Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tick Collection and Measurement
2.2. DNA Isolation and Quantitative Real-Time PCR
2.3. Reverse Line Blot
2.4. Statistical Analyses
3. Results
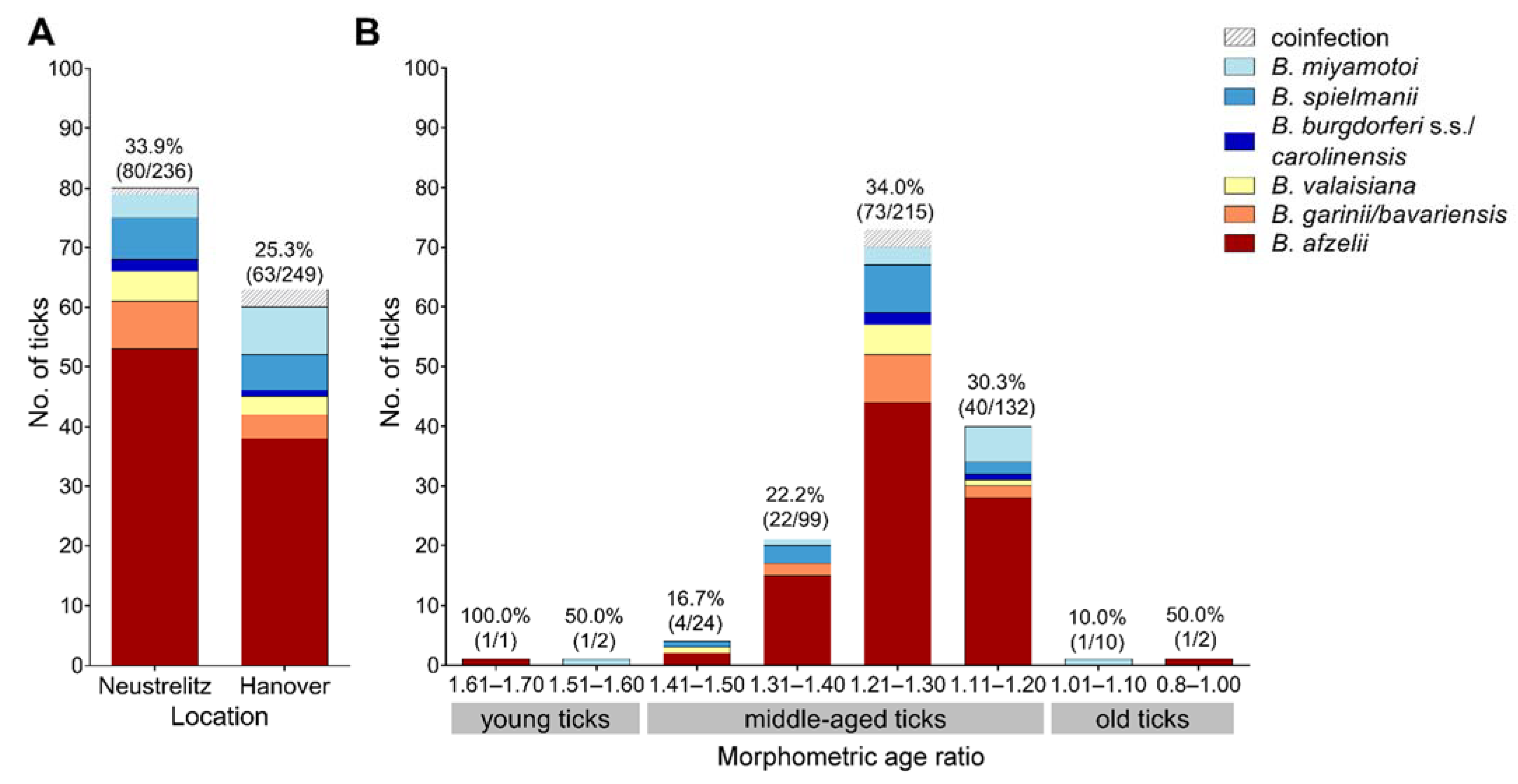
3.1. Borrelia Prevalence and Species Distribution
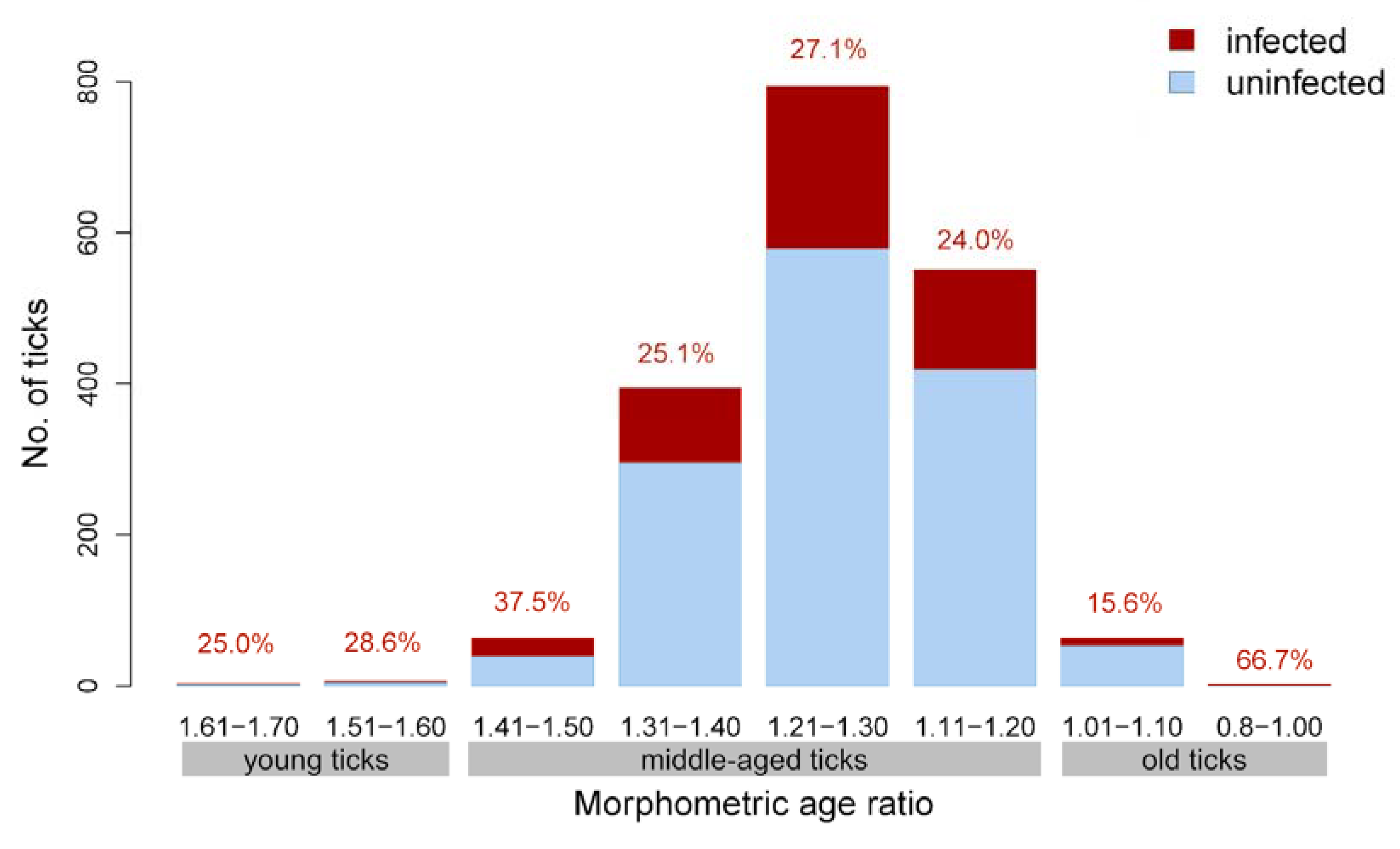
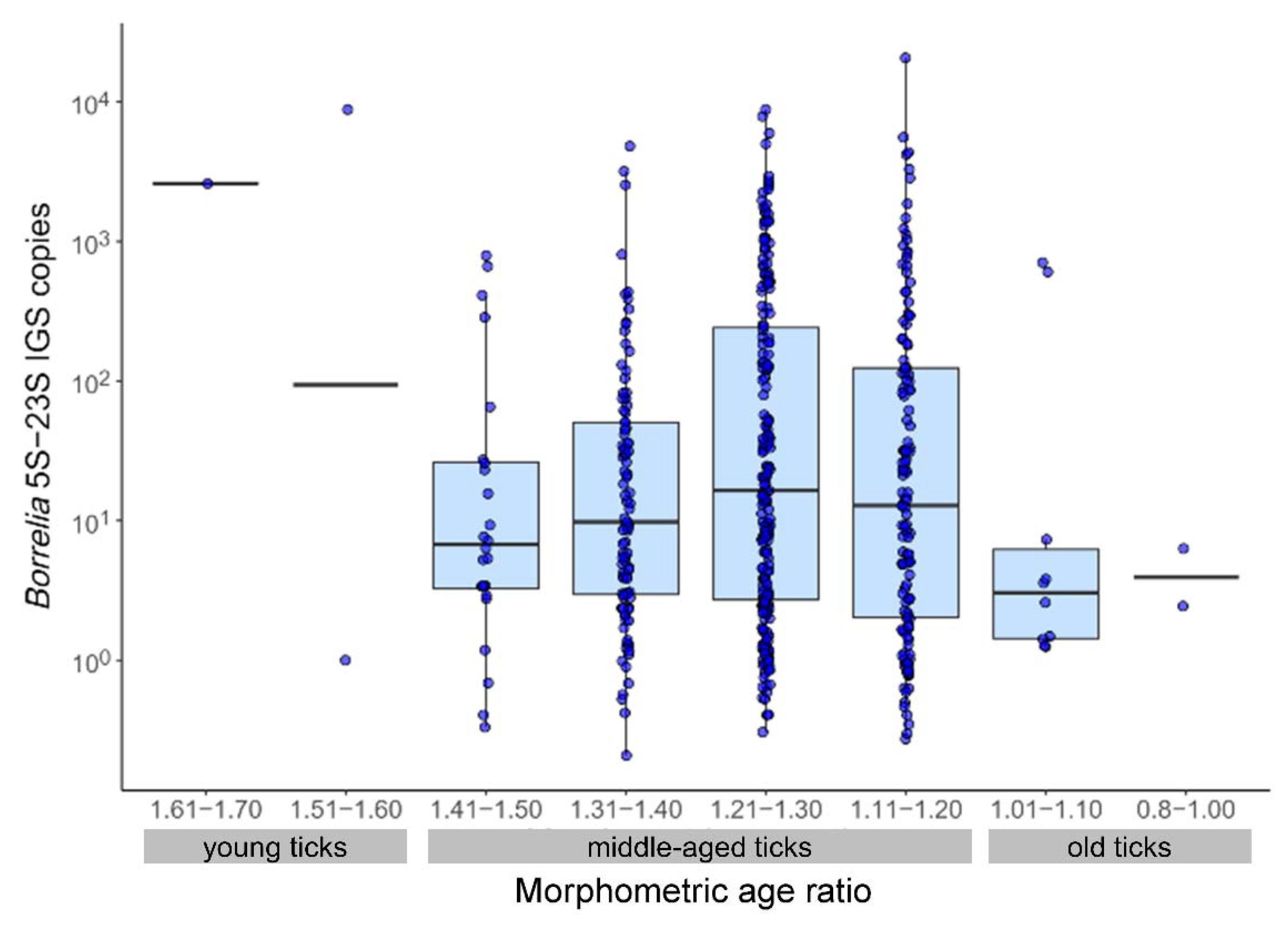
3.2. Relationship of Borrelia Prevalence and Infection Intensity with Tick Morphometric Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis. 2016, 7, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Pappas, C.J.; Iyer, R.; Petzke, M.M.; Caimano, M.J.; Radolf, J.D.; Schwartz, I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011, 7, e1002102. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, M.; Genné, D.; Belli, A.; Maluenda, E.; Sarr, A.; Voordouw, M.J. The abundance of the Lyme disease pathogen Borrelia afzelii declines over time in the tick vector Ixodes ricinus. Parasit. Vectors 2017, 10, 257. [Google Scholar] [CrossRef]
- Herrmann, C.; Gern, L. Survival of Ixodes ricinus (Acari: Ixodidae) under challenging conditions of temperature and humidity is influenced by Borrelia burgdorferi sensu lato infection. J. Med. Entomol. 2010, 47, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, S.; Wall, R. Metabolic rate and resource depletion in the tick Ixodes ricinus in response to temperature. Exp. Appl. Acarol. 2021, 83, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Pool, J.R.; Petronglo, J.R.; Falco, R.C.; Daniels, T.J. Energy usage of known-age blacklegged ticks (Acari: Ixodidae): What is the best method for determining physiological age? J. Med. Entomol. 2017, 54, 949–956. [Google Scholar] [CrossRef]
- Uspensky, I.; Kovalevskii, Y.V.; Korenberg, E.I. Physiological age of field-collected female taiga ticks, Ixodes persulcatus (Acari: Ixodidae), and their infection with Borrelia burgdorferi sensu lato. Exp. Appl. Acarol. 2006, 38, 201–209. [Google Scholar] [CrossRef]
