A Battery-Powered Fluid Manipulation System Actuated by Mechanical Vibrations
Abstract
:1. Introduction
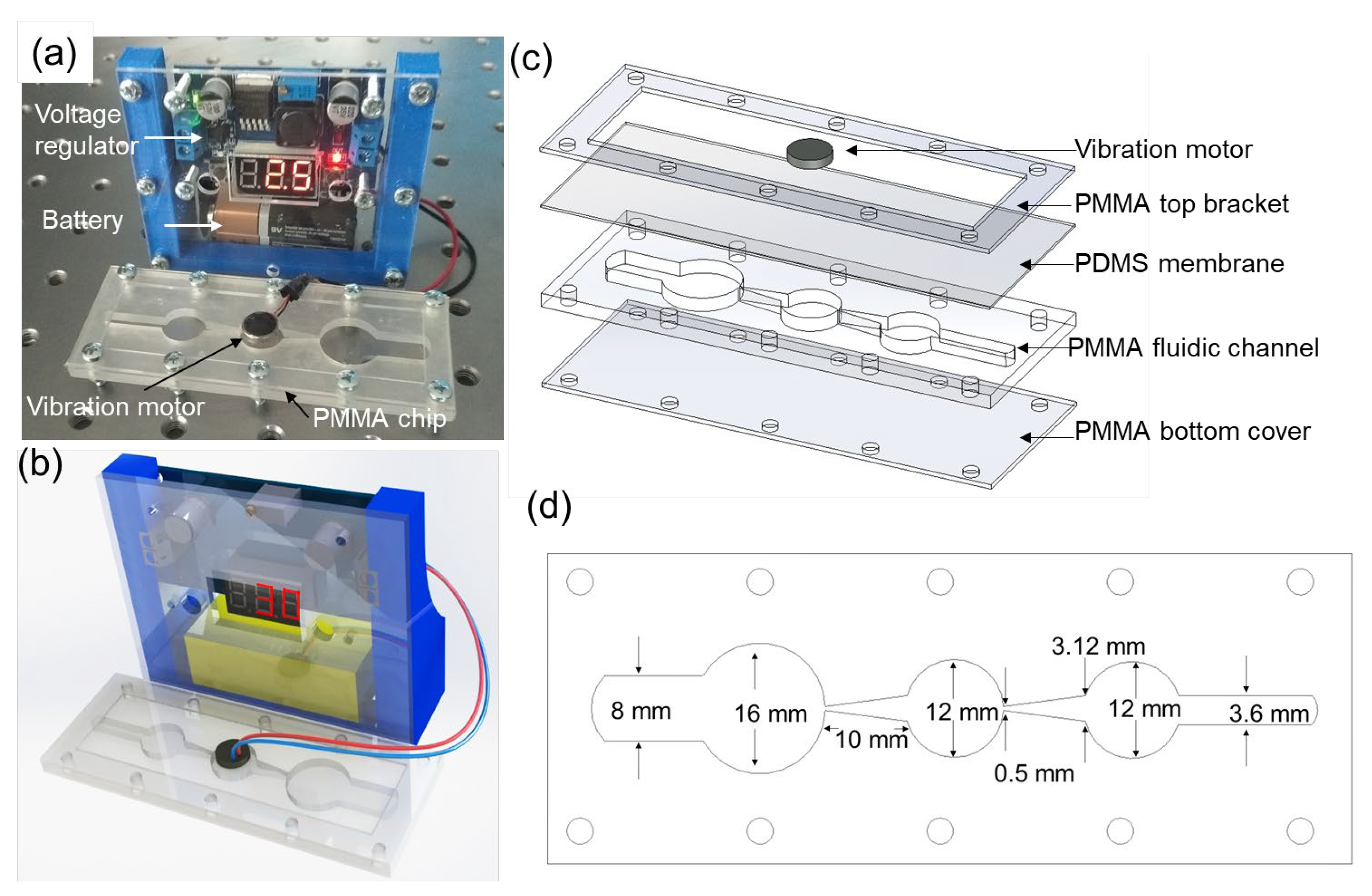
2. Materials and Methods
3. Results
3.1. Working Mechanism
3.2. Water Pumping Experiments
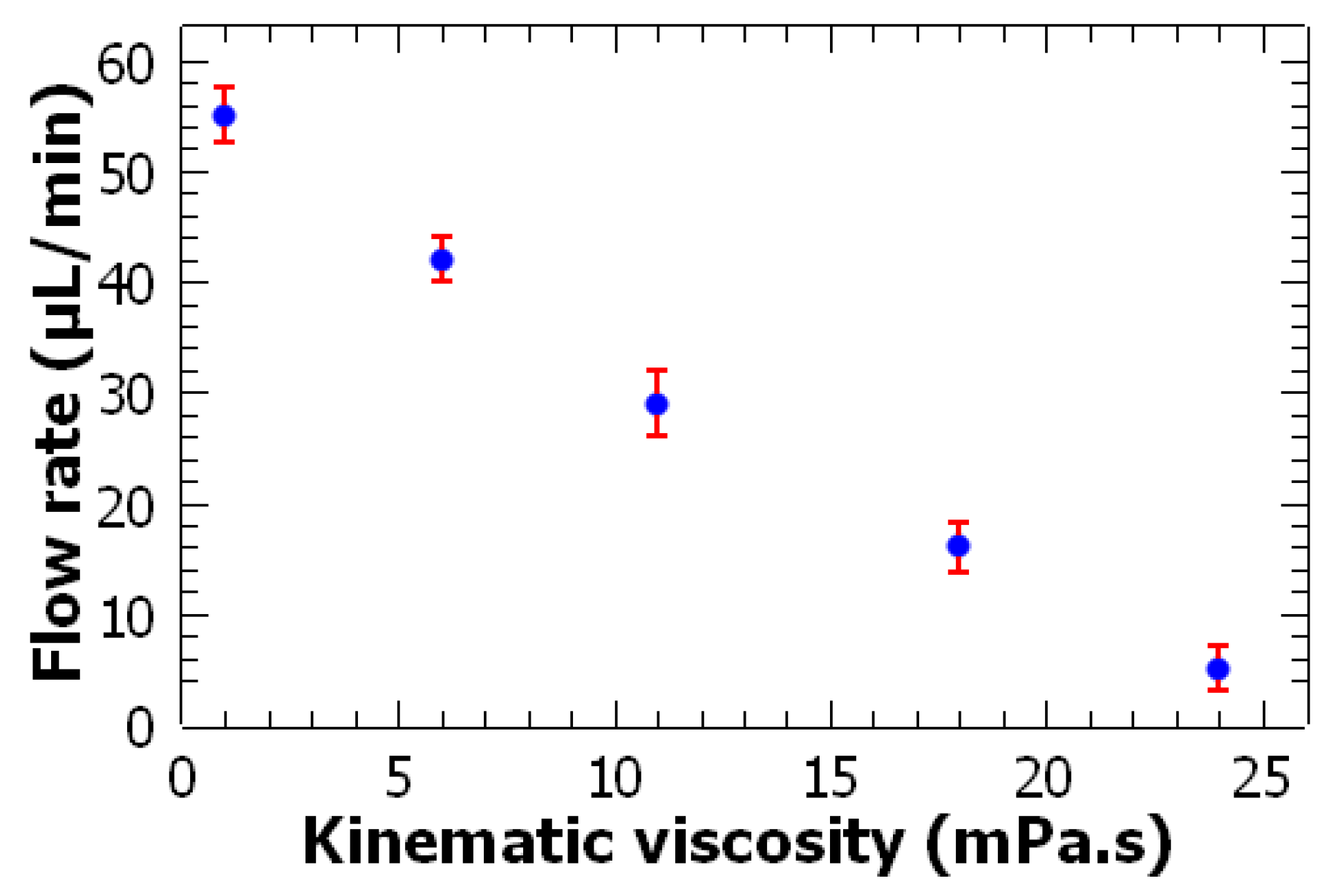
3.3. High Viscosity Fluid Pumping
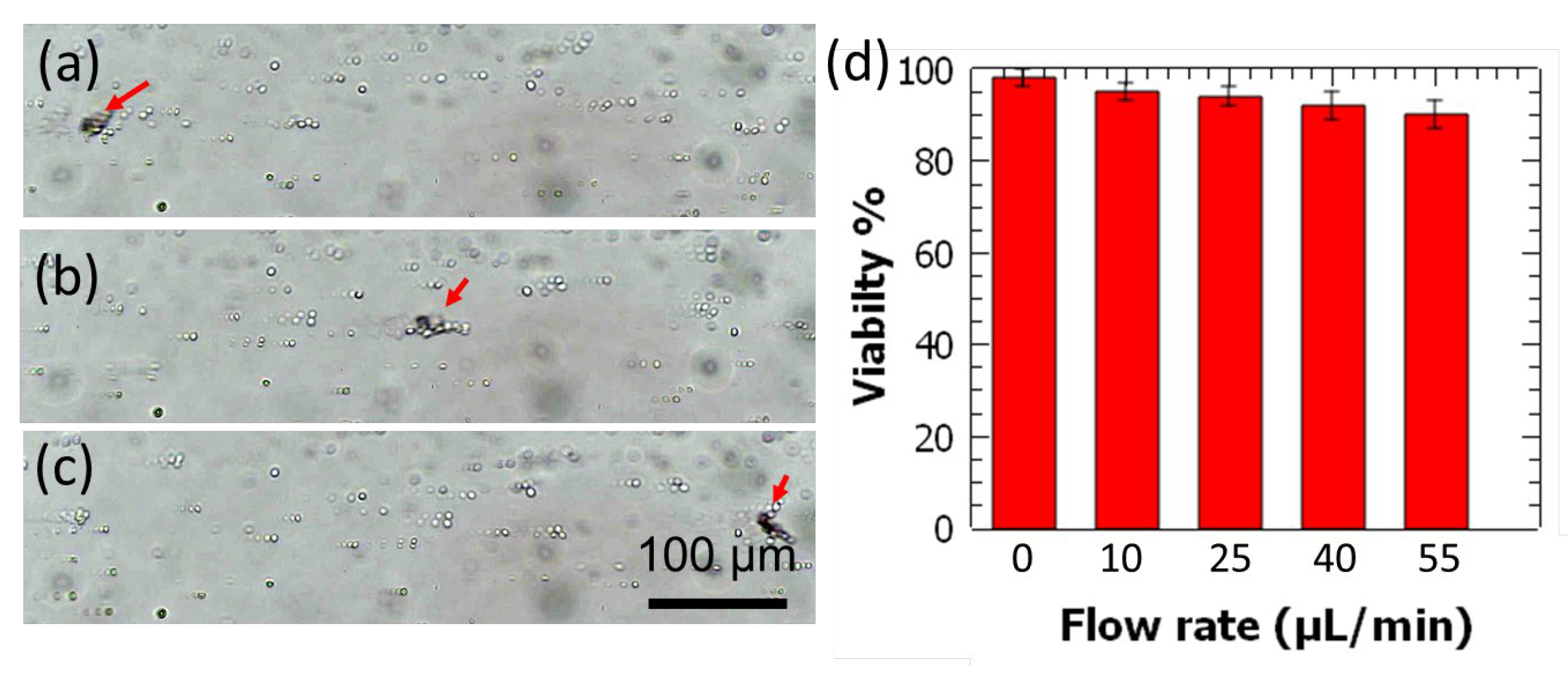
3.4. Cell Pumping and Viability Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Akkoyun, F.; Gucluer, S.; Ozcelik, A. Potential of the acoustic micromanipulation technologies for biomedical research. Biomicrofluidics 2021, 15, 061301. [Google Scholar] [CrossRef] [PubMed]
- Omori, K.; Fujimoto, N.; Kanda, T.; Wakimoto, S.; Seno, N. Core–Shell Droplet Generation Device Using a Flexural Bolt-Clamped Langevin-Type Ultrasonic Transducer. Actuators 2021, 10, 55. [Google Scholar] [CrossRef]
- Hossan, M.R.; Dutta, D.; Islam, N.; Dutta, P. Review: Electric field driven pumping in microfluidic device. Electrophoresis 2018, 39, 702–731. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.; Valencia, P.M.; Rodriguez, M.I.; Langer, R.; Farokhzad, O.C.; Karnik, R. Synthesis of Size-Tunable Polymeric Nanoparticles Enabled by 3D Hydrodynamic Flow Focusing in Single-Layer Microchannels. Adv. Mater. 2011, 23, H79–H83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.-H.; Ren, L.; Nama, N.; Li, S.; Li, P.; Yao, X.; Cuento, R.A.; Wei, C.H.; Chen, Y.; Xie, Y.; et al. An acoustofluidic sputum liquefier. Lab Chip 2015, 15, 3125–3131. [Google Scholar] [CrossRef]
- Tarn, M.; Lopez-Martinez, M.J.; Pamme, N. On-chip processing of particles and cells via multilaminar flow streams. Anal. Bioanal. Chem. 2013, 406, 139–161. [Google Scholar] [CrossRef]
- Weaver, W.; Kittur, H.; Dhar, M.; Di Carlo, D. Research highlights: Microfluidic point-of-care diagnostics. Lab Chip 2014, 14, 1962–1965. [Google Scholar] [CrossRef]
