Detrimental Effect of Ozone on Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Ozone Generating and Monitoring
2.3. Inoculation of the Test Surface
2.4. Ozone Treatment
2.5. Cell Viability
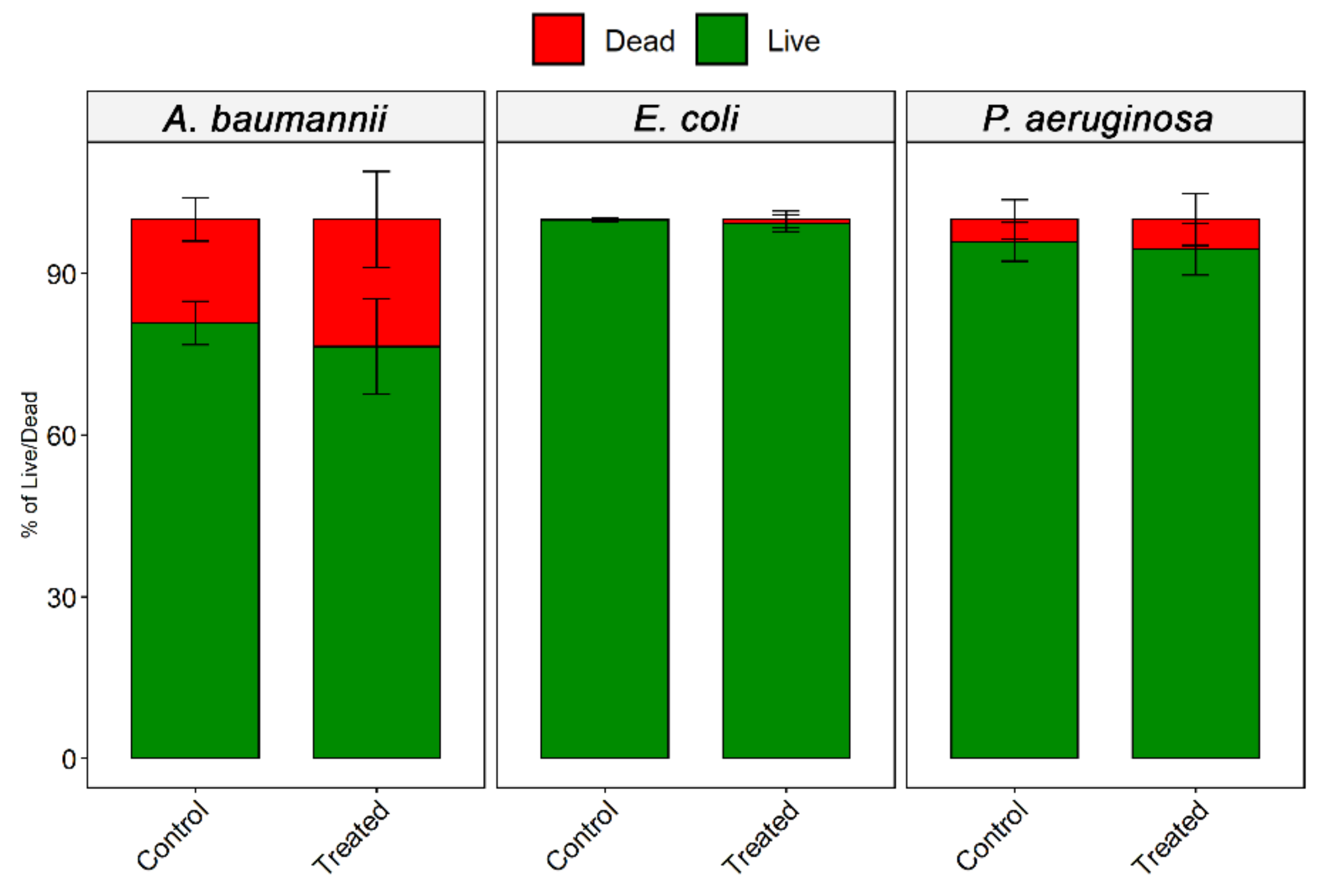
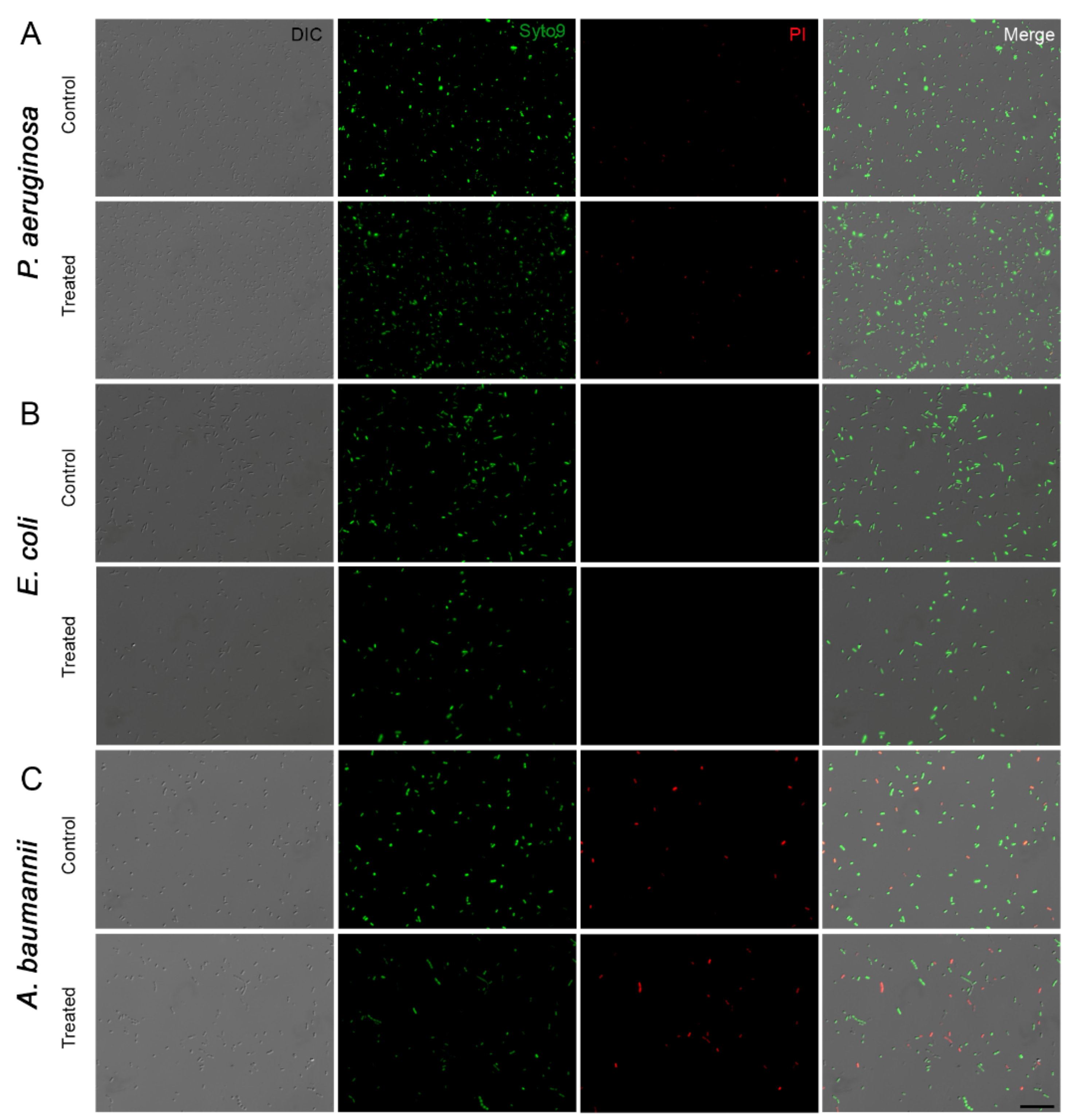
2.6. Live/Dead Assay
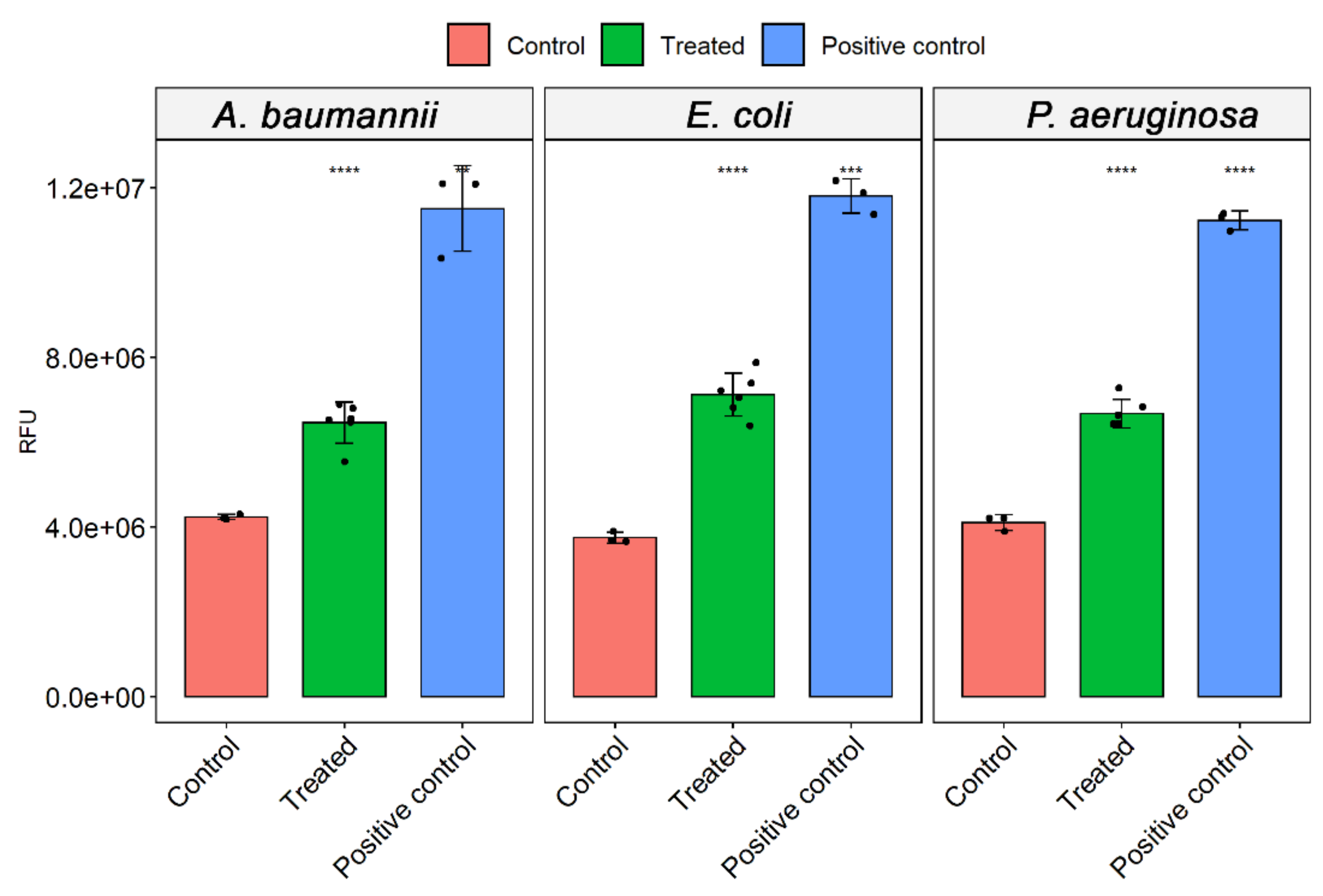
2.7. Measurement of Reactive Oxygen Species (ROS) Levels
2.8. Scanning Electron Microscopy (SEM)
