Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms
Abstract
1. Introduction
1.1. Overview of Electroactive Polymers (EAPs)
1.2. Importance of Self-Powered Actuators and Biosensors in Biomedical Diagnostics
1.3. Scope and Objectives of the Review
2. Fundamentals of Electroactive Polymers and Energy Harvesting Mechanisms
2.1. Representative Electroactive Polymers and Their Chemical Structures
2.2. Definition and Types of Electroactive Polymers (EAPs)
2.2.1. Energy Harvesting Mechanisms
2.2.2. Piezoelectricity
2.2.3. Triboelectricity
2.2.4. Ionic Conductivity-Based Mechanism
2.3. Advantages of Self-Powered Systems in Biomedical Applications
3. EAP-Based Self-Powered Actuators
3.1. Principles of Actuation
3.1.1. Mechanisms of Motion and Force Generation
3.1.2. Biomechanical Energy Harvesting in Actuators
3.2. Applications in Soft Robotics and Biohybrid Systems
3.3. Role in Medical Devices: Artificial Muscles, Prosthetics, and Rehabilitation
4. EAP-Based Self-Powered Biosensors
4.1. Sensing Mechanisms and Applications
4.1.1. Detection of Physiological Signals and Biomarkers
4.1.2. Real-Time Health Monitoring (e.g., Glucose, Lactate, and Sweat Analysis)
4.2. Integration with Flexible Electronics
4.2.1. Wearable and Implantable Sensors for Continuous Diagnostics
4.2.2. Non-Invasive Monitoring Technologies
4.3. Applications in Point-of-Care Diagnostics and Personalized Medicine
4.4. Quantitative Performance of EAP-Based Biosensors: Sensitivity, Specificity, and Detection Limits
5. Nanomaterial Integration for Enhanced EAP Performance
5.1. Nanocomposites and Nanostructured EAPs
5.1.1. Carbon Nanotubes (CNTs), Graphene, and 2D Materials
5.1.2. Metal Nanoparticles and Conductive Polymers
5.2. Enhancing Sensitivity, Durability, and Biocompatibility
5.3. Role of Nanomaterials in Energy Harvesting Efficiency
6. Challenges and Limitations in EAP-Based Systems
6.1. Material Durability and Stability
6.2. Power Generation and Storage Efficiency
6.3. Signal Optimization and Noise Reduction
6.4. Scalability and Manufacturing Challenges
7. Clinical Translation and Future Perspectives
7.1. Pathways for the Clinical Adoption of EAP-Based Devices
7.2. Regulatory Considerations for Implantable and Wearable Devices
7.3. Emerging Trends in Personalized Medicine and Smart Healthcare
7.4. Future Research Directions for EAP-Driven Biomedical Technologies
8. Conclusions
8.1. Summary of Key Findings and Innovations
8.2. Impact on the Future of Biomedical Diagnostics and Healthcare Technologies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Yeung, K.-W.; Tang, C.-Y.; Law, W.-C.; Tsui, G.C.-P.; Xie, X. Development of Ionic Liquid-Based Electroactive Polymer Composites Using Nanotechnology. Nanotechnol. Rev. 2021, 10, 99–116. [Google Scholar] [CrossRef]
- Bar-Cohen, Y.; Anderson, I.A. Electroactive Polymer (EAP) Actuators—Background Review. Mech. Soft Mater. 2019, 1, 5. [Google Scholar] [CrossRef]
- Bar-Cohen, Y.; Sherrit, S.; Lih, S.-S. Characterization of the Electromechanical Properties of EAP Materials. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; Bar-Cohen, Y., Ed.; SPIE: Bellingham, DC, USA, 2001; p. 319. [Google Scholar]
- Yi, J.; Yang, S.; Yue, L.; Lei, I.M. Digital Light Processing 3D Printing of Flexible Devices: Actuators, Sensors and Energy Devices. Microsyst. Nanoeng. 2025, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Ghevondyan, M.; Davtyan, M.; Aghayan, M. Dielectric Elastomer Actuators: Medical Applications Review. Discov. Mater. 2025, 5, 43. [Google Scholar] [CrossRef]
- Enyan, M.; Bing, Z.; Amu-Darko, J.N.O.; Issaka, E.; Otoo, S.L.; Agyemang, M.F. Advances in Smart Materials Soft Actuators on Mechanisms, Fabrication, Materials, and Multifaceted Applications: A Review. J. Thermoplast. Compos. Mater. 2025, 38, 302–370. [Google Scholar] [CrossRef]
- Runsewe, D.; Betancourt, T.; Irvin, J.A. Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review. Materials 2019, 12, 2629. [Google Scholar] [CrossRef]
- De Rossi, D.; Carpi, F.; Galantini, F. Functional Materials for Wearable Sensing, Actuating and Energy Harvesting. Adv. Sci. Technol. 2009, 57, 247–256. [Google Scholar]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanović, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Carpi, F.; Smela, E. (Eds.) Biomedical Applications of Electroactive Polymer Actuators; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Alkahtani, M.E.; Elbadawi, M.; Chapman, C.A.R.; Green, R.A.; Gaisford, S.; Orlu, M.; Basit, A.W. Electroactive Polymers for On-Demand Drug Release. Adv. Healthc. Mater. 2024, 13, 2301759. [Google Scholar] [CrossRef]
- Jyothish, K.J.; Mishra, S. A Survey on Robotic Prosthetics: Neuroprosthetics, Soft Actuators, and Control Strategies. ACM Comput. Surv. 2024, 56, 1–44. [Google Scholar] [CrossRef]
- Dias, D.; Paulo Silva Cunha, J. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Ryoo, H.-Y.; Shin, B.-S. Sustainable Wearables: Wearable Technology for Enhancing the Quality of Human Life. Sustainability 2016, 8, 466. [Google Scholar] [CrossRef]
