Design, Fabrication, and Experimental Validation of Optical Microbots
Abstract
1. Introduction
2. Design of Microbots
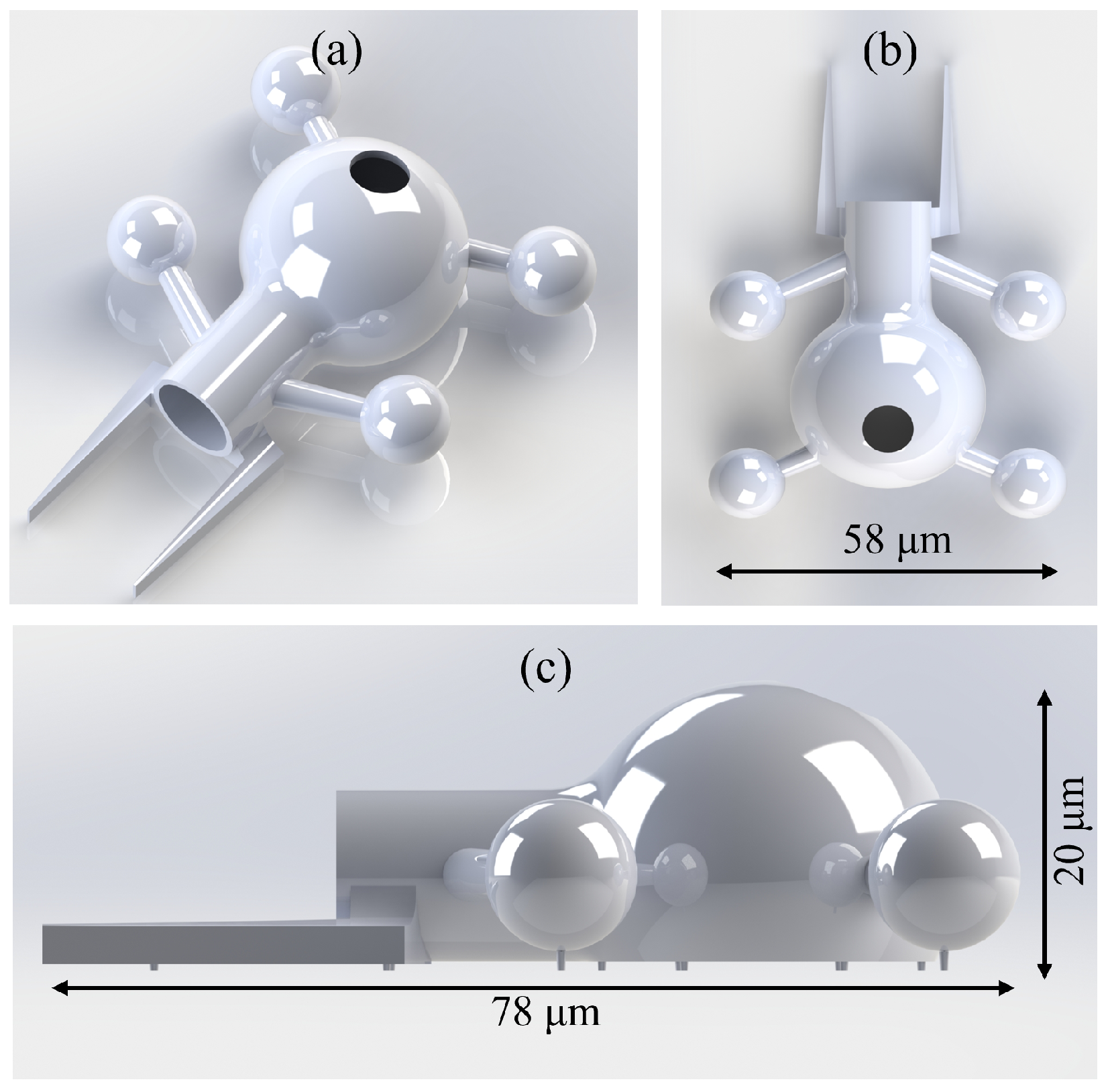
2.1. Design Version 1
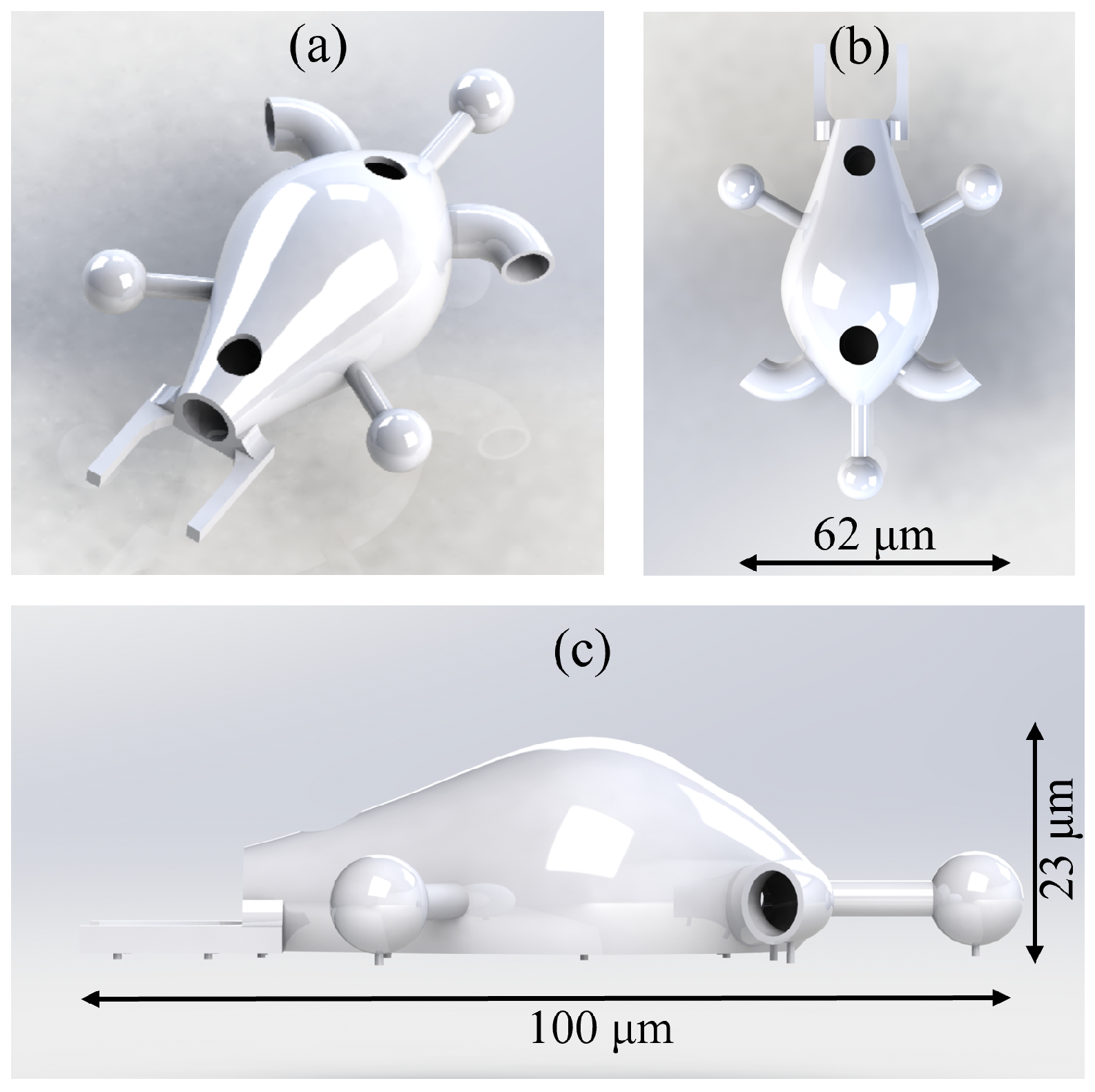
2.2. Design Version 2
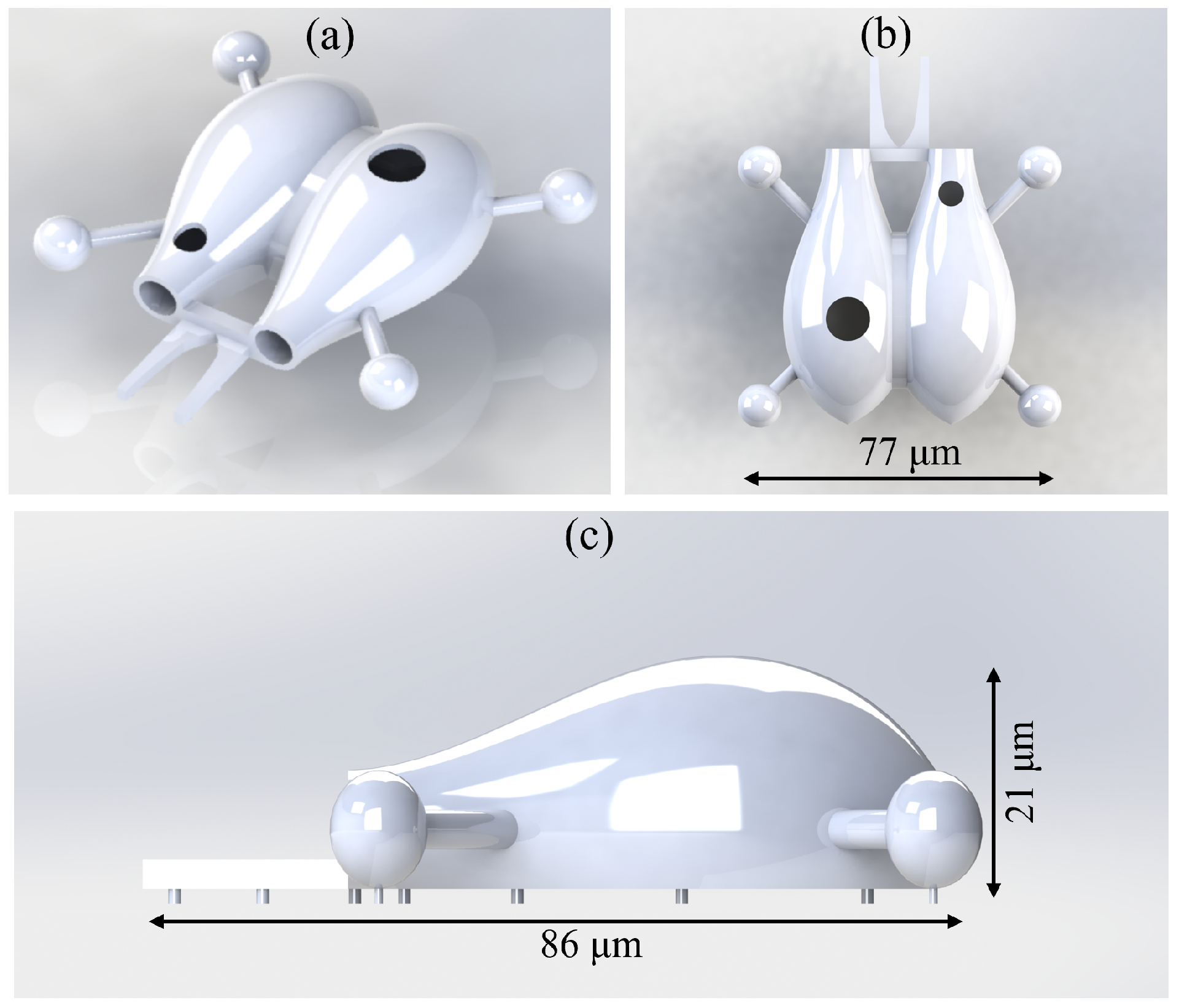
2.3. Design Version 3
3. Multiphysics Simulations
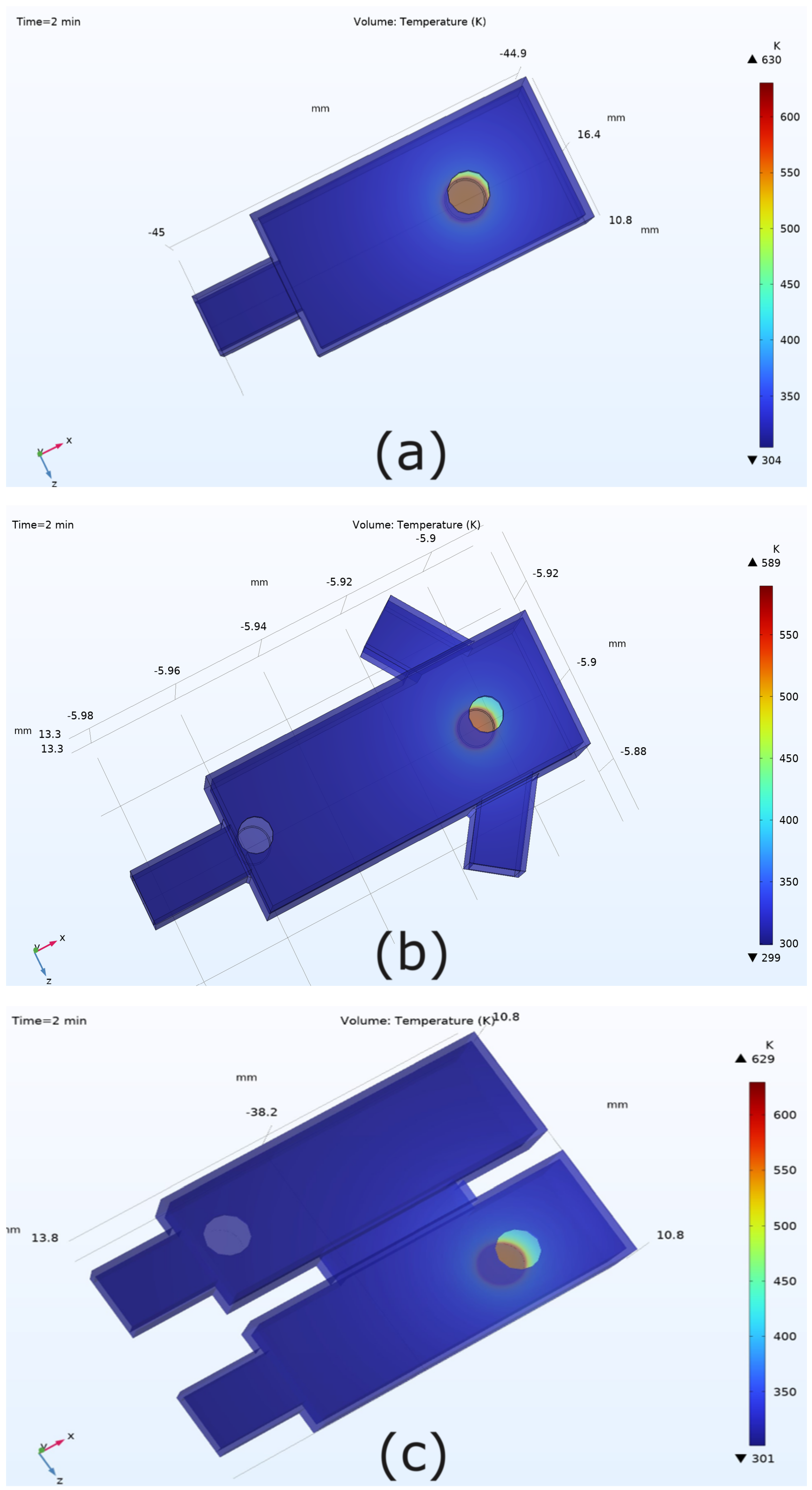
3.1. Temperature Distribution
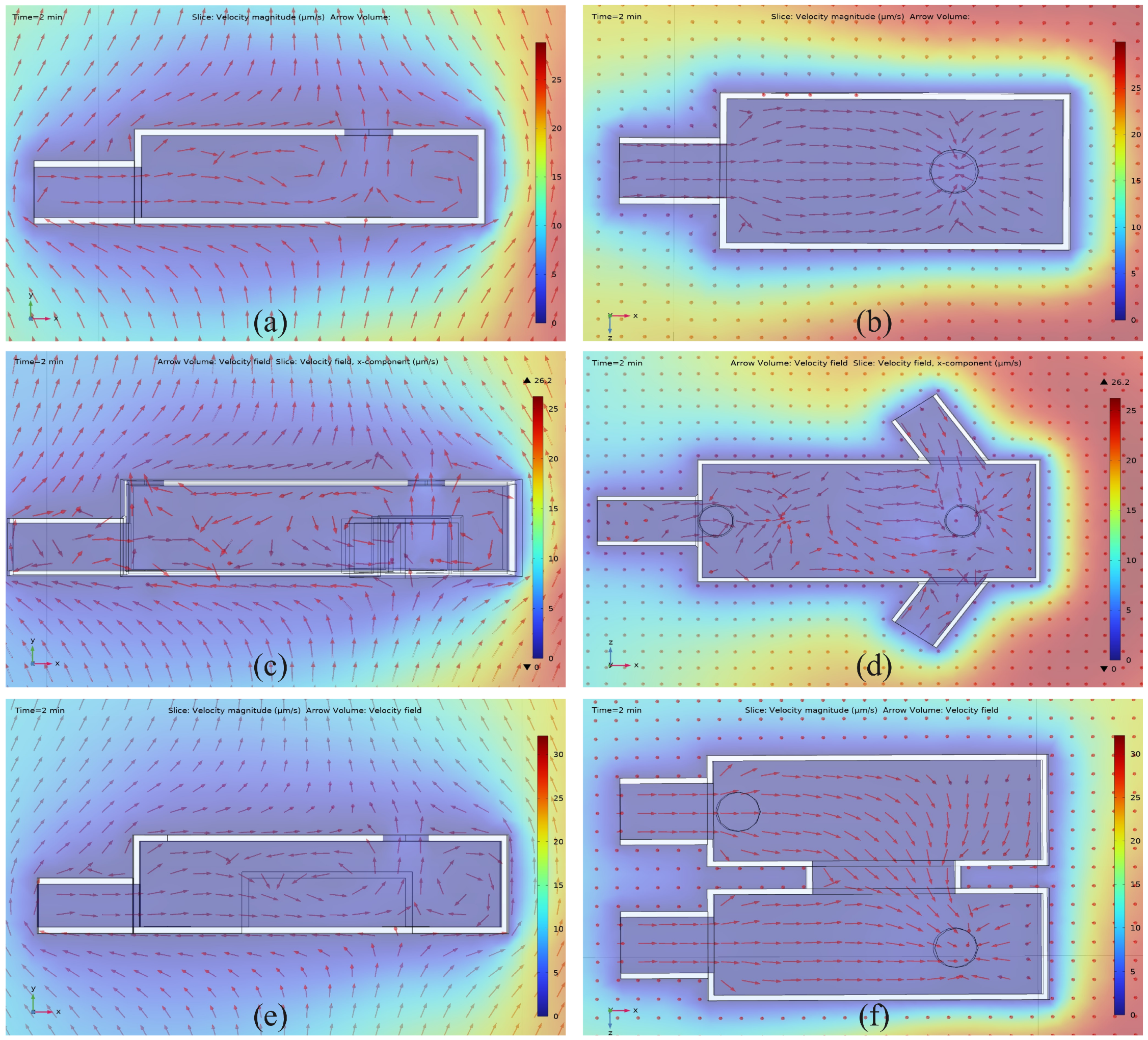
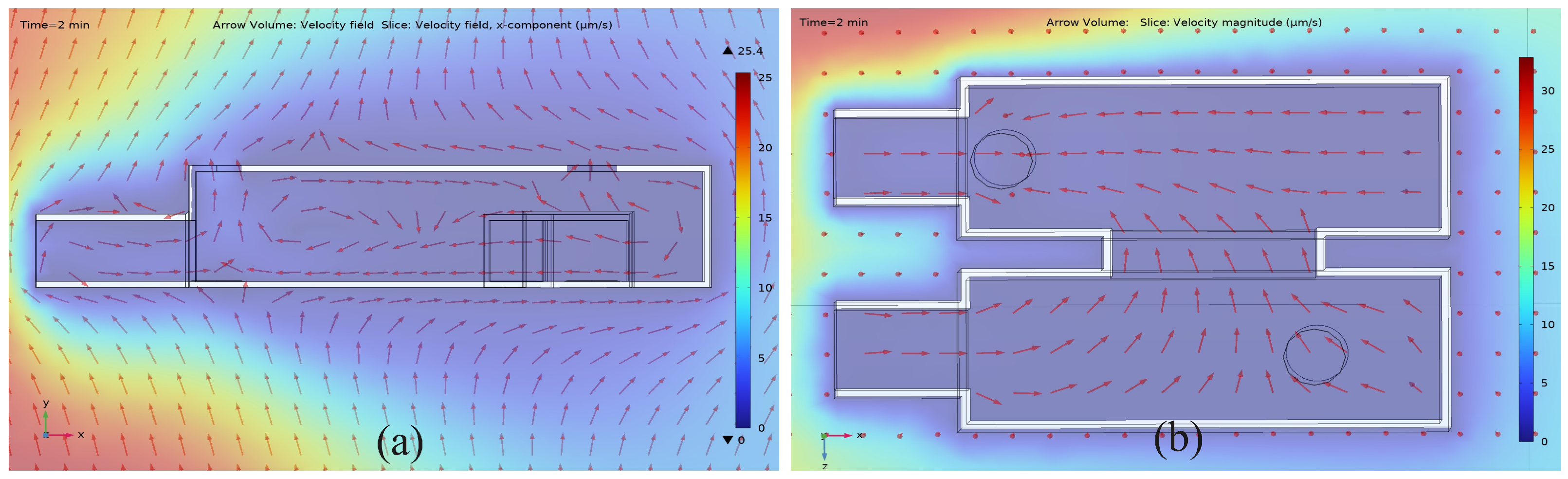
3.2. Microfluidic Flow Velocity Distribution
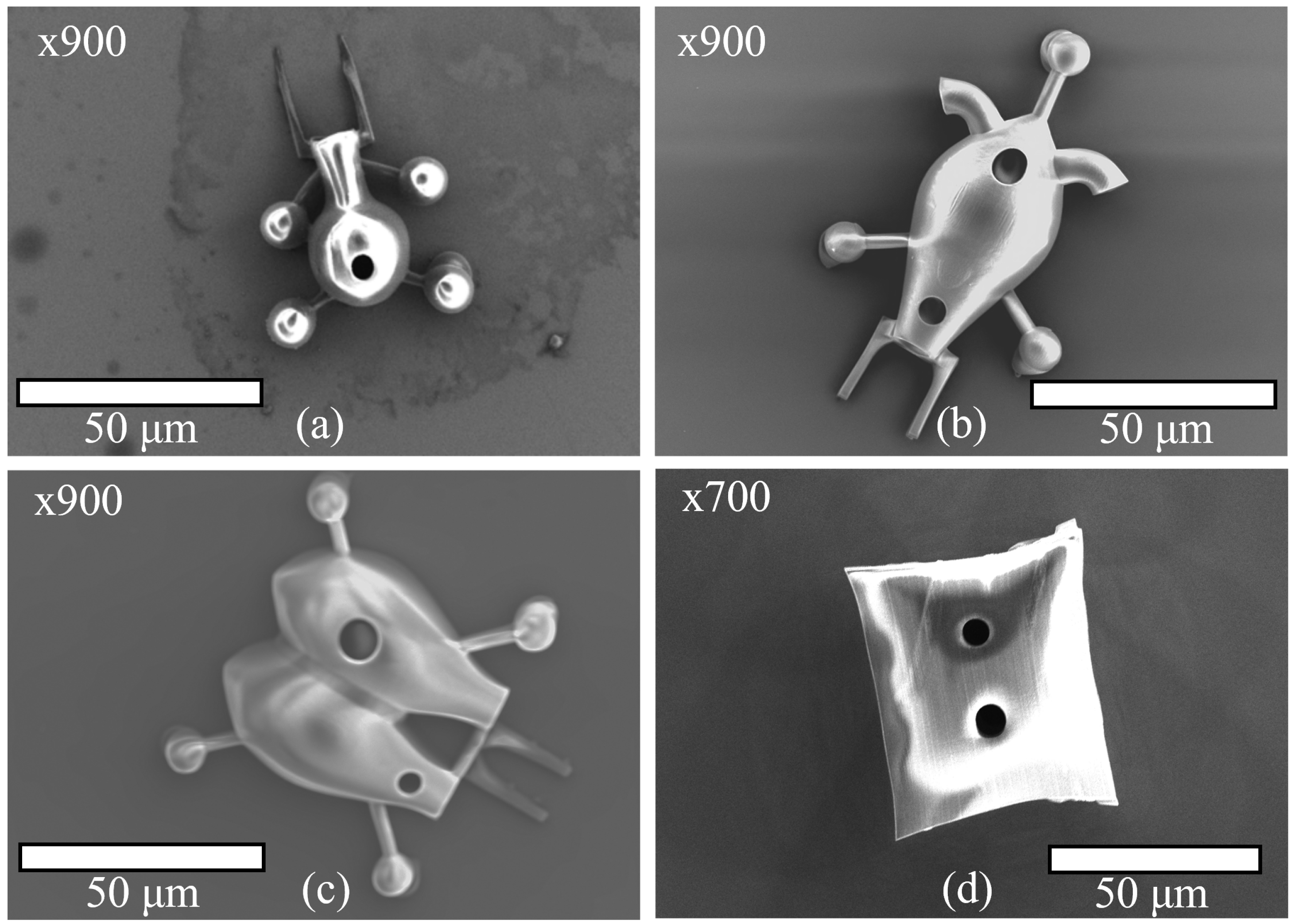
4. Microbot Fabrication
5. Experimental Results
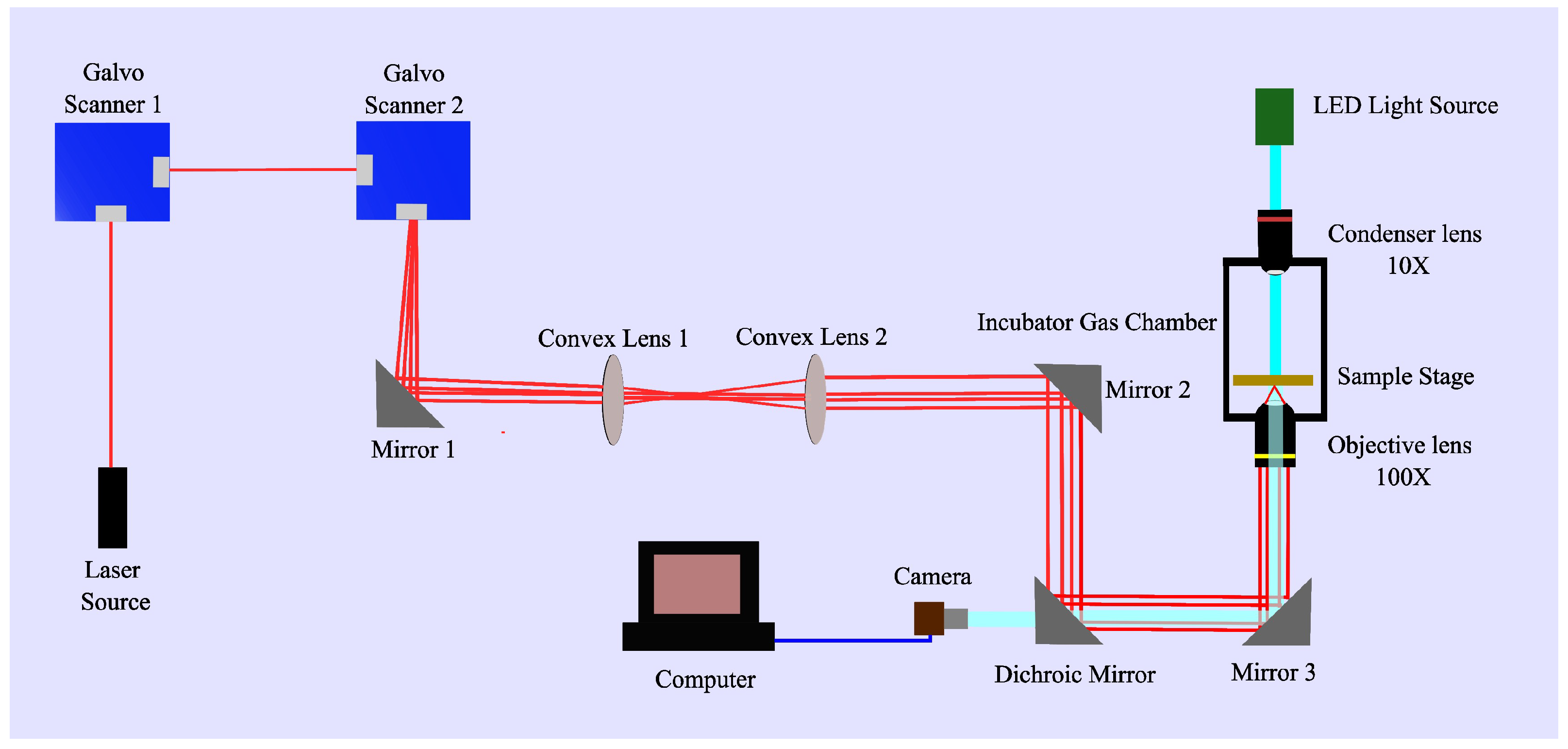
5.1. Experimental Setup
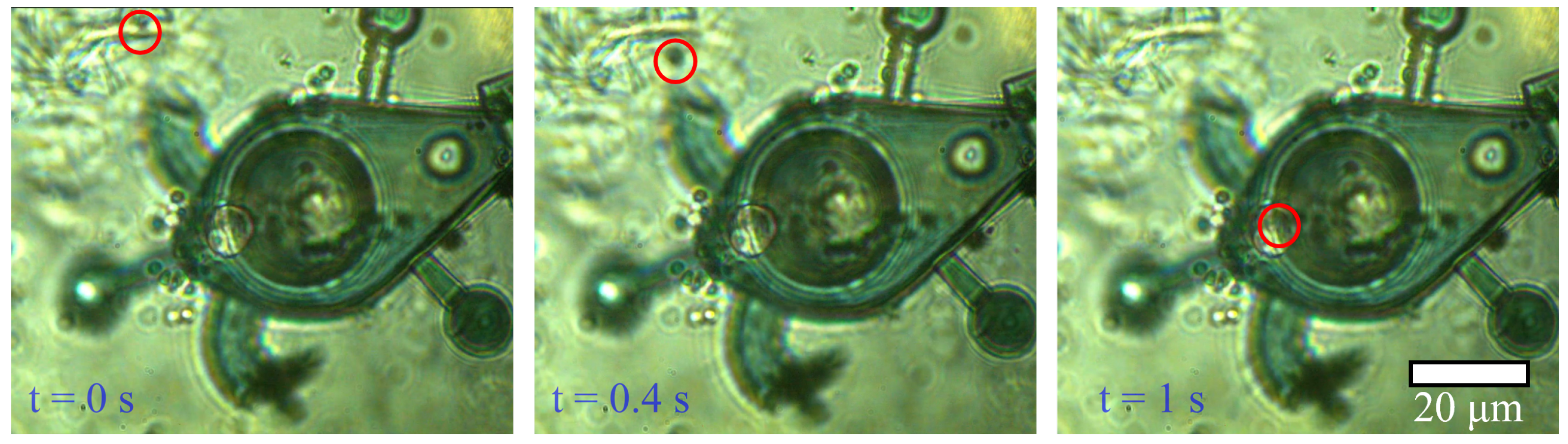
5.2. Microbot Extraction
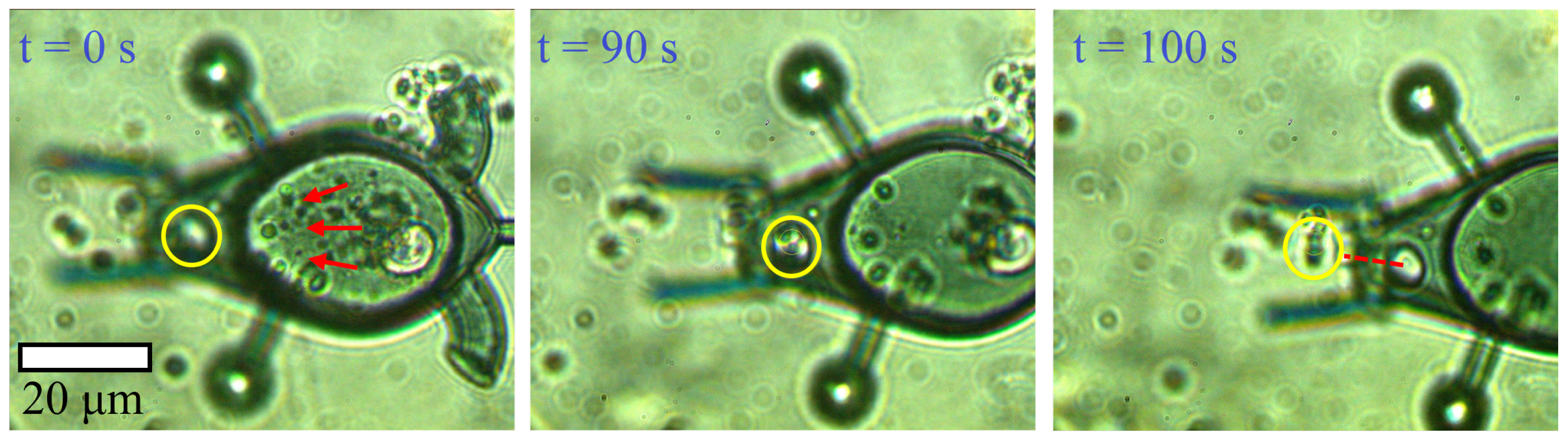
5.3. Laser-Induced Microparticle Loading and Unloading
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konara, K.; Amarasinghe, Y. Design and Simulation of a 4-DoF Vibratory Gyroscope. In Proceedings of the 2021 Moratuwa Engineering Research Conference (MERCon), Virtual, 27–29 July 2021; pp. 728–733. [Google Scholar]
- Perera, K.; Premachandra, H.; Amarasinghe, Y. Design of a Magnetostrictive Bimorph for Micromanipulation. In Proceedings of the 14th International Research Conference of the General Sir John Kotelawala Defence University, Dehiwala-Mount Lavinia, Sri Lanka, 9–10 September 2021. [Google Scholar]