- Strnad, M.; Rego, R.O.M. The need to unravel the twisted nature of the Borrelia burgdorferi sensu lato complex across Europe. Microbiology 2020, 166, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi—A human health risk? Eurosurveillance 2019, 24, 1800170. [Google Scholar] [CrossRef] [Green Version]
- Springer, A.; Raulf, M.-K.; Fingerle, V.; Strube, C. Borrelia prevalence and species distribution in ticks removed from humans in Germany, 2013–2017. Ticks Tick Borne Dis. 2020, 11, 101363. [Google Scholar] [CrossRef]
- Blazejak, K.; Raulf, M.-K.; Janecek, E.; Jordan, D.; Fingerle, V.; Strube, C. Shifts in Borrelia burgdorferi (s.l.) geno-species infections in Ixodes ricinus over a 10-year surveillance period in the city of Hanover (Germany) and Borrelia miyamotoi-specific Reverse Line Blot detection. Parasit. Vectors 2018, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippova, N.A. Ixodid Ticks (Ixodinae). Fauna USSR New Series 4 (4); Nauka: Moscow, Russia; Leningrad, Russia, 1977. [Google Scholar]
- Balashov, Y.S. Dynamics of stored nutritional substances and age determination in unfed ixodid ticks. Zool. Zhurnal 1961, 40, 1354–1363. [Google Scholar]
- Tappe, J.; Strube, C. Anaplasma phagocytophilum and Rickettsia spp. infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany): Revisited. Ticks Tick Borne Dis. 2013, 4, 432–438. [Google Scholar] [CrossRef]
- Strube, C.; Montenegro, V.M.; Epe, C.; Eckelt, E.; Schnieder, T. Establishment of a minor groove binder-probe based quantitative real time PCR to detect Borrelia burgdorferi sensu lato and differentiation of Borrelia spielmanii by ospA-specific conventional PCR. Parasit. Vectors 2010, 3, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tappe, J.; Jordan, D.; Janecek, E.; Fingerle, V.; Strube, C. Revisited: Borrelia burgdorferi sensu lato infections in hard ticks (Ixodes ricinus) in the city of Hanover (Germany). Parasit. Vectors 2014, 7, 441. [Google Scholar] [CrossRef] [Green Version]
