leishmania (Leishmania) Waltoni n.Sp. (Kinetoplastida: Trypanosomatidae), the Parasite Responsible for Diffuse Cutaneous Leishmaniasis in the Dominican Republic. Am. J. Trop. Med. Hyg. 2015, 93, 552–558. [Google Scholar] [CrossRef]
- Vianna, G. Sobre Uma Nova Especie de Leishmania (Nota Preliminar). Brasil-Medico 1911, 25, 411. [Google Scholar]
- Velez, L. La Uta Es Producida Por La Leishmania Peruviana. La Crónica Médica de Lima 1913, 463. [Google Scholar]
- Floch, H. Leishmania tropica guyanensis n. ssp., cause of cutaneous leishmaniasis in the Guinanas and Central America. Publ. Inst. Pasteur. Guyane Fr. Inini 1954, 15, 1–4. [Google Scholar]
- Silveira, F.T.; Shaw, J.J.; Braga, R.R.; Ishikawa, E. Dermal Leishmaniasis in the Amazon Region of Brazil: Leishmania (Viannaia) Lainsoni Sp.n., a New Parasite from the State of Pará. Mem. Inst. Oswaldo Cruz 1987, 82, 289–291. [Google Scholar] [CrossRef]
- Lainson, R.; Braga, R.R.; De Souza, A.A.; Pôvoa, M.M.; Ishikawa, E.A.; Silveira, F.T. Leishmania (Viannia) Shawi Sp. n., a Parasite of Monkeys, Sloths and Procyonids in Amazonian Brazil. Ann. Parasitol. Hum. Comp. 1989, 64, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.J. Leishmania (Viannia) Naiffi Sp. n., a Parasite of the Armadillo, Dasypus novemcinctus (L.) in Amazonian Brazil. Ann. Parasitol. Hum. Comp. 1989, 64, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.T.; Ishikawa, E.A.Y.; De Souza, A.A.A.; Lainson, R. An Outbreak of Cutaneous Leishmaniasis among Soldiers in Belém, Pará State, Brazil, Caused by Leishmania (Viannia) Lindenbergi n. Sp. A New Leishmanial Parasite of Man in the Amazon Region. Parasite 2002, 9, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Lainson, R.; Ishikawa, E.a.Y.; Shaw, J.J. Leishmania (Viannia) Utingensis n. Sp., a Parasite from the Sandfly Lutzomyia (Viannamyia) Tuberculata in Amazonian Brazil. Parasite 2003, 10, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Wenyon, D.M. Observations on the Intestinal Protozoa of Three Egyptian Lizards, with a Note on a Cell-Invading Fungus. Parasitology 1921, 12, 133–140. [Google Scholar] [CrossRef][Green Version]
- Mackie, F.P.; Das Gupta, B.M.; Swaminath, C.S. Progress Report on Kala-Azar. Indian J. Med. Res. 1923, 11, 591. [Google Scholar]
- Adler, S.; Theodor, O. Observations on Leishmania Ceramodactyli. N.SP. Trans. R. Soc. Trop. Med. Hyg. 1929, 22, 343–356. [Google Scholar] [CrossRef]
- Khodukin, N.T.; Sofiev, M.S. Leishmania of Some Lizards of Central Asia and Their Epidemiological Significance. Probl. Subtrop. Pathol. 1940, 4, 218–228. [Google Scholar]
- Killick-Kendrick, R.; Lainson, R.; Rioux, J.; Sarjanova, V.M. The Taxonomy of Leishmania-like Parasites of Reptiles. In Taxonomie et Phylogenèse. Applications Éco-Épidémiologiques; IMEEE: Montpellier, France, 1986; pp. 143–148. [Google Scholar]
- Heisch, R.B. On Leishmania Adleri Sp. Nov. from Lacertid Lizards (Latastia Sp.) in Kenya. Ann. Trop. Med. Parasitol. 1958, 52, 68–71. [Google Scholar] [CrossRef] [PubMed]
- McMillan, B. Leishmaniasis in the Sudan Republic. 22. Leishmania Hoogstraali Sp. n. in the Gecko. J. Parasitol. 1965, 51, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Ranque, P. Etude Morphologique et Biologique de Quelques Trypanosomidés Récoltés Au Senegal. Ph.D. Thesis, Aix-Marseille, Marseille, France, 1973. [Google Scholar]