- Acharya, R.; Dutta, S.D.; Patil, T.V.; Ganguly, K.; Randhawa, A.; Lim, K.-T. A Review on Electroactive Polymer–Metal Composites: Development and Applications for Tissue Regeneration. J. Funct. Biomater. 2023, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Ankit; Ho, T.Y.K.; Nirmal, A.; Kulkarni, M.R.; Accoto, D.; Mathews, N. Soft Actuator Materials for Electrically Driven Haptic Interfaces. Adv. Intell. Syst. 2022, 4, 2100061. [Google Scholar] [CrossRef]
- Dobos, A.M.; Filimon, A. Role of Metal Ion Implantation on Ionic Polymer Metal Composite Membranes. In Ionic Polymer Metal Composites for Sensors and Actuators; Springer: Berlin/Heidelberg, Germany, 2019; pp. 53–73. [Google Scholar]
- Guarino, V.; Zuppolini, S.; Borriello, A.; Ambrosio, L. Electro-Active Polymers (EAPs): A Promising Route to Design Bio-Organic/Bioinspired Platforms with on Demand Functionalities. Polymers 2016, 8, 185. [Google Scholar] [CrossRef]
- Costa, C.M.; Cardoso, V.F.; Martins, P.; Correia, D.M.; Gonçalves, R.; Costa, P.; Correia, V.; Ribeiro, C.; Fernandes, M.M.; Martins, P.M.; et al. Smart and Multifunctional Materials Based on Electroactive Poly(Vinylidene Fluoride): Recent Advances and Opportunities in Sensors, Actuators, Energy, Environmental, and Biomedical Applications. Chem. Rev. 2023, 123, 11392–11487. [Google Scholar] [CrossRef]
- Yan, B. Actuators for Implantable Devices: A Broad View. Micromachines 2022, 13, 1756. [Google Scholar] [CrossRef]
- Roy, S.; Azad, A.N.M.W.; Baidya, S.; Alam, M.K.; Khan, F. Powering Solutions for Biomedical Sensors and Implants Inside the Human Body: A Comprehensive Review on Energy Harvesting Units, Energy Storage, and Wireless Power Transfer Techniques. IEEE Trans. Power Electron. 2022, 37, 12237–12263. [Google Scholar] [CrossRef]
- Dong, L.; Closson, A.B.; Jin, C.; Trase, I.; Chen, Z.; Zhang, J.X.J. Vibration-Energy-Harvesting System: Transduction Mechanisms, Frequency Tuning Techniques, and Biomechanical Applications. Adv. Mater. Technol. 2019, 4, 1900177. [Google Scholar] [CrossRef]
- Sohail, A.; Ali, A.; Shaukat, H.; Bhatti, F.M.; Ali, S.; Kouritem, S.A.; Noori, M.; Altabey, W.A. Integrating Self-Powered Medical Devices with Advanced Energy Harvesting: A Review. Energy Strateg. Rev. 2024, 52, 101328. [Google Scholar] [CrossRef]
- Khandelwal, G.; Dahiya, R. Self-Powered Active Sensing Based on Triboelectric Generators. Adv. Mater. 2022, 34, 2200724. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, H.; Shetty, D.; Wagih, M.; Ghasempour, Y.; Palazzi, V.; Carvalho, N.B.; Correia, R.; Costanzo, A.; Vital, D.; Alimenti, F.; et al. Next-Generation IoT Devices: Sustainable Eco-Friendly Manufacturing, Energy Harvesting, and Wireless Connectivity. IEEE J. Microw. 2023, 3, 237–255. [Google Scholar] [CrossRef]
- Shaukat, H.; Ali, A.; Bibi, S.; Mehmood, S.; Altabey, W.A.; Noori, M.; Kouritem, S.A. Piezoelectric Materials: Advanced Applications in Electro-Chemical Processes. Energy Rep. 2023, 9, 4306–4324. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, S.J.; Park, S.H.; Lee, J.; Kim, H.; Kim, M.K. Recent Progress in Development and Applications of Ionic Polymer-Metal Composite. Micromachines 2022, 13, 1290. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.-N.; Melvin, A.A.; Choi, J.-W. Stimuli-Responsive Polymer Actuator for Soft Robotics. Polymers 2024, 16, 2660. [Google Scholar] [CrossRef]
- Anikwe, C.V.; Nweke, H.F.; Ikegwu, A.C.; Egwuonwu, C.A.; Onu, F.U.; Alo, U.R.; Teh, Y.W. Mobile and Wearable Sensors for Data-Driven Health Monitoring System: State-of-the-Art and Future Prospect. Expert Syst. Appl. 2022, 202, 117362. [Google Scholar] [CrossRef]
- Du, F.; Wang, S.; Chen, Z.; Li, Q. Stimuli Responsive Actuators: Recent Advances. J. Mater. Chem. C 2024, 12, 8217–8242. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Cho, S.-H.; Roh, G.; Park, H.-J.; Lee, Y.-J.; Jeon, H.-E.; Lee, Y.-S.; Bae, S.-H.; Youn, S.B.; et al. Assessing the Impact of mRNA Vaccination in Chronic Inflammatory Murine Model. npj Vaccines 2024, 9, 34. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Enhancing Vaccine Efficacy and Stability: A Review of the Utilization of Nanoparticles in mRNA Vaccines. Biomolecules 2024, 14, 1036. [Google Scholar] [CrossRef]
- Linh, V.T.N.; Han, S.; Koh, E.; Kim, S.; Jung, H.S.; Koo, J. Advances in Wearable Electronics for Monitoring Human Organs: Bridging External and Internal Health Assessments. Biomaterials 2025, 314, 122865. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.-T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive Polymers for Sensing. Interface Focus 2016, 6, 20160026. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qin, Q.; Han, Z.; Plamthottam, R.; Possinger, M.; Pei, Q. Dielectric Elastomer Artificial Muscle Materials Advancement and Soft Robotic Applications. SmartMat 2023, 4, e1203. [Google Scholar] [CrossRef]
- Kang, W.; Ji, G.; Huber, J.E. Mechanical Energy Harvesting: From Piezoelectric Effect to Ferroelectric/Ferroelastic Switching. Nano Energy 2025, 133, 110489. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Deng, L.; Zhu, X.; Xu, C.; Xie, L.; Yang, Q.; Zhang, H. Efficient Electrical Energy Conversion Strategies from Triboelectric Nanogenerators to Practical Applications: A Review. Nano Energy 2024, 132, 110383. [Google Scholar] [CrossRef]