- Sesen, M.; Devendran, C.; Malikides, S.; Alan, T.; Neild, A. Surface acoustic wave enabled pipette on a chip. Lab Chip 2016, 17, 438–447. [Google Scholar] [CrossRef]
- Wu, Y.; Ao, Z.; Chen, B.; Muhsen, M.; Bondesson, M.; Lu, X.; Guo, F. Acoustic assembly of cell spheroids in disposable capillaries. Nanotechnology 2018, 29, 504006. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Ao, Z.; Wu, Z.; Song, S.; Mackie, K.; Guo, F. Intelligent acoustofluidics enabled mini-bioreactors for human brain organoids. Lab Chip 2021, 21, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Lavrijsen, R.; Onck, P.; Toonder, J.M.D. Versatile microfluidic flow generated by moulded magnetic artificial cilia. Sens. Actuators B Chem. 2018, 263, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Noh, S.; Prisby, R.D.; Lee, J.B. An Implanted Magnetic Microfluidic Pump for In Vivo Bone Remodeling Applications. Micromachines 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revathi, S.; Padmanabhan, R. Design and Development of Piezoelectric Composite-Based Micropump. J. Microelectromech. Syst. 2018, 27, 1105–1113. [Google Scholar] [CrossRef]
- Ayan, B.; Ozcelik, A.; Bachman, H.; Tang, S.Y.; Xie, Y.; Wu, M.; Li, P.; Huang, T.J. Acoustofluidic coating of particles and cells. Lab Chip 2016, 16, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Ayan, B.; Nama, N.; Bian, Y.; Lata, J.P.; Guo, X.; Huang, T.J.; Tang, S.; Ayan, B.; Nama, N.; et al. On-Chip Production of Size-Controllable Liquid Metal Microdroplets Using Acoustic Waves. Small 2016, 12, 3861–3869. [Google Scholar] [CrossRef]
- Dolev, A.; Kaynak, M.; Sakar, M.S. Dynamics of entrapped microbubbles with multiple openings. Phys. Fluids 2022, 34, 012012. [Google Scholar] [CrossRef]
- Dolev, A.; Kaynak, M.; Sakar, M.S. On-Board Mechanical Control Systems for Untethered Microrobots. Adv. Intell. Syst. 2021, 3, 2000233. [Google Scholar] [CrossRef]