- Ovezmukhammedov, A.; Saf’janova, V.M. A New Species of Leishmania from Agama Caucásica in Turkmenia. Izv. Akad. Nauk. Turkm. SSR Biol. Nauk. 1987, 3, 21–27. [Google Scholar]
- Muniz, J.; Medina, H. Cutaneous leishmaniasis of the guinea pig, Leishmania enriettii n. sp. Hospital (Rio. J.) 1948, 33, 7–25. [Google Scholar]
- Desbois, N.; Pratlong, F.; Quist, D.; Dedet, J.-P. Leishmania (Leishmania) Martiniquensis n. Sp. (Kinetoplastida: Trypanosomatidae), Description of the Parasite Responsible for Cutaneous Leishmaniasis in Martinique Island (French West Indies). Parasite 2014, 21, 12. [Google Scholar] [CrossRef]
- Barratt, J.; Kaufer, A.; Peters, B.; Craig, D.; Lawrence, A.; Roberts, T.; Lee, R.; McAuliffe, G.; Stark, D.; Ellis, J. Isolation of Novel Trypanosomatid, Zelonia Australiensis Sp. Nov. (Kinetoplastida: Trypanosomatidae) Provides Support for a Gondwanan Origin of Dixenous Parasitism in the Leishmaniinae. PLoS Negl. Trop. Dis. 2017, 11, e0005215. [Google Scholar] [CrossRef]
- Jariyapan, N.; Daroontum, T.; Jaiwong, K.; Chanmol, W.; Intakhan, N.; Sor-Suwan, S.; Siriyasatien, P.; Somboon, P.; Bates, M.D.; Bates, P.A. Leishmania (Mundinia) Orientalis n. Sp. (Trypanosomatidae), a Parasite from Thailand Responsible for Localised Cutaneous Leishmaniasis. Parasit. Vectors 2018, 11, 351. [Google Scholar] [CrossRef]
- Herrer, A. Leishmania Hertigi Sp. n., from the Tropical Porcupine, Coendou Rothschildi Thomas. J. Parasitol. 1971, 57, 626–629. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J.J. Leishmanias of Neotropical Porcupines: Leishmania Hertigi Deanei Nov. Subsp. Acta Amaz. 1977, 7, 51–57. [Google Scholar] [CrossRef]
- Rossi, M.; Fasel, N. How to Master the Host Immune System? Leishmania Parasites Have the Solutions! Int. Immunol. 2018, 30, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Naiff, R.D.; Freitas, R.A.; Naiff, M.F.; Arias, J.R.; Barrett, T.V.; Momen, H.; Grimaldi Júnior, G. Epidemiological and Nosological Aspects of Leishmania Naiffi Lainson & Shaw, 1989. Mem. Inst. Oswaldo Cruz 1991, 86, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Lainson, R.; Shaw, J.J.; Silveira, F.T.; Braga, R.R.; Ishikawa, E.A. Cutaneous Leishmaniasis of Man Due to Leishmania (Viannia) Naiffi Lainson and Shaw, 1989. Ann. Parasitol. Hum. Comp. 1990, 65, 282–284. [Google Scholar]
- Guerra, J.A.d.O.; Prestes, S.R.; Silveira, H.; Coelho, L.I. de A.R.C.; Gama, P.; Moura, A.; Amato, V.; Barbosa, M. das G.V.; Ferreira, L.C. de L. Mucosal Leishmaniasis Caused by Leishmania (Viannia) Braziliensis and Leishmania (Viannia) Guyanensis in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2011, 5, e980. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.H.; Machado, P.R.L.; Lago, E.L.; Morgan, D.J.; Schriefer, A.; Bacellar, O.; Carvalho, E.M. Atypical Manifestations of Tegumentary Leishmaniasis in a Transmission Area of Leishmania Braziliensis in the State of Bahia, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 712–715. [Google Scholar] [CrossRef]