- Olvera, D.; Monaghan, M.G. Electroactive Material-Based Biosensors for Detection and Drug Delivery. Adv. Drug Deliv. Rev. 2021, 170, 396–424. [Google Scholar] [CrossRef]
- Wang, L.; Yao, X.; Zhang, Y.; Luo, G.; Wang, B.; Yu, X. Progress and Perspectives of Self-Powered Gas Sensors. Next Mater. 2024, 2, 100092. [Google Scholar] [CrossRef]
- He, T.; Guo, X.; Lee, C. Flourishing Energy Harvesters for Future Body Sensor Network: From Single to Multiple Energy Sources. iScience 2021, 24, 101934. [Google Scholar] [CrossRef]
- de Marzo, G.; Mastronardi, V.M.; Todaro, M.T.; Blasi, L.; Antonaci, V.; Algieri, L.; Scaraggi, M.; De Vittorio, M. Sustainable Electronic Biomaterials for Body-Compliant Devices: Challenges and Perspectives for Wearable Bio-Mechanical Sensors and Body Energy Harvesters. Nano Energy 2024, 123, 109336. [Google Scholar] [CrossRef]
- Kanaan, A.F.; Pinho, A.C.; Piedade, A.P. Electroactive Polymers Obtained by Conventional and Non-Conventional Technologies. Polymers 2021, 13, 2713. [Google Scholar] [CrossRef]
- Rahman, M.H.; Werth, H.; Goldman, A.; Hida, Y.; Diesner, C.; Lane, L.; Menezes, P.L. Recent Progress on Electroactive Polymers: Synthesis, Properties and Applications. Ceramics 2021, 4, 516–541. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Zang, W.; Jiang, Y.; Ning, N.; Tian, M. Fabrication of Multi-Layer Stacked Dielectric Elastomer Actuator with High Output Force by Co-Crosslinking of Electrode with DE Substrate. Compos. Commun. 2024, 47, 101874. [Google Scholar] [CrossRef]
- Almarri, N.; Chang, J.; Song, W.; Jiang, D.; Demosthenous, A. Piezoelectric Energy Harvesting and Ultra-Low-Power Management Circuits for Medical Devices. Nano Energy 2024, 131, 110196. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Park, S.; Mandal, T.K.; Joo, S.W. Innovative Wearable Electronics: Next-Generation Nitrogen-Doped Lutetium-Carbon Microspheres Composites for Robust Energy Harvesting. Small 2025, 21, 2407386. [Google Scholar] [CrossRef]
- Kumar, V.; Parvin, N.; Joo, S.W.; Mandal, T.K.; Park, S.S. Great Carbon Nano Materials Based Composites for Electronic Skin: Intelligent Sensing, and Self-Powered Nano Generators. Nano Energy 2025, 137, 110805. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Ren, Z.; Chang, L.; Xu, X.; Hu, Y. Endowing Actuators with Sensing Capability: Recent Progress on Perceptive Soft Actuators. Chem. Eng. J. 2024, 479, 147550. [Google Scholar] [CrossRef]
- Sarker, A.; Ul Islam, T.; Islam, M.R. A Review on Recent Trends of Bioinspired Soft Robotics: Actuators, Control Methods, Materials Selection, Sensors, Challenges, and Future Prospects. Adv. Intell. Syst. 2025, 7, 2400414. [Google Scholar] [CrossRef]
- Dewang, Y.; Sharma, V.; Baliyan, V.K.; Soundappan, T.; Singla, Y.K. Research Progress in Electroactive Polymers for Soft Robotics and Artificial Muscle Applications. Polymers 2025, 17, 746. [Google Scholar] [CrossRef]
- Bates, T.J.; Fergason, J.R.; Pierrie, S.N. Technological Advances in Prosthesis Design and Rehabilitation Following Upper Extremity Limb Loss. Curr. Rev. Musculoskelet. Med. 2020, 13, 485–493. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Lee, P.S. Functional Fibers and Fabrics for Soft Robotics, Wearables, and Human–Robot Interface. Adv. Mater. 2021, 33, 2002640. [Google Scholar] [CrossRef]
- Banyai, A.D.; Brișan, C. Robotics in Physical Rehabilitation: Systematic Review. Healthcare 2024, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lu, Z.; Hu, C.; Gao, Y.; Zhu, J.; Hu, W. Research Progress on Conductive Polymer Actuators: Mechanisms, Performance Improvement, and Applications. Mater. Today Commun. 2024, 41, 110828. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Zhang, X. Recent Progress in Preparation Process of Ionic Polymer-Metal Composites. Results Phys. 2021, 29, 104800. [Google Scholar] [CrossRef]
- Preethichandra, D.M.G.; Piyathilaka, L.; Sul, J.-H.; Izhar, U.; Samarasinghe, R.; Arachchige, S.D.; de Silva, L.C. Passive and Active Exoskeleton Solutions: Sensors, Actuators, Applications, and Recent Trends. Sensors 2024, 24, 7095. [Google Scholar] [CrossRef]
- Bhowmick, R.; Biswas, P.; Chattopadhyaya, M.; Sen, S. Graphene Based Elastomeric Composite Sensors. In Encyclopedia of Materials: Plastics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 656–662. [Google Scholar]
- Pan, M.; Liu, M.; Lei, J.; Wang, Y.; Linghu, C.; Bowen, C.; Hsia, K.J. Bioinspired Mechanisms and Actuation of Soft Robotic Crawlers. Adv. Sci. 2025, 12, 2416764. [Google Scholar] [CrossRef]
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Wang, S.; Zhang, S.; Liu, Q.; Wang, X.; Wang, G.; Zhang, Y.; et al. Carbon Nanotubes: A Powerful Bridge for Conductivity and Flexibility in Electrochemical Glucose Sensors. J. Nanobiotechnol. 2023, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Parvin, N.; Mishra, K.; Mohandoss, S.; Lee, Y.R. Sensitive and Selective Fluorometric Determination of DNA by Using Layered Hexagonal Nanosheets of a Covalent Organic Framework Prepared from p-Phenylenediamine and Benzene-1,3,5-Tricarboxaldehyde. Microchim. Acta 2019, 186, 833. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Yin, H.; Guo, M. A New Generation of Sensors for Non-Invasive Blood Glucose Monitoring. Am. J. Transl. Res. 2023, 15, 3825–3837. [Google Scholar]