- Kaynak, M.; Dirix, P.; Sakar, M.S. Addressable Acoustic Actuation of 3D Printed Soft Robotic Microsystems. Adv. Sci. 2020, 7, 2001120. [Google Scholar] [CrossRef]
- Vafaie, R.H.; Ghavifekr, H.B.; Van Lintel, H.; Brugger, J.; Renaud, P. Bi-directional ACET micropump for on-chip biological applications. Electrophoresis 2016, 37, 719–726. [Google Scholar] [CrossRef]
- Ozcelik, A.; Aslan, Z. A practical microfluidic pump enabled by acoustofluidics and 3D printing. Microfluid. Nanofluidics 2021, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Güçlüer, S. A Battery Powered on-Chip Peristaltic Pump for Lab-on-a-Chip Applications. Eur. Mech. Sci. 2021, 5, 201–205. [Google Scholar] [CrossRef]
- Uvarov, I.; Shlepakov, P.; Melenev, A.; Ma, K.; Svetovoy, V.; Krijnen, G. A Peristaltic Micropump Based on the Fast Electrochemical Actuator: Design, Fabrication, and Preliminary Testing. Actuators 2021, 10, 62. [Google Scholar] [CrossRef]
- Aishan, Y.; Yalikun, Y.; Shen, Y.; Yuan, Y.; Amaya, S.; Okutaki, T.; Osaki, A.; Maeda, S.; Tanaka, Y. A chemical micropump actuated by self-oscillating polymer gel. Sens. Actuators B Chem. 2021, 337, 129769. [Google Scholar] [CrossRef]
- Mao, Z.; Iizuka, T.; Maeda, S. Bidirectional electrohydrodynamic pump with high symmetrical performance and its application to a tube actuator. Sens. Actuators A Phys. 2021, 332, 113168. [Google Scholar] [CrossRef]
- Kellaris, N.; Venkata, V.G.; Smith, G.M.; Mitchell, S.K.; Keplinger, C. Peano-HASEL actuators: Muscle-mimetic, electrohydraulic transducers that linearly contract on activation. Sci. Robot. 2018, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Eluru, G.; Adhikari, J.V.; Chanda, P.; Gorthi, S.S. Hand-Powered Elastomeric Pump for Microfluidic Point-of-Care Diagnostics. Micromachines 2020, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.R.; Musgrove, H.B.; Throckmorton, A.L.; Pompano, R.R. Microscale impeller pump for recirculating flow in organs-on-chip and microreactors. Lab Chip 2021, 22, 605–620. [Google Scholar] [CrossRef]