- May, K.; Jordan, D.; Fingerle, V.; Strube, C. Borrelia burgdorferi sensu lato and co-infections with Anaplasma phagocytophilum and Rickettsia spp. in Ixodes ricinus in Hamburg, Germany. Med. Vet. Entomol. 2015, 29, 425–429. [Google Scholar] [CrossRef]
- Strube, C.; Schicht, S.; Schnieder, T. Borrelia burgdorferi sensu lato and Rickettsia spp. infections in hard ticks (Ixodes ricinus) in the region of Hanover (Germany). Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 512–517. [Google Scholar] [CrossRef]
- Alekseev, A.N.; Dubinina, H.V.; Van De Pol, I.; Schouls, L.M. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 2001, 39, 2237–2242. [Google Scholar] [CrossRef] [Green Version]
- Rijpkema, S.G.; Molkenboer, M.J.; Schouls, L.M.; Jongejan, F.; Schellekens, J.F. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 1995, 33, 3091–3095. [Google Scholar] [CrossRef] [Green Version]
- Gern, L.; Douet, V.; López, Z.; Rais, O.; Cadenas, F.M. Diversity of Borrelia genospecies in Ixodes ricinus ticks in a Lyme borreliosis endemic area in Switzerland identified by using new probes for reverse line blotting. Ticks Tick Borne Dis. 2010, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Poupon, M.A.; Lommano, E.; Humair, P.F.; Douet, V.; Rais, O.; Schaad, M.; Jenni, L.; Gern, L. Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl. Environ. Microbiol. 2006, 72, 976–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing, 4.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Kurokawa, C.; Lynn, G.E.; Pedra, J.H.F.; Pal, U.; Narasimhan, S.; Fikrig, E. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol. 2020, 18, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Voordouw, M.J.; Gern, L. Ixodes ricinus ticks infected with the causative agent of Lyme disease, Borrelia burgdorferi sensu lato, have higher energy reserves. Int. J. Parasitol. 2013, 43, 477–483. [Google Scholar] [CrossRef]