- Ding, Y.; Yang, L.; Wen, J.; Ma, Y.; Dai, G.; Mo, F.; Wang, J. A Comprehensive Review of Advanced Lactate Biosensor Materials, Methods, and Applications in Modern Healthcare. Sensors 2025, 25, 1045. [Google Scholar] [CrossRef]
- Ma, J.; Li, H.; Anwer, S.; Umer, W.; Antwi-Afari, M.F.; Xiao, E.B. Evaluation of Sweat-Based Biomarkers Using Wearable Biosensors for Monitoring Stress and Fatigue: A Systematic Review. Int. J. Occup. Saf. Ergon. 2024, 30, 677–703. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, L.; Chen, X.; Wu, W. Smart Wearable Systems for Health Monitoring. Sensors 2023, 23, 2479. [Google Scholar] [CrossRef] [PubMed]
- Clementi, G.; Neri, I.; Cottone, F.; Di Michele, A.; Mattarelli, M.; Sforna, L.; Chiappalupi, S.; Sorci, G.; Michelucci, A.; Catacuzzeno, L.; et al. Self-Powered Temperature Sensors Harnessing Membrane Potential of Living Cells. Nano Energy 2024, 121, 109211. [Google Scholar] [CrossRef]
- Arab Hassani, F.; Shi, Q.; Wen, F.; He, T.; Haroun, A.; Yang, Y.; Feng, Y.; Lee, C. Smart Materials for Smart Healthcare—Moving from Sensors and Actuators to Self-Sustained Nanoenergy Nanosystems. Smart Mater. Med. 2020, 1, 92–124. [Google Scholar] [CrossRef]
- Park, H.; Park, W.; Lee, C.H. Electrochemically Active Materials and Wearable Biosensors for the In Situ Analysis of Body Fluids for Human Healthcare. NPG Asia Mater. 2021, 13, 23. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring Towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Lakshmanan, K.; Liu, B.M. Impact of Point-of-Care Testing on Diagnosis, Treatment, and Surveillance of Vaccine-Preventable Viral Infections. Diagnostics 2025, 15, 123. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.-Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Manov, A.E.; Chauhan, S.; Dhillon, G.; Dhaliwal, A.; Antonio, S.; Donepudi, A.; Jalal, Y.N.; Nazha, J.; Banal, M.; House, J. The Effectiveness of Continuous Glucose Monitoring Devices in Managing Uncontrolled Diabetes Mellitus: A Retrospective Study. Cureus 2023, 15, e42545. [Google Scholar] [CrossRef]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, S.; Qin, Y.; Xia, X.; Sun, Y.; Han, G.; Shu, T.; Hu, L.; Zhang, Q. Flexible and Wearable Biosensors for Monitoring Health Conditions. Biosensors 2023, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.; Solomon, S.; Gao, W. Flexible Electronics and Devices as Human-Machine Interfaces for Medical Robotics. Adv. Mater. 2022, 34, e2107902. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, E.; Naseri, Z.; Deigner, H.-P.; Rahimi, H.; Altintas, Z. Approaches of Wearable and Implantable Biosensor Towards of Developing in Precision Medicine. Front. Med. 2024, 11, 1390634. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Chen, J. Wearable Biosensors for Non-Invasive Sweat Diagnostics. Biosensors 2021, 11, 245. [Google Scholar] [CrossRef]
- Wityk, P.; Terebieniec, A.; Nowak, R.; Łubiński, J.; Mroczyńska-Szeląg, M.; Wityk, T.; Kostrzewa-Nowak, D. Reusable Biosensor for Easy RNA Detection from Unfiltered Saliva. Sensors 2025, 25, 360. [Google Scholar] [CrossRef]
- Hosain, M.N.; Kwak, Y.-S.; Lee, J.; Choi, H.; Park, J.; Kim, J. IoT-Enabled Biosensors for Real-Time Monitoring and Early Detection of Chronic Diseases. Phys. Act. Nutr. 2024, 28, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Rheima, A.M.; Al-Sharify, Z.T.; Mohaimeed, A.A.; Kazem, M.A.A.-H.; Dhabab, J.M.; Athair, D.M.; Joseph, T.M.; Mahapatra, D.K.; Thomas, S.; Kianfar, E. Nano Biosensors: Classification, Electrochemistry, Nanostructures, and Optical Properties. Results Eng. 2024, 24, 103428. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, R.; Peng, I.; Xu, Z.; Jiang, Y. Wearable and Implantable Biosensors: Mechanisms and Applications in Closed-Loop Therapeutic Systems. J. Mater. Chem. B 2024, 12, 8577–8604. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Kwon, S.H.; Dong, L. Advancements in Flexible Biomechanical Energy Harvesting for smart Health Applications. Chem. Commun. 2025, 61, 2424–2449. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and Flexible Electrochemical Sensors for Sweat Analysis: A Review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef]
- Dong, C.; Ji, Y.; Fu, Z.; Qi, Y.; Yi, T.; Yang, Y.; Sun, Y.; Sun, H. Precision Management in Chronic Disease: An AI Empowered Perspective on Medicine-Engineering Crossover. iScience 2025, 28, 112044. [Google Scholar] [CrossRef]
- Sin, M.L.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and Challenges in Biosensor-Based Diagnosis of Infectious Diseases. Expert Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef]