- Rugani, J.N.; Quaresma, P.F.; Gontijo, C.F.; Soares, R.P.; Monte-Neto, R.L. Intraspecies Susceptibility of Leishmania (Viannia) Braziliensis to Antileishmanial Drugs: Antimony Resistance in Human Isolates from Atypical Lesions. Biomed. Pharm. 2018, 108, 1170–1180. [Google Scholar] [CrossRef]
- Quaresma, P.F.; de Brito, C.F.A.; Rugani, J.M.N.; Freire, J.d.M.; Baptista, R.d.P.; Moreno, E.C.; Gontijo, R.C.; Rego, F.D.; Diniz, J.E.; Melo, M.N.; et al. Distinct Genetic Profiles of Leishmania (Viannia) Braziliensis Associate with Clinical Variations in Cutaneous-Leishmaniasis Patients from an Endemic Area in Brazil. Parasitology 2018, 145, 1161–1169. [Google Scholar] [CrossRef]
- Lira, R.; Méndez, S.; Carrera, L.; Jaffe, C.; Neva, F.; Sacks, D. Leishmania Tropica: The Identification and Purification of Metacyclic Promastigotes and Use in Establishing Mouse and Hamster Models of Cutaneous and Visceral Disease. Exp. Parasitol. 1998, 89, 331–342. [Google Scholar] [CrossRef]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Daneshbod, Y. Three Leishmania/L. Species--L. Infantum, L. Major, L. Tropica—As Causative Agents of Mucosal Leishmaniasis in Iran. Pathog. Glob. Health. 2013, 107, 267–272. [Google Scholar] [CrossRef]
- Özbilgin, A.; Çulha, G.; Uzun, S.; Harman, M.; Topal, S.G.; Okudan, F.; Zeyrek, F.; Gündüz, C.; Östan, İ.; Karakuş, M.; et al. Leishmaniasis in Turkey: First Clinical Isolation of Leishmania Major from 18 Autochthonous Cases of Cutaneous Leishmaniasis in Four Geographical Regions. Trop. Med. Int. Health. 2016, 21, 783–791. [Google Scholar] [CrossRef]
- Srivastava, P.; Prajapati, V.K.; Vanaerschot, M.; Van der Auwera, G.; Dujardin, J.C.; Sundar, S. Detection of Leptomonas Sp. Parasites in Clinical Isolates of Kala-Azar Patients from India. Infect. Genet. Evol. 2010, 10, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.R.; de Santana, A.K.M.; Takamiya, N.T.; Takahashi, T.Y.; Rogerio, L.A.; Oliveira, C.A.B.; Milanezi, C.M.; Trombela, V.A.; Cruz, A.K.; Jesus, A.R.; et al. Non-Leishmania Parasite in Fatal Visceral Leishmaniasis-Like Disease, Brazil. Emerg. Infect. Dis. 2019, 25, 2088–2092. [Google Scholar] [CrossRef]
- Domagalska, M.A.; Dujardin, J.-C. Non-Leishmania Parasite in Fatal Visceral Leishmaniasis-like Disease, Brazil. Emerg. Infect. Dis. 2020, 26, 388. [Google Scholar] [CrossRef] [PubMed]
- Porfirio, G.E.d.O.; Santos, F.M.; de Macedo, G.C.; Barreto, W.T.G.; Campos, J.B.V.; Meyers, A.C.; André, M.R.; Perles, L.; de Oliveira, C.E.; Xavier, S.C.d.C.; et al. Maintenance of Trypanosoma Cruzi, T. Evansi and Leishmania Spp. by Domestic Dogs and Wild Mammals in a Rural Settlement in Brazil-Bolivian Border. Int. J. Parasitol. Parasites Wildl. 2018, 7, 398–404. [Google Scholar] [CrossRef] [PubMed]
- West, S.A.; Griffin, A.S.; Gardner, A. Evolutionary Explanations for Cooperation. Curr. Biol. 2007, 17, R661–R672. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A. Social Semantics: Altruism, Cooperation, Mutualism, Strong Reciprocity and Group Selection. J. Evol. Biol. 2007, 20, 415–432. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial Competition: Surviving and Thriving in the Microbial Jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef]
- Kinnula, H.; Mappes, J.; Sundberg, L.-R. Coinfection Outcome in an Opportunistic Pathogen Depends on the Inter-Strain Interactions. BMC Evol. Biol. 2017, 17, 77. [Google Scholar] [CrossRef]