- Paul, K.; Mallick, D.; Roy, S. Performance improvement of MEMS electromagnetic vibration energy harvester using optimized patterns of micromagnet arrays. IEEE Magn. Lett. 2021, 12, 2101805. [Google Scholar] [CrossRef]
- Cho, M.O.; Jang, W.; Lim, S.H. Fabrication and evaluation of a flexible MEMS-based microthermal flow sensor. Sensors 2021, 21, 8153. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.; Saitta, L.; Ongaro, A.E.; Cicala, G.; Kersaudy-Kerhoas, M.; Bucolo, M. Advanced technologies in the fabrication of a micro-optical light splitter. Micro 2023, 3, 338–352. [Google Scholar] [CrossRef]
- Mudugamuwa, A.; Roshan, U.; Hettiarachchi, S.; Cha, H.; Musharaf, H.; Kang, X.; Trinh, Q.T.; Xia, H.M.; Nguyen, N.T.; Zhang, J. Periodic Flows in Microfluidics. Small 2024, 20, 2404685. [Google Scholar] [CrossRef]
- Jamil, M.F.; Pokharel, M.; Park, K. Dexterous In-plane Manipulation of Light-controlled Microbots in Fluids. IEEE Robot. Autom. Lett. 2023, 8, 5600–5607. [Google Scholar] [CrossRef]
- Purcell, E.M. Life at low Reynolds number. In Physics and Our World: Reissue of the Proceedings of a Symposium in Honor of Victor F Weisskopf; World Scientific: Singapore, 2014; pp. 47–67. [Google Scholar]
- Qiu, T.; Gibbs, J.G.; Schamel, D.; Mark, A.G.; Choudhury, U.; Fischer, P. From nanohelices to magnetically actuated microdrills: A universal platform for some of the smallest untethered microrobotic systems for low Reynolds number and biological environments. In Proceedings of the Small-Scale Robotics, From Nano-to-Millimeter-Sized Robotic Systems and Applications: First International Workshop at ICRA 2013, Karlsruhe, Germany, 6 May 2013; Revised and Extended Papers 1. Springer: Berlin/Heidelberg, Germany, 2014; pp. 53–65. [Google Scholar]