3. Results
3.1. Monitoring of Ozone Concentration
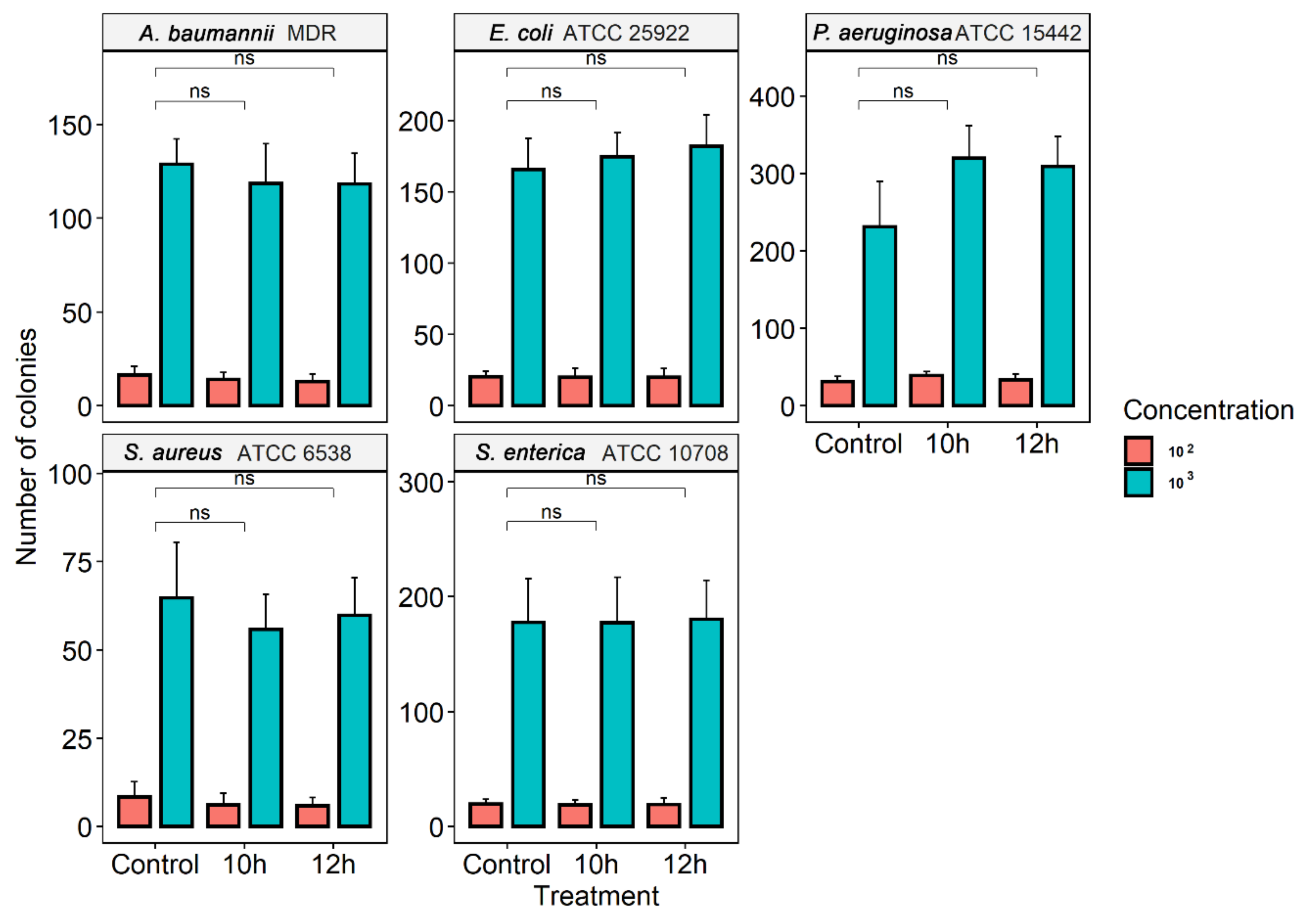
3.2. Ozone Treatment
3.3. Effect of Ozone
3.4. ROS Analysis
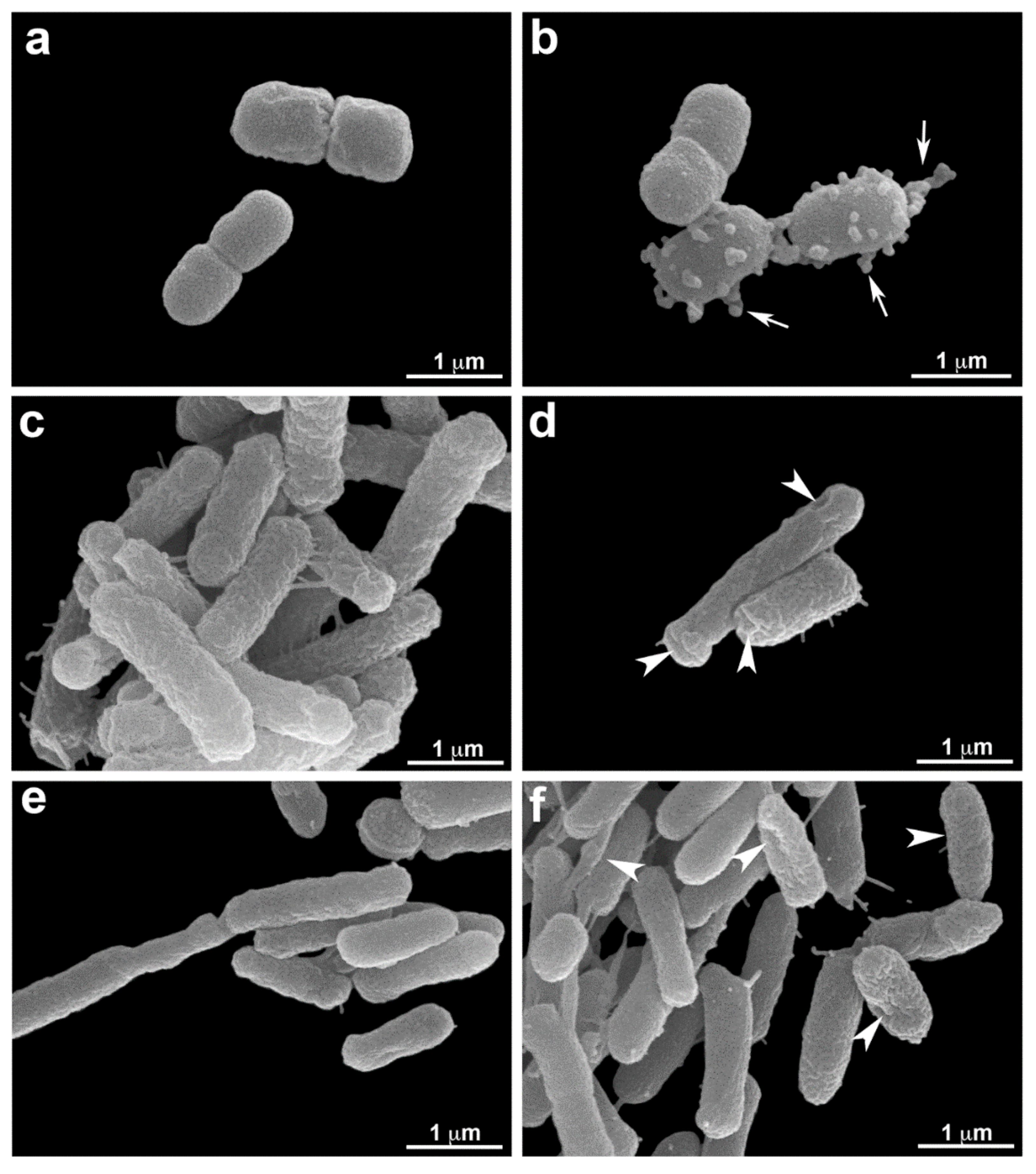
3.5. Scanning Electron Microscopy (SEM.)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, F.; Coelho, M.; Diniz, A.; Vicente, G.; Vieira, C. Velhos Problemas novos desafios. Rev. Technol. Hosp. 2011, 43, 30–32. [Google Scholar]
- Ratti, R.P.; Sousa, C.P. Staphylococcus aureus meticilina resistente MRSA e infecções nosocomiais. Vet. Ciên. Farm. Básica Apl. 2009, 30, 137–143. [Google Scholar]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Healthcare-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Word Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. 2011. Available online: https://apps.who.int/iris/handle/10665/80135 (accessed on 14 May 2021).