- Lefcort, H.; Durden, L.A. The effect of infection with Lyme disease spirochetes (Borrelia burgdorferi) on the phototaxis, activity, and questing height of the tick vector Ixodes scapularis. Parasitology 1996, 113, 97–103. [Google Scholar] [CrossRef]
- Romashchenko, A.V.; Ratushnyak, A.S.; Zapara, T.A.; Tkachev, S.E.; Moshkin, M.P. The correlation between tick (Ixodes persulcatus Sch.) questing behaviour and synganglion neuronal responses to odours. J. Insect Physiol. 2012, 58, 903–910. [Google Scholar] [CrossRef]
- Herrmann, C.; Gern, L. Do the level of energy reserves, hydration status and Borrelia infection influence walking by Ixodes ricinus (Acari: Ixodidae) ticks? Parasitology 2012, 139, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Alekseev, A.N.; Jensen, P.M.; Dubinina, H.V.; Smirnova, L.A.; Makrouchina, N.A.; Zharkov, S.D. Peculiarities of behaviour of taiga (Ixodes persulcatus) and sheep (Ixodes ricinus) ticks (Acarina: Ixodidae) determined by different methods. Folia Parasitol. 2000, 47, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Knoll, S.; Springer, A.; Hauck, D.; Schunack, B.; Pachnicke, S.; Fingerle, V.; Strube, C. Distribution of Borrelia burgdorferi s.l. and Borrelia miyamotoi in Ixodes tick populations in Northern Germany, co-infections with Rickettsiales and assessment of potential influencing factors. Med. Vet. Entomol. 2021, 35, 595–606. [Google Scholar] [CrossRef]