- Mia, A.K.; Meyyappan, M.; Giri, P.K. Two-Dimensional Transition Metal Dichalcogenide Based Biosensors: From Fundamentals to Healthcare Applications. Biosensors 2023, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Torop, J.; Peikolainen, A.-L.; Aabloo, A.; Koel, M.; Asaka, K.; Baughman, R. Electrochemically Driven Carbon-Based Materials as EAPs: Fundamentals and Device Configurations. In Electromechanically Active Polymers; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.-S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Yim, Y.-J.; Yoon, Y.-H.; Kim, S.-H.; Lee, J.-H.; Chung, D.-C.; Kim, B.-J. Carbon Nanotube/Polymer Composites for Functional Applications. Polymers 2025, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Alekseyev, N.I.; Khmelnitskiy, I.K.; Aivazyan, V.M.; Broyko, A.P.; Korlyakov, A.V.; Luchinin, V.V. Ionic EAP Actuators with Electrodes Based on Carbon Nanomaterials. Polymers 2021, 13, 4137. [Google Scholar] [CrossRef]
- Moreira, J.; Fernandes, M.M.; Carvalho, E.O.; Nicolau, A.; Lazic, V.; Nedeljković, J.M.; Lanceros-Mendez, S. Exploring Electroactive Microenvironments in Polymer-Based Nanocomposites to Sensitize Bacterial Cells to Low-Dose Embedded Silver Nanoparticles. Acta Biomater. 2022, 139, 237–248. [Google Scholar] [CrossRef]
- Imran, M.; Ahmed, S.; Abdullah, A.Z.; Hakami, J.; Chaudhary, A.A.; Rudayni, H.A.; Khan, S.; Khan, A.; Basher, N.S. Nanostructured Material-Based Optical and Electrochemical Detection of Amoxicillin Antibiotic. Luminescence 2023, 38, 1064–1086. [Google Scholar] [CrossRef]
- Singh, N.K.; Takashima, K.; Pandey, S.S. Enhancement in Capacitance of Ionic Type of EAP-Based Strain Sensors. Sensors 2023, 23, 9400. [Google Scholar] [CrossRef]
- Shang, J.; Zhou, C.; Jiang, C.; Huang, X.; Liu, Z.; Zhang, H.; Zhao, J.; Liang, W.; Zeng, B. Recent Developments in Nanomaterials for Upgrading Treatment of Orthopedics Diseases. Front. Bioeng. Biotechnol. 2023, 11, 1221365. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2020, 9, 1901287. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Gao, S.; Peng, S.; Shi, L.; Shah, S.P.; Li, W. Graphene Reinforced Cement-Based Triboelectric Nanogenerator for Efficient Energy Harvesting in Civil Infrastructure. Nano Energy 2024, 131, 110380. [Google Scholar] [CrossRef]
- Soto, D.; Orozco, J. Hybrid Nanobioengineered Nanomaterial-Based Electrochemical Biosensors. Molecules 2022, 27, 3841. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Wang, X.; Sun, X.; Li, Z. Enhanced Output Performance Piezoelectric Nanogenerators Based on Highly Polarized PVDF/TBAHP Tree-like Nanofiber Membranes for Energy Harvesting. Polymer 2024, 293, 126681. [Google Scholar] [CrossRef]
- Paramshetti, S.; Angolkar, M.; Al Fatease, A.; Alshahrani, S.M.; Hani, U.; Garg, A.; Ravi, G.; Osmani, R.A.M. Revolutionizing Drug Delivery and Therapeutics: The Biomedical Applications of Conductive Polymers and Composites-Based Systems. Pharmaceutics 2023, 15, 1204. [Google Scholar] [CrossRef]
- Perera, O.; Liyanapathirana, R.; Gargiulo, G.; Gunawardana, U. A Review of Soft Robotic Actuators and Their Applications in Bioengineering, with an Emphasis on HASEL Actuators’ Future Potential. Actuators 2024, 13, 524. [Google Scholar] [CrossRef]
- Rafiefard, N.; Fardindoost, S.; Kisomi, M.K.; Shooshtari, L.; Irajizad, A.; Seddighi, S.; Mohammadpour, R.; Vashaee, D. High-Performance Flexible and Stretchable Self-Powered Surface Engineered PDMS-TiO2 Nanocomposite Based Humidity Sensors Driven by Triboelectric Nanogenerator with Full Sensing Range. Sens. Actuators B Chem. 2023, 378, 133105. [Google Scholar] [CrossRef]
- Julius, A.; Malakondaiah, S.; Pothireddy, R.B. Polymer and Nanocomposite Fillers as Advanced Materials in Biomedical Applications. Nano Trends 2025, 9, 100087. [Google Scholar] [CrossRef]
- White, B.T.; Long, T.E. Advances in Polymeric Materials for Electromechanical Devices. Macromol. Rapid Commun. 2019, 40, 1800521. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. Latest Advances in Flexible Symmetric Supercapacitors: From Material Engineering to Wearable Applications. Acc. Chem. Res. 2020, 53, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Kumar, V.; Park, S.-S.; Mandal, T.K.; Joo, S.W. Enhanced Piezoelectric Energy Harvesting Using Hybrid Composites of MWCNTs and Partially-Reduced GO in RTV-SR for Stable Voltage Generation. Surf. Interfaces 2024, 44, 103681. [Google Scholar] [CrossRef]
- Parvin, N.; Merum, D.; Mandal, T.K.; Joo, S.W. Tunable Synthesis of Bimetallic Hybrid Multishelled Hollow Structure for High-Performance Aqueous Alkaline Batteries. J. Energy Storage 2023, 71, 108195. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Y.; Zhang, J.; Foroughi, J.; Peng, S.; Baughman, R.H.; Wang, Z.L.; Wang, C.H. Advanced Energy Harvesters and Energy Storage for Powering Wearable and Implantable Medical Devices. Adv. Mater. 2024, 36, 2404492. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Tong, Q.; Shan, B.; He, L.; Zhang, Y.; Wang, D. Active Electronic Skin: An Interface Towards Ambient Haptic Feedback on Physical Surfaces. npj Flex. Electron. 2024, 8, 25. [Google Scholar] [CrossRef]
- Chen, M.; Yazdani, M.; Murugappan, K. Non-Destructive Pest Detection: Innovations and Challenges in Sensing Airborne Semiochemicals. ACS Sens. 2024, 9, 5728–5747. [Google Scholar] [CrossRef]