- Nowak, M.A. Five Rules for the Evolution of Cooperation. Science 2006, 314, 1560–1563. [Google Scholar] [CrossRef]
- Khan, N.; Maezato, Y.; McClure, R.S.; Brislawn, C.J.; Mobberley, J.M.; Isern, N.; Chrisler, W.B.; Markillie, L.M.; Barney, B.M.; Song, H.-S.; et al. Phenotypic Responses to Interspecies Competition and Commensalism in a Naturally-Derived Microbial Co-Culture. Sci. Rep. 2018, 8, 297. [Google Scholar] [CrossRef]
- Seppälä, O.; Karvonen, A.; Valtonen, E.T.; Jokela, J. Interactions among Co-Infecting Parasite Species: A Mechanism Maintaining Genetic Variation in Parasites? Proc. Biol. Sci. 2009, 276, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.; Arora, G.; Khare, P.; Selvapandiyan, A. Selective Elimination of Leptomonas from the in Vitro Co-Culture with Leishmania. Parasitol. Int. 2015, 64, 1–5. [Google Scholar] [CrossRef]
- García-Hernández, R.; Gómez-Pérez, V.; Castanys, S.; Gamarro, F. Fitness of Leishmania Donovani Parasites Resistant to Drug Combinations. PLoS Negl. Trop. Dis. 2015, 9, e0003704. [Google Scholar] [CrossRef] [PubMed]
- Vanaerschot, M.; Dumetz, F.; Roy, S.; Ponte-Sucre, A.; Arevalo, J.; Dujardin, J.-C. Treatment Failure in Leishmaniasis: Drug-Resistance or Another (Epi-) Phenotype? Expert. Rev. Anti. Infect. Ther. 2014, 12, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.; Navarro, M.; Segovia, M. Evidence from Genotypic and Phenotypic Markers That an Attenuated Line Outgrows a Virulent One in a Mixed Population of Leishmania Major Promastigotes Cultured in Vitro. Ann. Trop. Med. Parasitol. 1995, 89, 477–484. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Oliveira, S.M.P.; Bertho, A.L.; Franco, A.M.R.; Rangel, E.F. Single and Concomitant Experimental Infections by Endotrypanum Spp. and Leishmania (Viannia) Guyanensis (Kinetoplastida: Trypanosomatidae) in the Neotropical Sand Fly Lutzomyia Longipalpis (Diptera: Psychodidae). Mem. Inst. Oswaldo Cruz 2006, 101, 851–856. [Google Scholar] [CrossRef]
- Andersson, D.I. The Biological Cost of Mutational Antibiotic Resistance: Any Practical Conclusions? Curr. Opin. Microbiol. 2006, 9, 461–465. [Google Scholar] [CrossRef]
- Veras, P.S.; Moulia, C.; Dauguet, C.; Tunis, C.T.; Thibon, M.; Rabinovitch, M. Entry and Survival of Leishmania Amazonensis Amastigotes within Phagolysosome-like Vacuoles That Shelter Coxiella Burnetii in Chinese Hamster Ovary Cells. Infect. Immun. 1995, 63, 3502–3506. [Google Scholar] [CrossRef]
- Pessoa, C.C.; Ferreira, É.R.; Bayer-Santos, E.; Rabinovitch, M.; Mortara, R.A.; Real, F. Trypanosoma Cruzi Differentiates and Multiplies within Chimeric Parasitophorous Vacuoles in Macrophages Coinfected with Leishmania Amazonensis. Infect. Immun. 2016, 84, 1603–1614. [Google Scholar] [CrossRef]
- Christodoulou, V.; Messaritakis, I.; Svirinaki, E.; Tsatsanis, C.; Antoniou, M. Leishmania Infantum and Toxoplasma Gondii: Mixed Infection of Macrophages in Vitro and in Vivo. Exp. Parasitol. 2011, 128, 279–284. [Google Scholar] [CrossRef]