- Lax, S.; Gilbert, J.A. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol. Med. 2015, 21, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Calfee, D.P. Crisis in hospital-acquired healthcare-associated infections. Annu. Rev. Med. 2011, 63, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Teerawattanapong, N.; Panich, P.; Kulpokin, D.; Ranong, S.N.; Kongpakwattana, K.; Saksinanon, A.; Goh, B.; Lee, L.; Apisarnthanarak, A.; Chaiyakunapruk, N. A systematic review of the burden of multidrug-resistant healthcare-associated infections among intensive care unit patients in Southeast Asia: The rise of multidrug-resistant Acinetobacter baumannii. Infect. Control Hosp. Epidemiol. 2018, 39, 525–533. [Google Scholar] [CrossRef]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Sin, M.A.; Plachouras, D.; Kinross, P.; Suetens, C.; et al. Healthcare-associated pneumonia in acute care hospitals in European Union/European economic area countries: An analysis of data from a point prevalence survey 2011 to 2012. Eurosurveillance 2018, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Agaba, P.; Tumukunde, J.; Tindimwewa, J.V.B.; Kwizera, A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: A cross-sectional study. BMC Res. Notes 2017, 10, 349. [Google Scholar] [CrossRef]
- Curcio, D.; Alí, A.; Duarte, A.; Pauta, A.D.; Ibáñez-Gúzman, C.; Sang, M.C.; Valencia, E.; Plano, F.; Paredes Oña, F.; Arancibia, F.; et al. Latin American antibiotic use in intensive care unit group. Prescription of antibiotics in intensive care units in Latin America: An observational study. J. Chemother. 2009, 21, 527–534. [Google Scholar] [CrossRef]
- Curcio, D.J. On behalf of the Latin American antibiotic use in intensive care unit group. Antibiotic prescription in intensive care units in Latin America. Rev. Argent. Microbiol. 2011, 43, 203–211. [Google Scholar]
- Monnet, D.L.; Archibald, L.K.; Phillips, L.; Tenover, F.C.; McGowan, J.E., Jr.; Gaynes, R.P. Antimicrobial use and resistance in eight U.S. hospitals: Complexities of analysis and modeling. Intensive care antimicrobial resistance epidemiology project and national N.I.S.S.H. Nosocomial infections surveillance system hospitals. Infect. Control Hosp. Epidemiol. 1998, 19, 388–394. [Google Scholar] [CrossRef]
- Fujimura, S.; Nakano, Y.; Sato, T.; Shirahata, K.; Watanabe, A. Relationship between the usage of carbapenem antibiotics and the incidence of imipenem-resistant Pseudomonas aeruginosa. J. Infect. Chemother. 2007, 13, 147–150. [Google Scholar] [CrossRef]
- Rogues, A.M.; Dumartin, C.; Amadéo, B.; Venier, A.G.; Marty, N.; Parneix, P.; Gachie, J.P. Relationship between rates of antimicrobial consumption and the incidence of antimicrobial resistance in Staphylococcus aureus and Pseudomonas aeruginosa isolates from 47 French hospitals. Infect. Control Hosp. Epidemiol. 2007, 28, 1389–1395. [Google Scholar] [CrossRef]
- Iosifidis, E.; Antachopoulos, C.; Tsivitanidou, M.; Katragkou, A.; Farmaki, E.; Tsiakou, M.; Kyriazi, T.; Sofianou, D.; Roilides, E. Differential correlation between rates of antimicrobial drug consumption and prevalence of antimicrobial resistance in a tertiary care hospital in Greece. Infect. Control Hosp. Epidemiol. 2008, 29, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Sales, V.M.; Oliveira, E.; Célia, R.; Gonçalves, F.R.; Melo, C.C. Análise microbiológica de superfícies inanimadas de uma Unidade de Terapia Intensiva e a segurança do paciente. Rev. Enfer Ref. 2014, 4, 45–53. [Google Scholar] [CrossRef][Green Version]
- Nogueira, C., Jr.; Padoveze, M.C.; Lacerda, R.A. Governmental surveillance system of healthcare-associated infection in Brazil. Rev. Esc. Enferm. USP 2014, 48, 657–662. [Google Scholar]
- Souza, E.S.; Belei, R.A.; Carrilho, C.M.D.M.; Matsuo, T.; Ogatta, S.F.Y.; Andrade, G.; Perugini, M.R.E.; Pieri, F.M.; Dessunti, E.M.; Kerbauy, G. Mortalidade e riscos associados a infecção relacionada à assistência à saúde. Texto Contexto Enferm. 2015, 24, 220–228. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of healthcare-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Healthcare-Associated Infections-Hospital Prevalence Survey. 2015. Available online: https://www.cdc.gov/hai/index.html (accessed on 18 April 2021).
- World Health Organization. Healthcare-Associated Infections Fact Sheet. 2016. Available online: http://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en. pdf (accessed on 18 May 2021).