- Lim, S.; Du, Y.; Lee, Y.; Panda, S.K.; Tong, D.; Jawed, M.K. Fabrication, control, and modeling of robots inspired by flagella and cilia. Bioinspiration Biomim. 2022, 18, 011003. [Google Scholar] [CrossRef]
- Palagi, S.; Fischer, P. Bioinspired microrobots. Nat. Rev. Mater. 2018, 3, 113–124. [Google Scholar] [CrossRef]
- Jamil, M.F.; Pokharel, M.; Park, K. Design and fabrication of untethered light-actuated microbots in fluid for biomedical applications. Appl. Mech. 2022, 3, 1240–1253. [Google Scholar] [CrossRef]
- Konara, M.; Mudugamuwa, A.; Dodampegama, S.; Roshan, U.; Amarasinghe, R.; Dao, D.V. Formation Techniques Used in Shape-Forming Microrobotic Systems with Multiple Microrobots: A Review. Micromachines 2022, 13, 1987. [Google Scholar] [CrossRef]
- Dodampegama, S.; Mudugamuwa, A.; Konara, M.; Perera, N.; De Silva, D.; Roshan, U.; Amarasinghe, R.; Jayaweera, N.; Tamura, H. A review on the motion of magnetically actuated bio-inspired microrobots. Appl. Sci. 2022, 12, 11542. [Google Scholar] [CrossRef]
- Han, K.; Shields, C.W., IV; Velev, O.D. Engineering of self-propelling microbots and microdevices powered by magnetic and electric fields. Adv. Funct. Mater. 2018, 28, 1705953. [Google Scholar] [CrossRef]
- Erdem, E.Y.; Chen, Y.M.; Mohebbi, M.; Suh, J.W.; Kovacs, G.T.; Darling, R.B.; Böhringer, K.F. Thermally actuated omnidirectional walking microrobot. J. Microelectromech. Syst. 2010, 19, 433–442. [Google Scholar] [CrossRef]