- Rao, C.H.; Avinash, K.; Varaprasad, B.K.S.V.L.; Goel, S. A Review on Printed Electronics with Digital 3D Printing: Fabrication Techniques, Materials, Challenges and Future Opportunities. J. Electron. Mater. 2022, 51, 2747–2765. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Bodaghi, M. A Review of Recent Manufacturing Technologies for Sustainable Soft Actuators. Int. J. Precis. Eng. Manuf. Technol. 2023, 10, 1661–1710. [Google Scholar] [CrossRef]
- Chen, S.; Tong, X.; Huo, Y.; Liu, S.; Yin, Y.; Tan, M.; Cai, K.; Ji, W. Piezoelectric Biomaterials Inspired by Nature for Applications in Biomedicine and Nanotechnology. Adv. Mater. 2024, 36, 2406192. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for Renewable Energy Applications: A Review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Jekateryńczuk, G.; Piotrowski, Z. A Survey of Sound Source Localization and Detection Methods and Their Applications. Sensors 2023, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, D.; Wang, K.; Li, Z.; Liu, S.; Peng, L.; Yang, D. Evolutionary Manufacturing Approaches for Advancing Flexible Perovskite Solar Cells. Joule 2024, 8, 944–969. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoo, S.; Kim, S.H.; Kim, Y.-M.; Han, S.I.; Lee, H. Nature-Inspired Surface Modification Strategies for Implantable Devices. Mater. Today Bio 2025, 31, 101615. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Rani, S.; Khandelwal, G.; Kumar, S.; Pillai, S.C.; Stylios, G.K.; Gadegaard, N.; Mulvihill, D.M. Flexible Self-Powered Supercapacitors Integrated with Triboelectric Nanogenerators. Energy Storage Mater. 2025, 74, 103977. [Google Scholar] [CrossRef]
- Oh, S.; Jekal, J.; Liu, J.; Kim, J.; Park, J.; Lee, T.; Jang, K. Bioelectronic Implantable Devices for Physiological Signal Recording and Closed-Loop Neuromodulation. Adv. Funct. Mater. 2024, 34, 2403562. [Google Scholar] [CrossRef]
- Frisch, E.; Clavier, L.; Belhamdi, A.; Vrana, N.E.; Lavalle, P.; Frisch, B.; Heurtault, B.; Gribova, V. Preclinical In Vitro Evaluation of Implantable Materials: Conventional Approaches, New Models and Future Directions. Front. Bioeng. Biotechnol. 2023, 11, 1193204. [Google Scholar] [CrossRef]
- Kaushal, J.B.; Raut, P.; Kumar, S. Organic Electronics in Biosensing: A Promising Frontier for Medical and Environmental Applications. Biosensors 2023, 13, 976. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, T.; Yao, Y.; Xu, L.; Zhao, Z.; Wang, Z.L. Stimulating Acrylic Elastomers by a Triboelectric Nanogenerator—Toward Self-Powered Electronic Skin and Artificial Muscle. Adv. Funct. Mater. 2016, 26, 4906–4913. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Kenny, E.E. Personalized Medicine and the Power of Electronic Health Records. Cell 2019, 177, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.V.; Ramasubramanian, B.; Sequerth, O.; Pilla, S.; Wang, T.; Mohanty, A.K.; Govindaraj, P.; Alhassan, S.M.; Salim, N.; Kingshott, P.; et al. Nanomaterial Advanced Smart Coatings: Emerging Trends Shaping the Future. Appl. Mater. Today 2025, 42, 102574. [Google Scholar] [CrossRef]
- Liza, L.; Kabir, M.H.; Jiang, L.; Jerrams, S.; Chen, S. The Technology of Wearable Flexible Textile-Based Strain Sensors for Monitoring Multiple Human Motions: Construction, Patterning and Performance. Sens. Diagn. 2023, 2, 1414–1436. [Google Scholar] [CrossRef]
- Mensah, G.A.; Czajkowski, S.M. Translational Science Matters: Forging Partnerships Between Biomedical and Behavioral Science to Advance the Public’s Health. Transl. Behav. Med. 2018, 8, 808–814. [Google Scholar] [CrossRef]
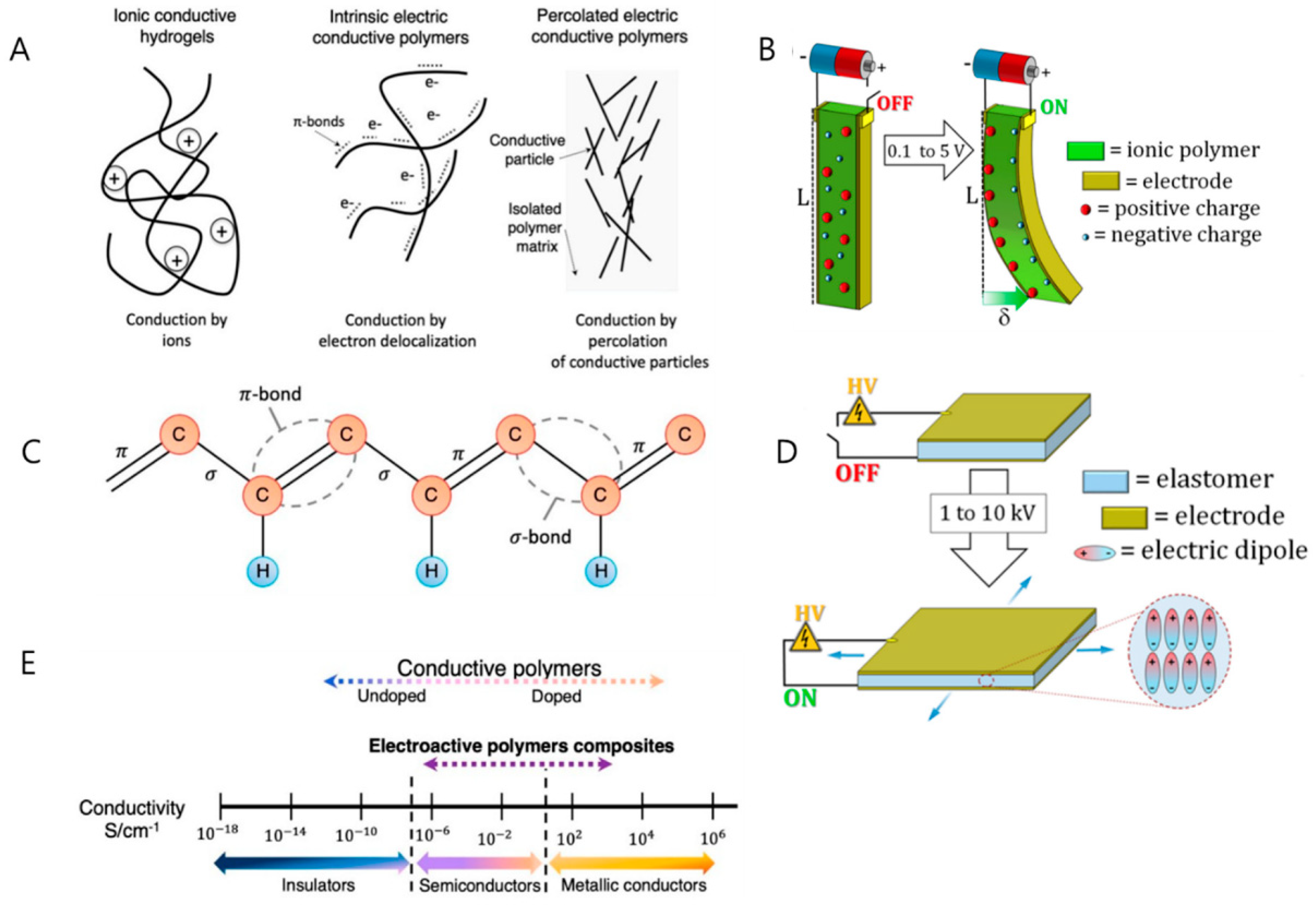
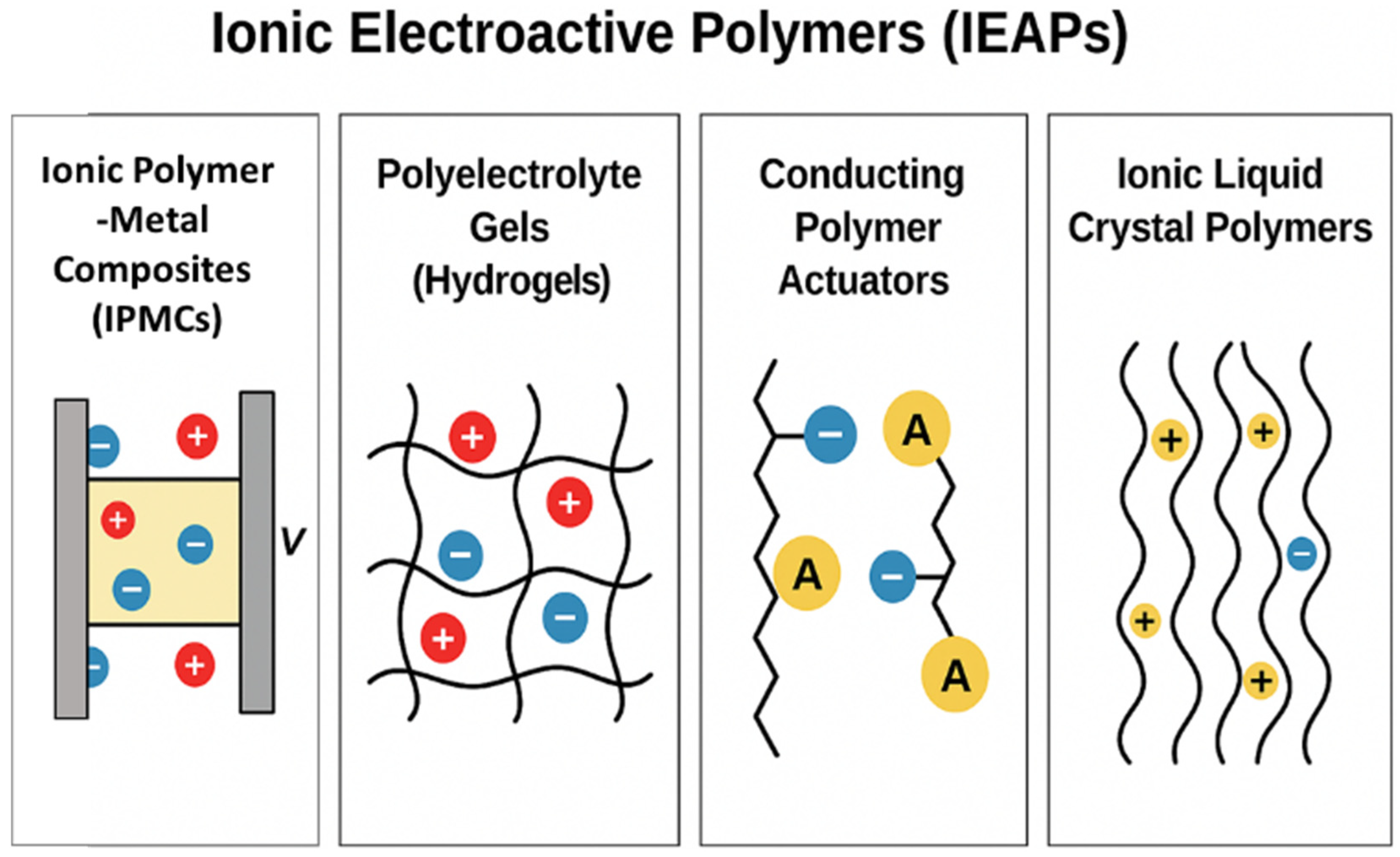


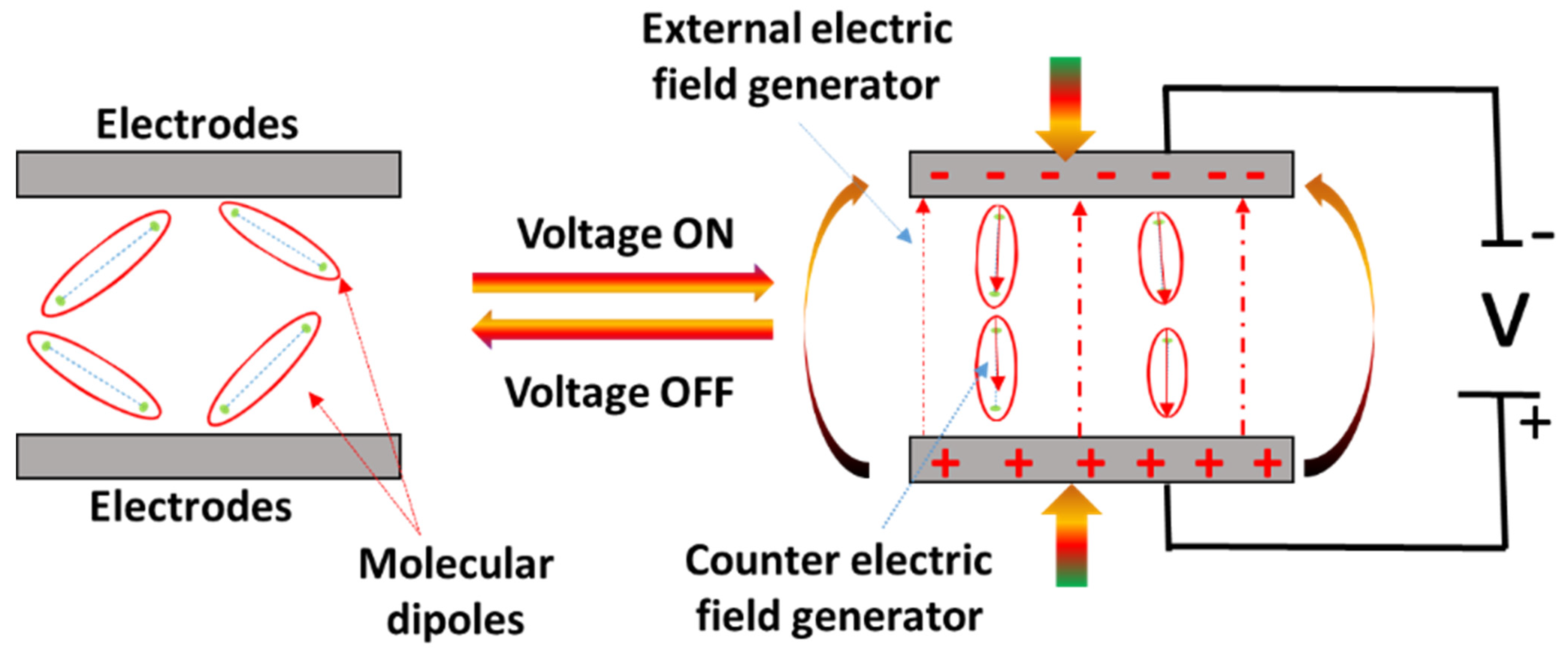
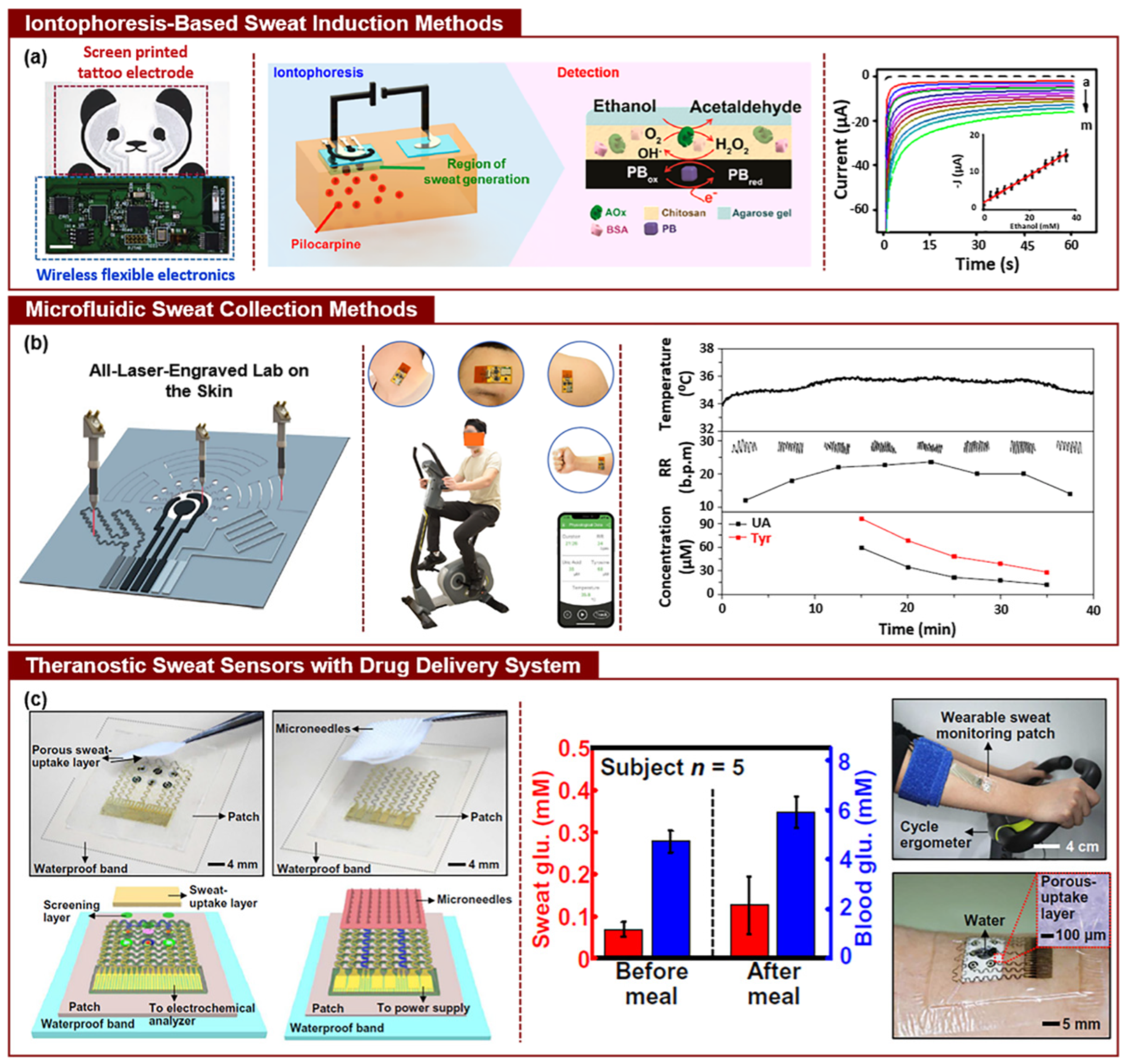
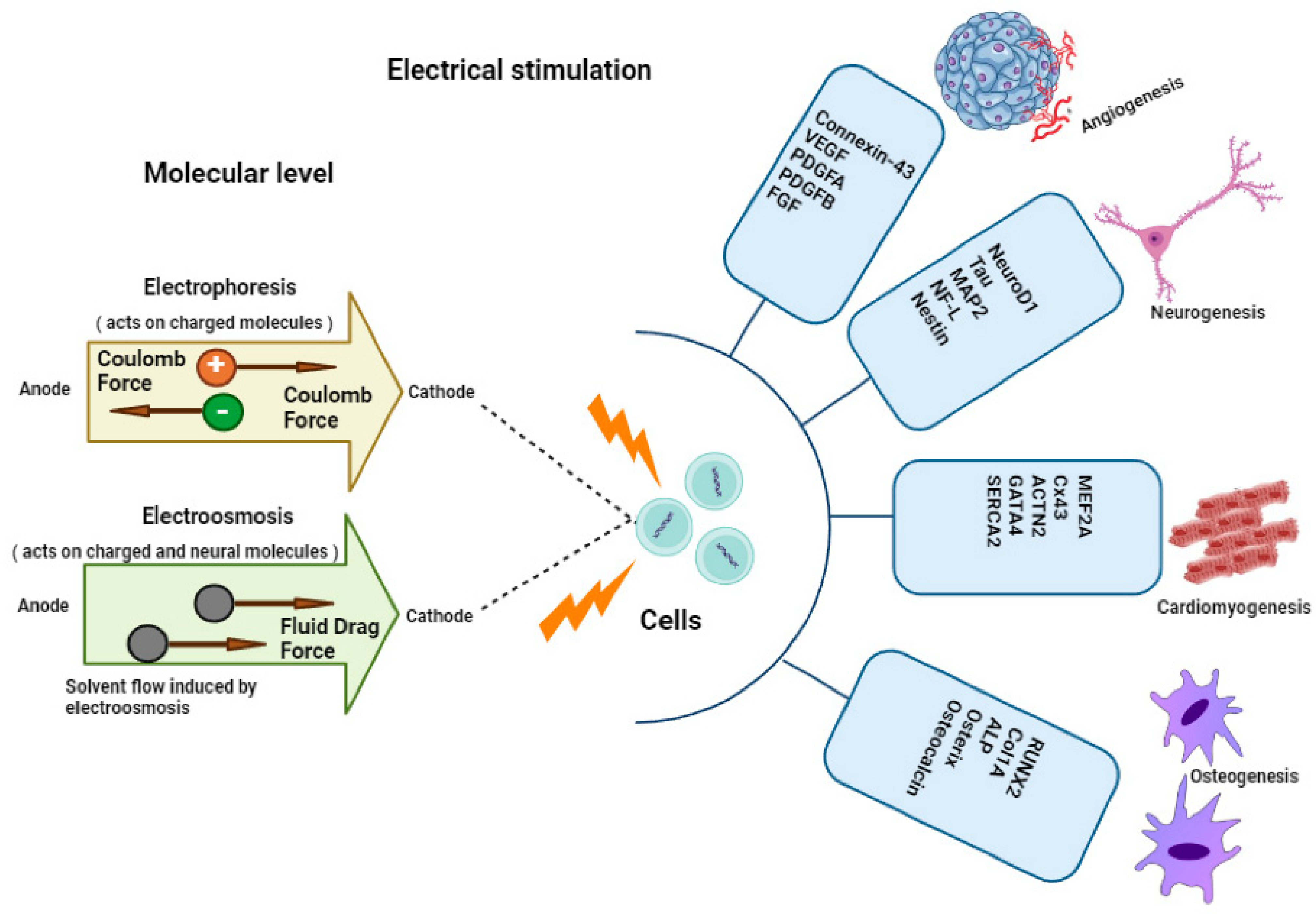

| Category | Type of EAP | Energy Harvesting Mechanism | Key Advantages for Biomedical Applications | Representative Polymer Structure | Ref. |
|---|---|---|---|---|---|
| Electroactive polymers (EAPs) | Ionic EAPs (IPMCs, hydrogels) | Ion movement within the polymer matrix due to applied voltage | - Soft, flexible, and biocompatible - Low-power operation - Suitable for sensing and actuating functions | Nafion (–[CF2–CF(CF3)]– SO3−H+) or hydrogel matrix (e.g., polyacrylamide) | [35] |
| Electronic EAPs (dielectric elastomers) | Electrical charge redistribution in response to an electric field | - High actuation strain - Large deformation - Suitable for prosthetics and artificial muscles | PDMS (–[Si(CH3)2–O]–) or VHB acrylic elastomer | [36] | |
| Energy harvesting mechanisms | Piezoelectricity | Generation of electrical charge due to mechanical stress/deformation | - Harvests energy from body motion - Suitable for low-frequency mechanical energy harvesting | PVDF (–[CH2–CF2]–) (β-phase) | [37] |
| Triboelectricity | Friction-induced charge generation between two materials with different electron affinities | - Effective for capturing ambient mechanical energy - Can be integrated into wearable systems | PTFE (–[CF2–CF2]–) with Nylon or PDMS pairs | [38] | |