- Rosenthal, V.D.; Al-Abdely, H.M.; El-Kholy, A.A.; AlKhawaja, S.A.A.; Leblebicioglu, H.; Mehta, Y.; Rai, V.; Hung, N.V.; Kanj, S.S.; Salama, M.F. International nosocomial infection control consortium report data summary of 50 countries for 2010–2015: Device-associated module. Am. J. Infect. Control. 2016, 44, 1495–1504. [Google Scholar] [CrossRef]
- Weber, D.J.; Raasch, R.; Rutala, W.A. Nosocomial infections in the I.C.U.: The growing importance of antibiotic-resistant pathogens. Chest 1999, 115, 34S–41S. [Google Scholar] [CrossRef]
- Mendes, W.; Pavão, A.L.B.; Martins, M.; Moura, M.L.O.; Travassos, C. Características de eventos adversos evitáveis em hospitais do Rio de Janeiro. Rev. Assoc. Med. Bras. 2013, 59, 42188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fortaleza, C.M.C.B.; Padoveze, M.C.; Kiffer, C.R.V.; Barth, A.L.; Carneiro, I.C.R.S.; Giamberardino, H.I.G.; Rodrigues, J.L.N.; Filho, L.S.; Gonçalves de Mello, M.J.; Severino Pereira, M.; et al. Multi-state survey of healthcare-associated infections in acute care hospitals in Brazil. J. Hosp. Infect. 2017, 96, 139–144. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar] [CrossRef]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Blot, S.; Antonelli, M.; Arvaniti, K.; Blot, K.; Creagh-Brown, B.; de Lange, D.; De Waele, J.; Deschepper, M.; Dikmen, Y.; Dimopoulos, G.; et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS,” a multinational observational cohort study and E.S.I.C.M. Trials Group Project. Int. Care Med. 2019, 45, 1703–1717. [Google Scholar] [CrossRef]
- Garner, J.S. The hospital infection control practices advisory committee. Guideline for isolation precautions in hospital. Infect. Control Hosp. Epidemiol. 1996, 17, 54–80. [Google Scholar] [CrossRef]
- Lisboa, T.; Faria, M.; Hoher, J.A.; Borges, L.A.A.; Gómez, J.; Schifelbain, L.; Dias, F.S.; Lisboa, J.; Friedman, G. Prevalência de infecção nosocomial em unidades de terapia intensiva do Rio Grande do Sul. Rev. Bras. Ter. Intensiva 2007, 19, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Junior, L.D.; Fernandes, H.S.; Moreno, R.; Jean-Louis Vincent, J. Prevalence and outcomes of infections in Brazilian I.C.U.s: A sub analysis of EPIC II study. Rev. Bras. Ter. Intensiva 2012, 24, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Braga, I.A.; Campos, P.A.; Gontijo-Filho, P.P.; Ribas, R.M. Multi-hospital point prevalence study of healthcare-associated infections in 28 adult intensive care units in Brazil. J. Hosp. Infect. 2018, 99, 318–324. [Google Scholar] [CrossRef]
- United Nations. Health Agency Steps Up Fight against ‘Invisible Pandemic’ of Antimicrobial Resistance. 2019. Available online: https://news.un.org/en/story/2019/06/1040741 (accessed on 21 May 2021).
- World Health Organization—Antibiotic Resistance. Newsroom. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibioticresis tance (accessed on 21 May 2021).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antimicrob. Resist. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf (accessed on 18 October 2021).
- Zaha, D.C.; Bungau, S.; Uivarosan, D.; Tit, D.M.; Maghiar, T.A.; Maghiar, O.; Pantis, C.; Fratila, O.; Rus, M.; Vesa, C.M. Antibiotic Consumption and Microbiological Epidemiology in Surgery Departments: Results from a Single Study Center. Antibiotics 2020, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Ginocchio, F.; Mikulska, M. New treatment options against gram-negative organisms. Crit. Care 2011, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research Discovery and Development of New Antibiotics 2017. Available online: https://www. who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 21 May 2021).
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in prevalence of healthcare-associated infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Zaha, D.C.; Kiss, R.; Hegedűs, C.; Gesztelyi, R.; Bombicz, M.; Muresan, M.; Pallag, A.; Zrinyi, M.; Pall, D.; Vesa, C.M.; et al. Recent Advances in Investigation, Prevention, and Management of Healthcare-Associated Infections (HAIs): Resistant Multidrug Strain Colonization and Its Risk Factors in an Intensive Care Unit of a University Hospital. Biomed. Res. Int. 2019, 2019, 2510875. [Google Scholar] [CrossRef]
- Oliveira, A.C. Infecções Hospitalares Epidemiologia Prevenção e Controle; Medsi: Rio de Janeiro, Brazil, 2005. [Google Scholar]
- Pelczar, M.J.; Chan, E.C.S.; Krieg, N.R. Microbiologia Conceitos e Aplicações; Makron Books: São Paulo, Brazil, 1997. [Google Scholar]
- Fernandes, A.T.; Fernandes, M.O.V.; Ribeiro Filho, N.; Graziano, K.U.; Gabrielloni, M.C.; Cavalcante, N.J.F.; Lacerda, R.A. Infecção Hospitalar e Suas Interfaces na Área da Saúde; Atheneu: São Paulo, Brazil, 2000. [Google Scholar]
- Rutala, W.; Gergen, M.F.; Tande, B.M.; Weber, D.J. Rapid hospital room decontamination using ultraviolet U.V. light with a nanostructured UV-reflective wall coating. Infect. Control Hosp. Epidemiol. 2013, 34, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning hospital room surfaces to prevent healthcare-associated infections. Ann. Int. Med. 2015, 163, 598–607. [Google Scholar] [CrossRef]