- Nocentini, S.; Parmeggiani, C.; Martella, D.; Wiersma, D.S. Optically driven soft micro robotics. Adv. Opt. Mater. 2018, 6, 1800207. [Google Scholar] [CrossRef]
- Ge, Q.; Li, Z.; Wang, Z.; Kowsari, K.; Zhang, W.; He, X.; Zhou, J.; Fang, N.X. Projection micro stereolithography based 3D printing and its applications. Int. J. Extrem. Manuf. 2020, 2, 022004. [Google Scholar] [CrossRef]
- Ertugrul, I. The fabrication of micro beam from photopolymer by digital light processing 3D printing technology. Micromachines 2020, 11, 518. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Piqué, A.; Charipar, K.M. Laser-induced forward transfer applications in micro-engineering. In Handbook of Laser Micro-and Nano-Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1325–1359. [Google Scholar]
- Bunea, A.I.; del Castillo Iniesta, N.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D printing by two-photon polymerization: Configurations and parameters for the nanoscribe system. Micro 2021, 1, 164–180. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Sarabi, M.R.; Birtek, M.T.; Seyfi, S.; Sitti, M.; Tasoglu, S. 3D-printed microrobots from design to translation. Nat. Commun. 2022, 13, 5875. [Google Scholar] [CrossRef]
- Carlotti, M.; Mattoli, V. Functional materials for two-photon polymerization in microfabrication. Small 2019, 15, 1902687. [Google Scholar] [CrossRef]