- Huang, P.-H.; Nama, N.; Mao, Z.; Li, P.; Rufo, J.; Chen, Y.; Xie, Y.; Wei, C.-H.; Wang, L.; Huang, T.J. A reliable and programmable acoustofluidic pump powered by oscillating sharp-edge structures. Lab Chip 2014, 14, 4319–4323. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Wu, M.; Lin, Y.; Zhao, W.; Xu, J. Acoustic bubble-based bidirectional micropump. Microfluid. Nanofluidics 2020, 24, 29. [Google Scholar] [CrossRef]
- Wu, Z.; Cai, H.; Ao, Z.; Nunez, A.; Liu, H.; Bondesson, M.; Guo, S.; Guo, F. A Digital Acoustofluidic Pump Powered by Localized Fluid-Substrate Interactions. Anal. Chem. 2019, 91, 7097–7103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, X.; Zhang, J.; Zhang, X.; Xie, J.; Jin, L.; Liu, Z.; Zhuang, J.; Ren, W.; Ye, Z. A wearable, nozzle-diffuser microfluidic pump based on high-performance ferroelectric nanocomposites. Sens. Actuators B Chem. 2021, 347, 130611. [Google Scholar] [CrossRef]
- Zhao, B.; Cui, X.; Ren, W.; Xu, F.; Liu, M.; Ye, Z.-G. A Controllable and Integrated Pump-enabled Microfluidic Chip and Its Application in Droplets Generating. Sci. Rep. 2017, 7, 11319. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Jiang, D. Dynamic Characterization of a Valveless Micropump Considering Entrapped Gas Bubbles. J. Heat Transf. 2013, 135, 091403. [Google Scholar] [CrossRef]
- Wiranata, A.; Kanno, M.; Chiya, N.; Okabe, H.; Horii, T.; Fujie, T.; Hosoya, N.; Maeda, S. High-Frequency, low-voltage oscillations of dielectric elastomer actuators. Appl. Phys. Express 2021, 15, 011002. [Google Scholar] [CrossRef]
- Hiruta, T.; Hosoya, N.; Maeda, S.; Kajiwara, I. Experimental validation of vibration control in membrane structures using dielectric elastomer actuators in a vacuum environment. Int. J. Mech. Sci. 2020, 191, 106049. [Google Scholar] [CrossRef]