- Blythe, D.; Keenlyside, D.; Dawson, S.J.; Galloway, A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (M.R.S.A.). J. Hosp. Infect. 1998, 38, 67–69. [Google Scholar] [CrossRef]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.T.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus M.R.S.A.: A comparison between conventional terminal cleaning and hydrogen peroxide vapor decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Fan, L.; Song, J.; Hildebrand, P.D.; Forney, C.F. Interaction of ozone and negative air ions to control micro-organisms. J. Appl. Microbiol. 2002, 93, 144–148. [Google Scholar] [CrossRef]
- De Boer, H.E.L.; Van Elzelingen-Dekker, C.M.; Van Rheenen-Vernerg, C.M.; Spanjaard, L. Use of gaseous ozone for eradication of methicillin-resistant Staphylococcus aureus from the home environment of a colonized hospital employee. Infect. Control Hosp. Epidemiol. 2006, 27, 1120–1122. [Google Scholar] [CrossRef][Green Version]
- Hudson, J.B.; Sharma, M.; Petric, M. Inactivation of Norovirus by ozone gas in conditions relevant to healthcare. J. Hosp. Infect. 2007, 66, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Hudson, J.B. Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control. 2008, 36, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Elvis, A.M.; Ekta, J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A. New developments in disinfection and sterilization. Am. J. Infect. Control 2016, 44, e23–e27. [Google Scholar] [CrossRef]
- Huth, K.C.; Quirling, M.; Maier, S.; Kamereck, K.; Alkhayer, M.; Paschos, E.; Welsch, U.; Miethke, T.; Brand, K.; Hickel, R. Effectiveness of ozone against endodontopathogenic microorganisms in a root canal biofilm model. Int. Endod. J. 2009, 42, 3–13. [Google Scholar] [CrossRef]
- Rosenblum, J.; Ge, C.; Bohrerova, Z.; Yousef, A.; Lee, J. Ozonation as a clean technology for fresh produce industry and environment: Sanitizer efficiency and wastewater quality. J. Appl. Microbiol. 2012, 113, 837–845. [Google Scholar] [CrossRef]
- Martinelli, M.; Giovannangeli, F.; Rotunno, S.; Trombetta, C.M.; Montomoli, E. Water and air ozone treatment as an alternative sanitizing technology. J. Prev. Med. Hyg. 2017, 58, E48–E52. [Google Scholar] [CrossRef]
- Bitter, K.; Vlassakids, A.; Niepel, M.; Hoedke, D.; Schulze, J.; Neumann, K.; Moter, A.; Noetzel, J. Effects of diode laser gaseous ozone and medical dressings on Enterococcus faecalis biofilms in the root canal ex vivo. Biomed. Res. Int. 2017, 2017, 6321850. [Google Scholar] [CrossRef]
- Lim, Y.; Lee, H.; Woodby, B.; Valacchi, G. Ozonated Oils, and Cutaneous Wound Healing. Curr. Pharm. Des. 2019, 25, 2264–2278. [Google Scholar] [CrossRef] [PubMed]
- Breidablik, H.J.; Lysebo, D.E.; Johannessen, L.; Skare, Å.; Andersen, J.R.; Kleiven, O. Effects of hand disinfection with alcohol hand rub, ozonized water, or soap and water: Time for reconsideration? J. Hosp. Infect. 2020, 105, 213–215. [Google Scholar] [CrossRef]
- Tonon, C.C.; Panariello, B.H.D.; Spolidorio, D.M.P.; Gossweiler, A.G.; Duarte, S.J. Antibiofilm effect of ozonized physiological saline solution on peri-implant-related biofilm. Periodontology 2021, 92, 1151–1162. [Google Scholar] [CrossRef]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Juneja, V.; Castillo, A. Inactivation of Salmonella and Shiga toxin-producing Escherichia coli (S.T.E.C.) from the surface of alfalfa seeds and sprouts by combined antimicrobial treatments using ozone and electrolyzed water. Food Res. Int. 2020, 136, 109488. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, A.; Gurol, M.D. Application of gaseous ozone for inactivation of Bacillus subtilis spores. J. Air Waste Manag. Assoc. 2006, 56, 179–185. [Google Scholar] [CrossRef]
- Hudson, J.B.; Sharma, M.; Vimalanathan, S. Development of a practical method for using ozone gas as a virus decontaminating agent. Ozone Sci. Eng. 2009, 31, 216–223. [Google Scholar] [CrossRef]
- Najafi, M.B.H.; Khodaparast, M.H.H. Efficacy of ozone to reduce microbial populations in date fruits. Food Control 2009, 20, 27–30. [Google Scholar] [CrossRef]
- Li, C.-S.; Wang, Y.-C. Surface germicidal effects of ozone for microorganisms. AIHA J. 2003, 64, 533–537. [Google Scholar] [CrossRef]
- Tseng, C.; Li, C. Inactivation of surface viruses by gaseous ozone. J. Environ. Health 2008, 70, 56–63. [Google Scholar] [PubMed]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Berrington, A.W.; Pedler, S.J. Investigation of gaseous ozone for M.R.S.A. decontamination of hospital side-rooms. J. Hosp. Infect. 1998, 40, 61–65. [Google Scholar]
- Cardoso, C.C.; Fiorini, J.E.; Gurjão, J.W.B.; Amaral, L.A. Disinfection of hospital laundry using ozone: Microbiological evaluation. Infect. Control Hosp. Epidemiol. 2000, 21, 248. [Google Scholar] [CrossRef]
- Cestonaro, L.V.; Marcolan, A.M.; Rossato-Grando, L.G.; Anzolin, A.P.; Goethel, G.; Angélica Vilani, A.; Garcia, S.C.; Bertol, C.D. Ozone generated by air purifier in low concentrations: Friend or foe? Environ. Sci. Pollut. Res. Int. 2017, 24, 22673–22678. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. Is ozone therapy therapeutic? Perspect. Biol. Med. 1998, 42, 131–143. [Google Scholar] [CrossRef]
- Bocci, V. Biological and clinical effects of ozone: Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 1999, 56, 270–279. [Google Scholar]
- Di Paolo, N.; Bocci, V.; Gaggiotti, E. Ozone therapy editorial review. Int. J. Artif. Organs 2004, 27, 168–175. [Google Scholar] [CrossRef]