- Lee, J.G.; Raj, R.R.; Day, N.B.; Shields, C.W., IV. Microrobots for biomedicine: Unsolved challenges and opportunities for translation. ACS Nano 2023, 17, 14196–14204. [Google Scholar] [CrossRef]
- Jamil, M.F.; Pokharel, M.; Park, K. Light-controlled microbots in biomedical application: A review. Appl. Sci. 2022, 12, 11013. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Elsayed, M.; Nan, F.; Liang, W.; Valley, J.K.; Liu, L.; Huang, Q.; Wu, M.C.; Wheeler, A.R. Optoelectronic tweezers: A versatile toolbox for nano-/micro-manipulation. Chem. Soc. Rev. 2022, 51, 9203–9242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Min, C.; Dou, X.; Wang, X.; Urbach, H.P.; Somekh, M.G.; Yuan, X. Plasmonic tweezers: For nanoscale optical trapping and beyond. Light Sci. Appl. 2021, 10, 59. [Google Scholar] [CrossRef]
- Bradac, C. Nanoscale optical trapping: A review. Adv. Opt. Mater. 2018, 6, 1800005. [Google Scholar] [CrossRef]
- Ashkin, A.; Dziedzic, J.M.; Bjorkholm, J.E.; Chu, S. Observation of a single-beam gradient force optical trap for dielectric particles. Opt. Lett. 1986, 11, 288–290. [Google Scholar] [CrossRef]
- Villangca, M.J.; Palima, D.; Banas, A.R.; Glückstad, J. Light-driven micro-tool equipped with a syringe function. Light Sci. Appl. 2016, 5, e16148. [Google Scholar] [CrossRef]
- Rivas, D.; Mallick, S.; Sokolich, M.; Das, S. Cellular manipulation using rolling microrobots. In Proceedings of the 2022 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Toronto, ON, Canada, 25–29 July 2022; pp. 1–6. [Google Scholar]