| Ionic conductivity-based mechanism | Ion migration within the polymer matrix driven by applied voltage or stress | - High sensitivity for ionic concentration detection - Useful for biosensing applications | Poly(3,4-ethylenedioxythiophene):PEDOT or Polypyrrole (PPy) | [39] | |
| Advantages of self-powered systems | Autonomy and continuous operation | No need for external power sources or frequent battery replacements | - Ensures continuous, long-term monitoring of health data - Eliminates dependence on battery replacement | PEDOT:PSS or PANI blends | [40] |
| Sustainability | Energy harvesting from ambient sources (body movement and environmental energy) | - Reduces environmental impact - Provides sustainable power for biomedical systems | Ecoflex elastomer with embedded conductive fillers | [41] | |
| Biocompatibility and flexibility | Soft, stretchable materials that conform to the body’s surface | - Comfortable and non-invasive for the patient - Ideal for implantable and wearable medical devices | Gelatin methacrylate (GelMA), PU | [42] | |
| Minimization of external devices | Eliminates the need for bulky external power supplies | - Reduces size and weight of medical devices - Ideal for everyday use in continuous monitoring systems | CNT-based PANI/PVDF composite films | [34] |
| Actuator Type | Actuation Mechanism | Representative Materials | Typical Strain (%) | Operating Voltage | Response Time | Biomedical Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Dielectric elastomer (DEAP) | Electrostatic (Coulombic force) | VHB 4910, silicone, and polyacrylate | 100–300 | 1–5 kV | <1 ms | Artificial muscles, cardiac compression sleeves, and soft prosthetics | [55,56] |
| Ionic polymer–metal composite (IPMC) | Ionic migration (bending) | Nafion and Pt/Au electrodes | 5–15 | 1–5 V | ~0.1–1 s | Micropumps, artificial cilia, and smart catheters | [56] |
| Conductive polymer (CP) | Electrochemical actuation | Polypyrrole (PPy) and Polyaniline (PANI) | 2–10 | 1–2 V | 1–10 s | Drug delivery pumps and neural interface actuators | [5] |
| Carbon nanotube (CNT) yarn actuator | Electrothermal/electrochemical | CNTs with electrolyte-infused matrices | 1–10 | <2 V | <1 s | Prosthetic fingers and biohybrid muscles | [57] |
| Graphene-based EAPs | Electrostatic or hybrid | Graphene oxide–elastomer composites | 20–100 | <500 V | ~ms | Wearable exosuits, soft grippers, and implantable biosensors | [58] |
| Hydrogel ionic EAPs | Osmotic/ionic swelling | Polyacrylamideand alginate-based hydrogels | 10–50 | 1–3 V | 0.5–5 s | Artificial muscles, biohybrid robots, and tissue engineering scaffolds | [59] |
| Application Area | Sensing Mechanism | Target Biomarkers/Function | Sensitivity/Detection Limit | Operating Conditions | Limitations | Ref. |
|---|---|---|---|---|---|---|
| General biosensing | Piezoelectricity, ionic conductivity, and electrochemical response | Converts physiological signals into electrical output | Up to 350 µA/mM·cm2; detection limits ~0.1 µM–10 µM | 10 Hz–1 kHz; skin temperature; ambient humidity | Signal drift, limited power, and material degradation | [39] |
| Biomarker detection | Ion exchange and electrochemical redox reaction | Glucose, lactate, electrolytes, and cortisol | 110–320 µA/mM·cm2 (depending on target); LOD ~0.5 µM (lactate) | Sweat-based sensing; normal pH ~5.5–7.0 | Selectivity in complex fluids and interference | [20] |
| Real-time health monitoring | Ionic conduction and piezoelectricity | Continuous analyte sensing (glucose and lactate) | Continuous tracking; dynamic range up to 20 mM | Sweat, ISF, and breath; temperature 32–37 °C | Accuracy affected by motion and sweat variability | [75] |
| Flexible electronics integration | Mechanical–electrical transduction | Energy harvesting and real-time biosensing | Power density ~3–5 µW/cm2 (TENG-based) | Stretchable, wearable skin patch format | Low energy conversion efficiency | [76] |
| Wearable and implantable devices | Triboelectricity and EAP deformation | Smart patches, skin electronics, and glucose sensors | Specificity > 95%, sensitivity varies by fluid | Implanted under skin or adhered on epidermis | Biocompatibility, encapsulation, and long-term stability | [77] |
| Non-invasive monitoring | Breath, sweat, and ISF sampling with EAP films | pH, Na+, K+, and glucose | Electrochemical sensors: ~120 µA/mM·cm2; LOD < 1 µM | Temperature-dependent; humidity-sensitive | Requires calibration, environmental interferences | [78] |
| Point-of-care (PoC) diagnostics | Self-powered electrochemical sensing | Infection markers, glucose, and inflammation | Rapid response (<5 min); LOD ~1–10 µM | No external power; portable conditions | Data variability and limited analyte coverage | [79] |
| Personalized medicine | Continuous real-time feedback loop | Patient-specific biomarker trends | Depends on algorithm and biosensor combo | Wearable/implantable platforms | Privacy, algorithm bias, and power management | [80] |