- Real, F.; Mortara, R.A. The Diverse and Dynamic Nature of Leishmania Parasitophorous Vacuoles Studied by Multidimensional Imaging. PLoS Negl. Trop. Dis. 2012, 6, e1518. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, R.D.; Yemma, J.J.; Grogl, M.; Tesh, R.B.; Martin, T.I. Evidence of Sexual Reproduction in the Protozoan Parasite Leishmania (Kinetoplastida: Trypanosomatidae). Am. J. Trop. Med. Hyg. 1994, 51, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lanotte, G.; Rioux, J.A. Cell fusion in Leishmania (Kinetoplastida, Trypanosomatidae). C R Acad Sci III 1990, 310, 285–288. [Google Scholar]
- Belli, A.A.; Miles, M.A.; Kelly, J.M. A Putative Leishmania Panamensis/Leishmania Braziliensis Hybrid Is a Causative Agent of Human Cutaneous Leishmaniasis in Nicaragua. Parasitology 1994, 109 Pt 4, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.C.; Bañuls, A.L.; Llanos-Cuentas, A.; Alvarez, E.; DeDoncker, S.; Jacquet, D.; Le Ray, D.; Arevalo, J.; Tibayrenc, M. Putative Leishmania Hybrids in the Eastern Andean Valley of Huanuco, Peru. Acta Trop. 1995, 59, 293–307. [Google Scholar] [CrossRef]
- Delgado, O.; Cupolillo, E.; Bonfante-Garrido, R.; Silva, S.; Belfort, E.; Grimaldi Júnior, G.; Momen, H. Cutaneous Leishmaniasis in Venezuela Caused by Infection with a New Hybrid between Leishmania (Viannia) Braziliensis and L. (V.) Guyanensis. Mem. Inst. Oswaldo Cruz 1997, 92, 581–582. [Google Scholar] [CrossRef]
- Tojal da Silva, A.C.; Cupolillo, E.; Volpini, A.C.; Almeida, R.; Romero, G.A.S. Species Diversity Causing Human Cutaneous Leishmaniasis in Rio Branco, State of Acre, Brazil. Trop. Med. Int. Health 2006, 11, 1388–1398. [Google Scholar] [CrossRef]
- Lima, A.C.S.; Gomes, C.M.C.; Tomokane, T.Y.; Campos, M.B.; Zampieri, R.A.; Jorge, C.L.; Laurenti, M.D.; Silveira, F.T.; Corbett, C.E.P.; Floeter-Winter, L.M. Molecular Tools Confirm Natural Leishmania (Viannia) Guyanensis/L. (V.) Shawi Hybrids Causing Cutaneous Leishmaniasis in the Amazon Region of Brazil. Genet. Mol. Biol. 2021, 44, e20200123. [Google Scholar] [CrossRef]
- Evans, D.A.; Kennedy, W.P.; Elbihari, S.; Chapman, C.J.; Smith, V.; Peters, W. Hybrid Formation within the Genus Leishmania? Parassitologia 1987, 29, 165–173. [Google Scholar]
- Kelly, J.M.; Law, J.M.; Chapman, C.J.; Van Eys, G.J.; Evans, D.A. Evidence of Genetic Recombination in Leishmania. Mol. Biochem. Parasitol. 1991, 46, 253–263. [Google Scholar] [CrossRef]
- Ravel, C.; Cortes, S.; Pratlong, F.; Morio, F.; Dedet, J.-P.; Campino, L. First Report of Genetic Hybrids between Two Very Divergent Leishmania Species: Leishmania Infantum and Leishmania Major. Int. J. Parasitol. 2006, 36, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Volf, P.; Benkova, I.; Myskova, J.; Sadlova, J.; Campino, L.; Ravel, C. Increased Transmission Potential of Leishmania Major/Leishmania Infantum Hybrids. Int. J. Parasitol. 2007, 37, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Cortes, S.; Esteves, C.; Maurício, I.; Maia, C.; Cristovão, J.M.; Miles, M.; Campino, L. In Vitro and in Vivo Behaviour of Sympatric Leishmania (V.) Braziliensis, L. (V.) Peruviana and Their Hybrids. Parasitology 2012, 139, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, F.; Savill, N.J.; Imamura, H.; Sanders, M.; Maes, I.; Cooper, S.; Mateus, D.; Jara, M.; Adaui, V.; Arevalo, J.; et al. Ecological Divergence and Hybridization of Neotropical Leishmania Parasites. Proc. Natl. Acad. Sci. USA 2020, 117, 25159–25168. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.A.; Yeo, M.; Mauricio, I.L. Genetics. Leishmania Exploit Sex. Science 2009, 324, 187–189. [Google Scholar] [CrossRef]