- Hu, S.; Xie, H.; Wei, T.; Chen, S.; Sun, D. Automated indirect transportation of biological cells with optical tweezers and a 3D printed microtool. Appl. Sci. 2019, 9, 2883. [Google Scholar] [CrossRef]
- Shishkin, I.; Markovich, H.; Roichman, Y.; Ginzburg, P. Auxiliary optomechanical tools for 3d cell manipulation. Micromachines 2020, 11, 90. [Google Scholar] [CrossRef]
- Avci, E.; Grammatikopoulou, M.; Yang, G.Z. Laser-printing and 3D optical-control of untethered microrobots. Adv. Opt. Mater. 2017, 5, 1700031. [Google Scholar] [CrossRef]
- Gao, W.; Bai, Y.; Yang, Y.; Jia, L.; Mi, Y.; Cui, W.; Liu, D.; Shakoor, A.; Zhao, L.; Li, J.; et al. Intelligent sensing for the autonomous manipulation of microrobots toward minimally invasive cell surgery. Appl. Phys. Rev. 2024, 11, 041302. [Google Scholar] [CrossRef]
- Hayakawa, T.; Fukada, S.; Arai, F. Fabrication of an on-chip nanorobot integrating functional nanomaterials for single-cell punctures. IEEE Trans. Robot. 2013, 30, 59–67. [Google Scholar] [CrossRef]
- Lekka, M.; Pogoda, K.; Gostek, J.; Klymenko, O.; Prauzner-Bechcicki, S.; Wiltowska-Zuber, J.; Jaczewska, J.; Lekki, J.; Stachura, Z. Cancer cell recognition–mechanical phenotype. Micron 2012, 43, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Grexa, I.; Fekete, T.; Molnár, J.; Molnár, K.; Vizsnyiczai, G.; Ormos, P.; Kelemen, L. Single-cell elasticity measurement with an optically actuated microrobot. Micromachines 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Fukada, S.; Onda, K.; Maruyama, H.; Masuda, T.; Arai, F. 3D fabrication and manipulation of hybrid nanorobots by laser. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 2594–2599. [Google Scholar]