- Raulf, M.-K.; Jordan, D.; Fingerle, V.; Strube, C. Association of Borrelia and Rickettsia spp. and bacterial loads in Ixodes ricinus ticks. Ticks Tick Borne Dis. 2018, 9, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liveris, D.; Brei, B.; Wu, H.; Falco, R.C.; Fish, D.; Schwartz, I. Real-Time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the northeastern United States. Appl. Environ. Microbiol. 2003, 69, 4561–4565. [Google Scholar] [CrossRef] [Green Version]
- Răileanu, C.; Tauchmann, O.; Vasić, A.; Wöhnke, E.; Silaghi, C. Borrelia miyamotoi and Borrelia burgdorferi (sensu lato) identification and survey of tick-borne encephalitis virus in ticks from north-eastern Germany. Parasit. Vectors 2020, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Coipan, E.C.; Fonville, M.; Tijsse-Klasen, E.; van der Giessen, J.W.B.; Takken, W.; Sprong, H.; Takumi, K. Geodemographic analysis of Borrelia burgdorferi sensu lato using the 5S–23S rDNA spacer region. Infect. Genet. Evol. 2013, 17, 216–222. [Google Scholar] [CrossRef]
- Alasmari, S.; Wall, R. Determining the total energy budget of the tick Ixodes ricinus. Exp. Appl. Acarol. 2020, 80, 531–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Age Category | Alloscutal/Scutal Index | Age Group According to Balashov [14] |
|---|---|---|
| 1 | 1.61−1.70 | II (young ticks) |
| 2 | 1.51−1.60 | II (young ticks) |
| 3 | 1.41−1.50 | III (middle-aged ticks) |
| 4 | 1.31−1.40 | III (middle-aged ticks) |
| 5 | 1.21−1.30 | III (middle-aged ticks) |
| 6 | 1.11−1.20 | III (middle-aged ticks) |
| 7 | 1.01−1.10 | IV (old ticks) |
| 8 | 0.8−1.00 | IV (old ticks) |
| Probe | Target Species | Borrelia Strain Used as Positive Control | Probe References |
|---|---|---|---|
| SL2 | B. burgdorferi s.l. | - | [17] |
| AF | B. afzelii | PBas | [21] |
| GA | B. garinii, B. bavariensis | PWudII, PBi | [21] |
| BisNE2 | B. bissettiae | DN127 | [22] |
| SS | B. burgdorferi s.s., B. carolinensis | PAbe, SCW-22T | [22] |
| LusiNE2 | B. lusitaniae | Poti B2 | [22] |
| SpiNE3 | B. spielmanii | PHap | [23] |