- Kato, H.; Cáceres, A.G.; Gomez, E.A.; Tabbabi, A.; Mizushima, D.; Yamamoto, D.S.; Hashiguchi, Y. Prevalence of Genetically Complex Leishmania Strains With Hybrid and Mito-Nuclear Discordance. Front. Cell Infect. Microbiol. 2021, 11, 625001. [Google Scholar] [CrossRef]
- Sterkers, Y.; Lachaud, L.; Crobu, L.; Bastien, P.; Pagès, M. FISH Analysis Reveals Aneuploidy and Continual Generation of Chromosomal Mosaicism in Leishmania Major. Cell Microbiol. 2011, 13, 274–283. [Google Scholar] [CrossRef]
- Bussotti, G.; Gouzelou, E.; Côrtes Boité, M.; Kherachi, I.; Harrat, Z.; Eddaikra, N.; Mottram, J.C.; Antoniou, M.; Christodoulou, V.; Bali, A.; et al. Leishmania Genome Dynamics during Environmental Adaptation Reveal Strain-Specific Differences in Gene Copy Number Variation, Karyotype Instability, and Telomeric Amplification. mBio 2018, 9, e01399-18. [Google Scholar] [CrossRef]
- Prieto Barja, P.; Pescher, P.; Bussotti, G.; Dumetz, F.; Imamura, H.; Kedra, D.; Domagalska, M.; Chaumeau, V.; Himmelbauer, H.; Pages, M.; et al. Haplotype Selection as an Adaptive Mechanism in the Protozoan Pathogen Leishmania Donovani. Nat. Ecol. Evol. 2017, 1, 1961–1969. [Google Scholar] [CrossRef]
- Imamura, H.; Monsieurs, P.; Jara, M.; Sanders, M.; Maes, I.; Vanaerschot, M.; Berriman, M.; Cotton, J.A.; Dujardin, J.-C.; Domagalska, M.A. Evaluation of Whole Genome Amplification and Bioinformatic Methods for the Characterization of Leishmania Genomes at a Single Cell Level. Sci. Rep. 2020, 10, 15043. [Google Scholar] [CrossRef]
- Parsons, M.; Ruben, L. Pathways Involved in Environmental Sensing in Trypanosomatids. Parasitol. Today 2000, 16, 56–62. [Google Scholar] [CrossRef]
- Marsh, L.; Neiman, A.M.; Herskowitz, I. Signal Transduction during Pheromone Response in Yeast. Annu. Rev. Cell Biol. 1991, 7, 699–728. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.; Cayla, M.; Ivens, A.; Mony, B.M.; MacGregor, P.; Silvester, E.; McWilliam, K.; Matthews, K.R. Non-Linear Hierarchy of the Quorum Sensing Signalling Pathway in Bloodstream Form African Trypanosomes. PLoS Pathog. 2018, 14, e1007145. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, A.-L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and Microvesicles in Normal Physiology, Pathophysiology, and Renal Diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.B.; Bell, C.R.; Bibb, K.E.; Gu, L.; Coats, M.T.; Matthews, Q.L. Pathogens and Their Effect on Exosome Biogenesis and Composition. Biomedicines 2018, 6, 79. [Google Scholar] [CrossRef]
- de Souza, W.; Barrias, E.S. Membrane-Bound Extracellular Vesicles Secreted by Parasitic Protozoa: Cellular Structures Involved in the Communication between Cells. Parasitol. Res. 2020, 119, 2005–2023. [Google Scholar] [CrossRef]
- Eliaz, D.; Kannan, S.; Shaked, H.; Arvatz, G.; Tkacz, I.D.; Binder, L.; Waldman Ben-Asher, H.; Okalang, U.; Chikne, V.; Cohen-Chalamish, S.; et al. Exosome Secretion Affects Social Motility in Trypanosoma Brucei. PLoS Pathog. 2017, 13, e1006245. [Google Scholar] [CrossRef]