- Yang, Y.; Long, K.; Chu, Y.; Lu, H.; Wang, W.; Zhan, C. Photoresponsive Drug Delivery Systems: Challenges and Progress. Adv. Funct. Mater. 2024, 34, 2402975. [Google Scholar] [CrossRef]
- Zhang, S.; Scott, E.Y.; Singh, J.; Chen, Y.; Zhang, Y.; Elsayed, M.; Chamberlain, M.D.; Shakiba, N.; Adams, K.; Yu, S.; et al. The optoelectronic microrobot: A versatile toolbox for micromanipulation. Proc. Natl. Acad. Sci. USA 2019, 116, 14823–14828. [Google Scholar] [CrossRef]
- Miao, X.; Wilson, B.K.; Lin, L.Y. Localized surface plasmon assisted microfluidic mixing. Appl. Phys. Lett. 2008, 92, 124108. [Google Scholar] [CrossRef]
- Zhang, K.; Jian, A.; Zhang, X.; Wang, Y.; Li, Z.; Tam, H.y. Laser-induced thermal bubbles for microfluidic applications. Lab Chip 2011, 11, 1389–1395. [Google Scholar] [CrossRef]
- Liu, G.L.; Kim, J.; Lu, Y.; Lee, L.P. Optofluidic control using photothermal nanoparticles. Nat. Mater. 2006, 5, 27–32. [Google Scholar] [CrossRef]
- Qian, Y.; Neale, S.L.; Marsh, J.H. Microparticle manipulation using laser-induced thermophoresis and thermal convection flow. Sci. Rep. 2020, 10, 19169. [Google Scholar] [CrossRef]
- Engay, E.; Bunea, A.I.; Chouliara, M.; Bañas, A.; Glückstad, J. Natural convection induced by an optically fabricated and actuated microtool with a thermoplasmonic disk. Opt. Lett. 2018, 43, 3870–3873. [Google Scholar] [CrossRef]