| SpiNE3T | B. spielmanii | PHap | this study |
| VSNE | B. valaisiana | VS116 | [23] |
| BisNE1 | B. kurtenbachii | 25015 | [22] |
| MIYA | B. miyamotoi | HT31 | [12] |
| Month | Hanover | Neustrelitz | Total |
|---|---|---|---|
| March | 35/151 (23.2%) | 48/153 (31.4%) | 83/304 (27.3%) |
| April | 46/150 (30.7%) | 31/148 (20.9%) | 77/298 (25.8%) |
| May | 29/147 (19.7%) | 30/149 (20.1%) | 59/296 (19.9%) |
| June | 31/147 (21.1%) | 44/108 (40.7%) | 75/255 (29.4%) |
| July | 40/148 (27.0%) | 42/141 (29.8%) | 82/289 (28.4%) |
| August | 21/134 (15.7%) | 21/63 (33.3%) | 42/197 (21.3%) |
| September | 31/118 (26.3%) | 3/8 (37.5%) | 34/126 (27.0%) |
| October | 16/68 (23.5%) | 17/49 (34.7%) | 33/117 (28.2%) |
| Variable | Estimate | Std. Error | z-Value | p-Value |
|---|---|---|---|---|
| Intercept | −1.92 | 1.34 | −1.43 | 0.154 |
| Month | ||||
| March | Reference | − | − | − |
| April | −0.07 | 0.19 | −0.36 | 0.716 |
| May | −0.41 | 0.20 | −2.06 | 0.040 |
| June | 0.12 | 0.19 | 0.62 | 0.536 |
| July | 0.07 | 0.19 | 0.37 | 0.715 |
| August | −0.06 | 0.28 | −0.21 | 0.836 |
| September | 0.36 | 0.31 | 1.15 | 0.250 |
| October | 0.28 | 0.30 | 0.91 | 0.362 |
| Morphometric age ratio | ||||
| 1.61–1.70 | Reference | − | − | − |
| 1.51–1.60 | 0.16 | 1.44 | 0.11 | 0.910 |
| 1.41–1.50 | 0.51 | 1.19 | 0.42 | 0.672 |
| 1.31–1.40 | −0.18 | 1.17 | −0.15 | 0.881 |
| 1.21–1.30 | −0.01 | 1.17 | −0.01 | 0.996 |
| 1.11–1.20 | −0.18 | 1.17 | −0.15 | 0.878 |
| 1.01–1.10 | −0.73 | 1.21 | −0.60 | 0.550 |
| 0.80–1.00 | 1.71 | 1.69 | 1.01 | 0.312 |
| Ixodes ITS2 copies 1 | 0.07 | 0.05 | 1.49 | 0.136 |
| Variable | Estimate | Std. Error | Df | t-Value | p-Value |
|---|---|---|---|---|---|
| Intercept | 2.25 | 2.76 | 466.20 | 0.81 | 0.416 |
| Month | |||||
| March | Reference | − | − | − | − |
| April | −0.45 | 0.38 | 439.99 | −1.18 | 0.239 |
| May | 1.02 | 0.41 | 468.98 | 2.50 | 0.013 |