- Douanne, N.; Dong, G.; Douanne, M.; Olivier, M.; Fernandez-Prada, C. Unravelling the Proteomic Signature of Extracellular Vesicles Released by Drug-Resistant Leishmania Infantum Parasites. PLoS Negl. Trop. Dis. 2020, 14, e0008439. [Google Scholar] [CrossRef]
- Cantanhêde, L.M.; da Silva Júnior, C.F.; Ito, M.M.; Felipin, K.P.; Nicolete, R.; Salcedo, J.M.V.; Porrozzi, R.; Cupolillo, E.; Ferreira, R.d.G.M. Further Evidence of an Association between the Presence of Leishmania RNA Virus 1 and the Mucosal Manifestations in Tegumentary Leishmaniasis Patients. PLoS Negl. Trop. Dis. 2015, 9, e0004079. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular Communication: Diverse Structures for Exchange of Genetic Information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mony, B.M.; Matthews, K.R. Assembling the Components of the Quorum Sensing Pathway in African Trypanosomes. Mol. Microbiol. 2015, 96, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Tagoe, D.N.A.; Kalejaiye, T.D.; de Koning, H.P. The Ever Unfolding Story of CAMP Signaling in Trypanosomatids: Vive La Difference! Front. Pharmacol. 2015, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Rojas, F.; Matthews, K.R. Quorum Sensing in African Trypanosomes. Curr. Opin. Microbiol. 2019, 52, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; DeMarco, S.F.; Rehmann, R.; Wenzler, T.; Florini, F.; Roditi, I.; Hill, K.L. Flagellar CAMP Signaling Controls Trypanosome Progression through Host Tissues. Nat. Commun. 2019, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Cayla, M.; McDonald, L.; MacGregor, P.; Matthews, K. An Atypical DYRK Kinase Connects Quorum-Sensing with Posttranscriptional Gene Regulation in Trypanosoma Brucei. Elife 2020, 9, e51620. [Google Scholar] [CrossRef]
- Rocha, V.P.C.; Dacher, M.; Young, S.A.; Kolokousi, F.; Efstathiou, A.; Späth, G.F.; Soares, M.B.P.; Smirlis, D. Leishmania Dual-Specificity Tyrosine-Regulated Kinase 1 (DYRK1) Is Required for Sustaining Leishmania Stationary Phase Phenotype. Mol. Microbiol. 2020, 113, 983–1002. [Google Scholar] [CrossRef]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 2019, 5, 17. [Google Scholar] [CrossRef]
- Coakley, G.; Maizels, R.M.; Buck, A.H. Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015, 31, 477–489. [Google Scholar] [CrossRef]
- Fu, H.; Elena, R.C.; Marquez, P.H. The Roles of Small RNAs: Insights from Bacterial Quorum Sensing. ExRNA 2019, 1, 32. [Google Scholar] [CrossRef]
- Briggs, E.M.; Rojas, F.; McCulloch, R.; Matthews, K.R.; Otto, T.D. Single-Cell Transcriptomic Analysis of Bloodstream Trypanosoma Brucei Reconstructs Cell Cycle Progression and Developmental Quorum Sensing. Nat. Commun. 2021, 12, 5268. [Google Scholar] [CrossRef] [PubMed]
- Acuña, S.M.; Floeter-Winter, L.M.; Muxel, S.M. MicroRNAs: Biological Regulators in Pathogen-Host Interactions. Cells 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.d.A.; Ramos-Sanchez, E.M.; Muxel, S.M.; Lagos, D.; Reis, L.C.; Pereira, V.R.A.; Brito, M.E.F.; Zampieri, R.A.; Kaye, P.M.; Floeter-Winter, L.M.; et al. MiR-548d-3p Alters Parasite Growth and Inflammation in Leishmania (Viannia) Braziliensis Infection. Front. Cell. Infect. Microbiol. 2021, 11, 687647. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).