- Alinezhad, H.G.; Reihani, S.N.S. Optimal condition for optical trapping of large particles: Tuning the laser power and numerical aperture of the objective. J. Opt. Soc. Am. B 2019, 36, 3053–3059. [Google Scholar] [CrossRef]
- Jamil, M.F.; Konara, M.; Pokhare, M.; Park, K. Design and Fabrication of Multi-Functional Optical Microbots. In Proceedings of the 2024 21st International Conference on Ubiquitous Robots (UR), New York, NY, USA, 24–27 June 2024; pp. 330–334. [Google Scholar]
- Crocker, J.C.; Grier, D.G. Methods of digital video microscopy for colloidal studies. J. Colloid Interface Sci. 1996, 179, 298–310. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konara, M.; Pokharel, M.; Sagar, M.M.; Kim, Y.; Park, K. Design, Fabrication, and Experimental Validation of Optical Microbots. Actuators 2025, 14, 229. https://doi.org/10.3390/act14050229
Konara M, Pokharel M, Sagar MM, Kim Y, Park K. Design, Fabrication, and Experimental Validation of Optical Microbots. Actuators. 2025; 14(5):229. https://doi.org/10.3390/act14050229
Chicago/Turabian StyleKonara, Menaka, Mishal Pokharel, Md Mainuddin Sagar, Yeongjin Kim, and Kihan Park. 2025. "Design, Fabrication, and Experimental Validation of Optical Microbots" Actuators 14, no. 5: 229. https://doi.org/10.3390/act14050229
APA StyleKonara, M., Pokharel, M., Sagar, M. M., Kim, Y., & Park, K. (2025). Design, Fabrication, and Experimental Validation of Optical Microbots. Actuators, 14(5), 229. https://doi.org/10.3390/act14050229