- Carvalho, C.F.; Brioschi, M.L.; Teixeira, M.J. Uso da termografia na avaliação da ozonioterapia como tratamento da epicondilite lateral. Pan. Am. J. Med. Thermol. 2015, 2, 90–93. [Google Scholar] [CrossRef]
- Manoto, S.L.; Maepa, M.J.; Motaung, S.K. Medical ozone therapy as a potential treatment modality for regeneration of damaged articular cartilage in osteoarthritis. Saudi J. Biol. Sci. 2016, 25, 672–679. [Google Scholar] [CrossRef]
- Silva, R.A.; Garotti, J.E.R.; Silva, R.S.B.; Navarini, A.; Pacheco, A.M., Jr. Analysis of the bactericidal effect of ozone pneumo peritoneum. Acta Cir. Bras. 2009, 24, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Henley-Smith, C.J.; Canha, M.N.; Oosthuizen, C.B.; Barrington, D. Viability reagent presto blue in comparison with other available reagents utilized in cytotoxicity and antimicrobial assays. Int. J. Microbiol. 2013, 2013, 420601. [Google Scholar] [CrossRef]
- Riss, T.; Moravec, R.; Niles, A. Development for cell viability and apoptosis for high-throughput screening. In A Practical Guide to Assay Development and High-Throughput Screening in Drug Discovery; Chen, T., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 109–110. [Google Scholar]
- Riss, T.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L.; Markossian, S.; Grossman, A.; Brimacombe, K.; et al. Cell Viability Assays. In The Assay Guidance Manual; Sittampalam, G., Ed.; Eli Lilly Co.; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016; pp. 10–12. [Google Scholar]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT WST and Resazurin. Methods Mol. Biol. 2017, 1601, 1–17. [Google Scholar] [PubMed]
- Rangel, K.; Lechuga, G.C.; Souza, A.L.A.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; De-Simone, S.G. Pan-drug resistant Acinetobacter baumannii but not other strains are resistant to the bee venom peptide melittin. Antibiotics 2020, 9, 178. [Google Scholar] [CrossRef]
- Lara, L.S.; Moreira, C.S.; Calvet, C.M.; Lechuga, G.C.; Souza, R.S.; Bourguignon, S.C.; Ferreira, V.F.; Rocha, D.; Pereira, M.C.S. Efficacy of 2-hydroxy-3-phenyl sulfanyl methyl-[14]-naphthoquinone derivatives against different Trypanosoma cruzi discrete type units: Identification of a promising hit compound. Eur. J. Med. Chem. 2018, 144, 572–581. [Google Scholar] [CrossRef]
- Nogales, C.G.; Ferreira, M.B.; Lage-Marques, J.L. Comparison of the antimicrobial activity of three different concentrations of aqueous ozone on Pseudomonas aeruginosa, Staphylococcus aureus, and Enterococcus faecalis- in vitro study. Rev. Esp. Ozonoterapia 2014, 4, 9–15. [Google Scholar]
- Tormin, S.C.; Navarrini, A.; Almeida, J.O.C.F.; Travassos, L.H.R.; Negri, M.V.G.; Silva, R.A. Análise do efeito bactericida do ozônio sobre bactérias multirresistentes. Arq. Med. Hosp. Fac. Ciências Médicas Santa Casa São Paulo 2016, 61, 138–141. [Google Scholar]
- Tomasino, S. Development and assessment of disinfectant efficacy test methods for regulatory purposes. Am. J. Infect. Control 2013, 41, S72–S76. [Google Scholar] [CrossRef] [PubMed]
- Mcclurkin, J.D.; Maier, D.E.; Ileleji, K.E. Half-life time of ozone as a function of air movement and conditions in a sealed container. J. Stored Prod. Res. 2013, 55, 41–47. [Google Scholar] [CrossRef]
- Nemmich, S.; Tilmatine, A.; Dey, Z.; Hammadi, N.; Nassour, K.; Messal, S. Optimal sizing of a D.B.D. Ozone generator using response surface modeling ozone: Science and engineering. J. Int. Ozone Assoc. 2015, 37, 3–8. [Google Scholar] [CrossRef]
- Osha; Occupational Safety and Health Administration; U.S. Department of Health and Human Services. 1992. Available online: http://www.osha.gov/dts/chemicalsampling/data/CH_259 300.html (accessed on 14 May 2021).
- Environmental Protection Agency. 2015. Available online: http://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners#generators-effective (accessed on 21 May 2021).
- BRASIL. Ministério do Trabalho e Emprego. Norma Regulamentadora (NR) 15 NR 15 Anexo 11: Atividades e Operações Insalubres. 2016. Available online: http://www.mte.gov.br/legislacao/normas_regulamentadoras/nr_15_anexo11.pdf (accessed on 14 May 2021).
- ECO Sensors Company. 2020. Available online: https://www.ecosensors.com/company/ (accessed on 21 May 2021).
- ECO Sensors Company. 2020. Available online: http://www.ecosensors.com/wpcontent/uploads/old/MO-100.pdf (accessed on 21 May 2021).
- Masaoka, T.; Kubota, Y.; Namiuchi, S.; Takubo, T.; Ueda, T.; Shibata, H.; Nakamura, H.; Yoshitake, J.; Yamayoshi, T.; Doi, H.; et al. Ozone decontamination of bio clean rooms. Appl. Environ. Microbiol. 2002, 43, 509–513. [Google Scholar] [CrossRef]
- Dyas, A.; Boughton, B.J.; Das, B.C. Ozone killing action against bacterial and fungal species; microbiological testing of a domestic ozone generator. J. Clin. Pathol. 1983, 36, 1102–1104. [Google Scholar] [CrossRef]
- Ishizaki, K.; Sawadaishi, K.; Miura, K.; Shinriki, N. Effect of ozone on plasmid D.N.A. of Escherichia coli in situ. Water Res. 1987, 21, 823–827. [Google Scholar] [CrossRef]
- Whistler, P.E.; Sheldon, B.W. Comparison of ozone and formaldehyde as poultry hatchery disinfectants. Poult. Sci. 1989, 68, 1345–1350. [Google Scholar] [CrossRef]
- World Health Organization. Water Treatment and Pathogen Control. 2004. Available online: https://www.who.int/water_ sanitation_health/water-quality/guidelines/en/watreatpath3. pdf (accessed on 14 May 2021).