| June | 1.71 | 0.38 | 468.08 | 4.53 | <0.001 |
| July | 2.16 | 0.38 | 468.61 | 5.70 | <0.001 |
| August | 0.79 | 0.59 | 416.62 | 1.33 | 0.184 |
| September | 1.83 | 0.63 | 177.98 | 2.89 | 0.004 |
| October | 1.27 | 0.64 | 441.67 | 1.99 | 0.048 |
| Morphometric age ratio | |||||
| 1.61−1.70 | Reference | − | − | − | − |
| 1.51−1.60 | −0.88 | 2.88 | 468.13 | −0.31 | 0.760 |
| 1.41−1.50 | −4.50 | 2.39 | 468.58 | −1.88 | 0.060 |
| 1.31−1.40 | −3.78 | 2.35 | 468.97 | −1.61 | 0.109 |
| 1.21−1.30 | −3.41 | 2.35 | 468.96 | −1.45 | 0.147 |
| 1.11−1.20 | −3.85 | 2.35 | 468.96 | −1.64 | 0.102 |
| 1.01−1.10 | −4.45 | 2.45 | 468.96 | −1.81 | 0.070 |
| 0.80−1.00 | −6.48 | 2.85 | 468.71 | −2.27 | 0.023 |
| Ixodes ITS2 copies 1 | 0.25 | 0.10 | 361.62 | 2.52 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Jordan, D.; Glass, A.; Kahl, O.; Fingerle, V.; Girl, P.; Chitimia-Dobler, L.; Strube, C. Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs. Microorganisms 2022, 10, 166. https://doi.org/10.3390/microorganisms10010166
Springer A, Jordan D, Glass A, Kahl O, Fingerle V, Girl P, Chitimia-Dobler L, Strube C. Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs. Microorganisms. 2022; 10(1):166. https://doi.org/10.3390/microorganisms10010166
Chicago/Turabian StyleSpringer, Andrea, Daniela Jordan, Antje Glass, Olaf Kahl, Volker Fingerle, Philipp Girl, Lidia Chitimia-Dobler, and Christina Strube. 2022. "Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs" Microorganisms 10, no. 1: 166. https://doi.org/10.3390/microorganisms10010166
APA StyleSpringer, A., Jordan, D., Glass, A., Kahl, O., Fingerle, V., Girl, P., Chitimia-Dobler, L., & Strube, C. (2022). Borrelia Infections in Ageing Ticks: Relationship with Morphometric Age Ratio in Field-Collected Ixodes ricinus Nymphs. Microorganisms, 10(1), 166. https://doi.org/10.3390/microorganisms10010166