| Topic | Nanocomposites and Nanostructured EAPs | Carbon Nanotubes (CNTs), Graphene, and 2D Materials | Metal Nanoparticles and Conductive Polymers | Enhancing Sensitivity, Durability, and Biocompatibility | Role of Nanomaterials in Energy Harvesting Efficiency | Ref. |
|---|---|---|---|---|---|---|
| Key nanomaterials involved | Nanofillers (e.g., CNTs, graphene, and metal nanoparticles) integrated into electroactive polymer matrices | Carbon nanotubes (CNTs), graphene, and 2D materials (MoS2, TMDs) | Metal nanoparticles (Au, Ag, and Cu) and conductive polymers (PPy, PANI) | Nanomaterials (e.g., CNTs and graphene) enhance sensitivity, durability, and biocompatibility | CNTs, graphene, and metal nanoparticles enhance piezoelectric and triboelectric properties | [99] |
| Mechanical properties | Improved flexibility and mechanical strength | Exceptional mechanical strength, flexibility, and tensile properties | Enhanced mechanical properties and flexibility of EAPs | Improved mechanical durability under repeated deformation | Enhanced mechanical performance with improved energy harvesting ability | [31] |
| Electrical conductivity | Improved electrical conductivity and charge distribution | High conductivity, ideal for piezoelectric and triboelectric properties | Improved conductivity through nanostructured fillers | Enhanced electrical properties increase sensor and actuator performance | Increased conductivity improves energy conversion efficiency | [100] |
| Biocompatibility | Potential biocompatibility issues with certain fillers | Graphene and CNTs demonstrate good biocompatibility for biomedical use | Biocompatibility of gold nanoparticles and conductive polymers is well established | Reduced inflammatory responses, suitable for implantable devices | Biocompatibility ensures safe use in wearable and implantable applications | [101] |
| Application areas | Used for soft robotics, biohybrids, and smart biomedical devices | Used in sensors, actuators, wearable electronics, and biohybrid systems | Applied in glucose sensors, biosensors, and implantable electronics | Ideal for long-term health monitoring devices and non-invasive biosensors | Used in self-powered actuators and biosensors for continuous monitoring | [102] |
| Performance improvements | Enhanced actuation and sensor performance due to improved properties | High piezoelectric and triboelectric properties for energy harvesting | Increased sensitivity and response times for biosensing applications | Enhanced long-term durability, with superior sensor performance | Improved energy harvesting efficiency leads to longer operational times | [103] |
| Challenges | Dispersion and alignment of nanomaterials and stability issues | Potential cytotoxicity of CNTs and graphene and scalability issues | Conductive polymers may degrade over time and metal nanoparticle leaching | Ensuring long-term stability and biocompatibility in vivo | Ensuring efficiency over long-term use and under dynamic biomechanical conditions | [104] |
| Challenges and Limitations | Key Issues | Recent Advances/Research | Ref. |
|---|---|---|---|
| Material durability and stability | Long-term performance and biocompatibility concerns. EAPs degrade over time due to mechanical stress and environmental factors. Nanomaterial integration may introduce toxicity. | Development of surface modifications, protective coatings, and new stable materials. Use of biocompatible coatings and nanomaterials for improved safety. | [115] |
| Power generation and storage efficiency | Energy harvesting mechanisms often have limited output and efficiency. Integrating efficient energy storage systems is challenging. | Advances in energy storage, such as supercapacitors and flexible batteries. Optimization of energy harvesting systems for continuous operation. | [116] |
| Signal optimization and noise reduction | High sensitivity of EAP sensors leads to noise and interference, affecting accuracy. Environmental disturbances can impact sensor readings. | Advanced signal processing techniques, such as filtering and noise cancelation. Hybrid systems with smart materials for improved performance. | [117] |
| Scalability and manufacturing challenges | Difficulty in large-scale production and maintaining uniformity. High cost of fabrication. | Scalable manufacturing techniques such as roll-to-roll printing. Development of cost-effective production methods and optimized polymer synthesis. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms. Actuators 2025, 14, 257. https://doi.org/10.3390/act14060257
Parvin N, Joo SW, Jung JH, Mandal TK. Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms. Actuators. 2025; 14(6):257. https://doi.org/10.3390/act14060257
Chicago/Turabian StyleParvin, Nargish, Sang Woo Joo, Jae Hak Jung, and Tapas Kumar Mandal. 2025. "Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms" Actuators 14, no. 6: 257. https://doi.org/10.3390/act14060257
APA StyleParvin, N., Joo, S. W., Jung, J. H., & Mandal, T. K. (2025). Electroactive Polymers for Self-Powered Actuators and Biosensors: Advancing Biomedical Diagnostics Through Energy Harvesting Mechanisms. Actuators, 14(6), 257. https://doi.org/10.3390/act14060257