- Fontes, B.; Heimbecker, A.M.C.; Brito, G.S.; Costa, S.F.; Van Der Heijden, I.M.; Levin, A.S.; Rasslan, R. Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Almaz, M.E.; Sönmez, I.Ş. Ozone therapy in the management and prevention of caries. J. Formos Med. Assoc. 2015, 114, 3–11. [Google Scholar] [CrossRef]
- Yamayoshi, T.; Tatsumi, N. Microbicidal effects of ozone solution on methicillin-resistant Staphylococcus aureus. Drugs Exp. Clin. Res. 1993, 19, 59–64. [Google Scholar]
- Komanapalli, I.R.; Lau, B.H. Inactivation of bacteriophage lambda Escherichia coli and Candida albicans by ozone. Appl. Microbiol. Biotechnol. 1998, 49, 766–769. [Google Scholar] [CrossRef]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal properties of ozone and its potential application as a terminal disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? Jpn. Med. Assoc. J. 2002, 45, 271–276. [Google Scholar]
- Vatansever, F.; De Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics photodynamic therapy and beyond. FEMS Microbiol. Ver. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Cheng, T.J.; Kao, H.P.; Chan, C.C.; Chang, W.P. Effects of ozone on D.N.A. single-strand breaks and 8-oxoguanine formation in A549 cells. Environ. Res. 2003, 93, 279–284. [Google Scholar] [CrossRef]
- Mathineu-Nolf, M. Poisons in the air: A cause of chronic disease in children. J. Toxicol. Clin. Toxicol. 2002, 40, 483–491. [Google Scholar] [CrossRef]
- Bocci, V. Ozone as Janus: This controversial gas can be either toxic or medically useful. Mediat. Inflamm. 2004, 13, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The ozone paradox: Ozone is a strong oxidant as well as a medical drug. Med. Res. Rev. 2009, 29, 646–682. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; Fortino, V.; Bocci, V. The dual action of ozone on the skin. Br. J. Dermatol. 2005, 153, 1096–1100. [Google Scholar] [CrossRef]
- Velano, H.E.; Nascimento, L.C.; De Barros, L.M.; Panzeri, H. Avaliação in vitro da atividade antibacteriana da água ozonizada frente ao Staphylococcus aureus. Pesq. Odontol. Bras. 2001, 151, 18–22. [Google Scholar] [CrossRef]
- Park, J.S.; Sung, B.J.; Yoon, K.S.; Jeong, C.S. The bactericidal effect of an ionizer under low concentration of ozone. BMC Microbiol. 2016, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment from Basic Principles to Applications; I.W.A. Publishing: London, UK, 2012. [Google Scholar]
- Hamelin, C.; Chung, Y.S. Optimal conditions for mutagenesis by ozone in Escherichia coli K12. Mutat. Res. 1974, 24, 271–279. [Google Scholar] [CrossRef]
- Hamelin, C.; Sarhan, F.; Chung, Y.S. Ozone induced D.N.A. degradation in different D.N.A. polymerase I mutants of Escherichia coli K12. Biochem. Biophys. Res. Commun. 1977, 77, 220–224. [Google Scholar] [CrossRef]
- Hamelin, C.; Sarhan, F.; Chung, Y.S. Induction of deoxyribonucleic acid degradation in Escherichia coli by ozone. Experientia 1978, 34, 1578–1579. [Google Scholar] [CrossRef]
- Scott, D.B.M.; Lesher, E.C. Effect of ozone on survival and permeability of Escherichia coli. J. Bacteriol. 1963, 85, 567–576. [Google Scholar] [CrossRef]
- Hunt, N.K.; Mariñas, B.J. Inactivation of Escherichia coli with ozone: Chemical and inactivation kinetics. Water Res. 1999, 33, 2633–2641. [Google Scholar] [CrossRef]
- Dodd, M.C. Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment. J. Environ. Monit. 2012, 14, 1754–1771. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Broadwater, W.T.; Hoehn, R.C.; King, P.H. Sensitivity of three selected bacterial species to ozone. Appl. Microbiol. 1973, 26, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Casolari, A. Physiological Models in Microbiology; Bazin, M.J., Prosser, J.I., Eds.; C.R.C. Press: Boca Raton, FL, USA, 1988; pp. 1–44. [Google Scholar]
- Patil, S.; Valdramidis, V.P.; Karatzas, K.A.G.; Cullen, P.J.; Bourke, P. Assessing the microbial oxidative stress mechanism of ozone treatment through the responses of Escherichia coli mutants. J. Appl. Microbiol. 2011, 111, 136–144. [Google Scholar] [CrossRef]
- Xu, P.; Janex, M.L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Patil, S.; Bourke, P.; Frías, J.M.; Kumar, T.B.; Cullen, P.J. Inactivation of Escherichia coli in orange juice using ozone. Innov. Food Sci. Emerg. Technol. 2009, 10, 551–557. [Google Scholar] [CrossRef]
- Tang, K.W.; Dziallas, C.; Grossart, H.P. Zooplankton and aggregates as refuge for aquatic bacteria: Protection from U.V. heat and ozone stresses used for water treatment. Environ. Microbiol. 2011, 13, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Pak, G.; Salcedo, D.E.; Lee, H.; Oh, J.; Maeng, S.K.; Song, K.G.; Hong, S.W.; Kim, H.C.; Chandran, K.; Kim, S. Comparison of antibiotic resistance removal efficiencies using ozone disinfection under different pH and suspended solids and humic substance concentrations. Environ. Sci. Technol. 2016, 50, 7590–7600. [Google Scholar] [CrossRef]
- Tseng, C.; Li, C. Ozone for inactivation of aerosolized bacteriophages. Aerosol. Sci. Technol. 2006, 40, 683–689. [Google Scholar] [CrossRef]
- Cannon, J.L.; Kotwall, G.; Wang, Q. Inactivation of norovirus surrogates after exposure to atmospheric ozone. Ozone Sci. Eng. 2013, 35, 217–219. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 2022, 10, 40. https://doi.org/10.3390/microorganisms10010040
Rangel K, Cabral FO, Lechuga GC, Carvalho JPRS, Villas-Bôas MHS, Midlej V, De-Simone SG. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms. 2022; 10(1):40. https://doi.org/10.3390/microorganisms10010040
Chicago/Turabian StyleRangel, Karyne, Fellipe O. Cabral, Guilherme C. Lechuga, João P. R. S. Carvalho, Maria H. S. Villas-Bôas, Victor Midlej, and Salvatore G. De-Simone. 2022. "Detrimental Effect of Ozone on Pathogenic Bacteria" Microorganisms 10, no. 1: 40. https://doi.org/10.3390/microorganisms10010040
APA StyleRangel, K., Cabral, F. O., Lechuga, G. C., Carvalho, J. P. R. S., Villas-Bôas, M. H. S., Midlej, V., & De-Simone, S. G. (2022). Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms, 10(1), 40. https://doi.org/10.3390/microorganisms10010040