- Iwai, K.; Shih, K.C.; Lin, X.; Brubaker, T.A.; Sochol, R.D.; Lin, L. Finger-powered microfluidic systems using multilayer soft lithography and injection molding processes. Lab Chip 2014, 14, 3790–3799. [Google Scholar] [CrossRef]
- Yuan, Y.; Yalikun, Y.; Ota, N.; Tanaka, Y. Property Investigation of Replaceable PDMS Membrane as an Actuator in Microfluidic Device. Actuators 2018, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; van der Wijngaart, W.; Nilsson, P.; Enoksson, P.; Stemme, G. A valve-less diffuser micropump for microfluidic analytical systems. Sens. Actuators B Chem. 2001, 72, 259–265. [Google Scholar] [CrossRef]
- Ao, Z.; Cai, H.; Wu, Z.; Song, S.; Karahan, H.; Kim, B.; Lu, H.-C.; Kim, J.; Mackie, K.; Guo, F. Tubular human brain organoids to model microglia-mediated neuroinflammation. Lab Chip 2021, 21, 2751–2762. [Google Scholar] [CrossRef]
- Ao, Z.; Cai, H.; Wu, Z.; Krzesniak, J.; Tian, C.; Lai, Y.Y.; Mackie, K.; Guo, F. Human Spinal Organoid-on-a-Chip to Model Nociceptive Circuitry for Pain Therapeutics Discovery. Anal. Chem. 2021, 94, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.; Davis, G.L.; Oden, Z.M.; Razavi, M.R.; Fateh, A.; Ghazanfari, M.; Abdolrahimi, F.; Poorazar, S.; Sakhaie, F.; Olsen, R.J.; et al. Portable, Battery-Operated, Low-Cost, Bright Field and Fluorescence Microscope. PLoS ONE 2010, 5, e11890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.; Connacher, W.; Stambaugh, M.; Zhang, N.; Zhang, S.; Mei, J.; Jain, A.; Alluri, S.; Leung, V.; Rajapaksa, A.E.; et al. Practical microcircuits for handheld acoustofluidics. Lab Chip 2021, 21, 1352–1363. [Google Scholar] [CrossRef] [PubMed]
- Boyd-Moss, M.; Baratchi, S.; Di Venere, M.; Khoshmanesh, K. Self-contained microfluidic systems: A review. Lab Chip 2016, 16, 3177–3192. [Google Scholar] [CrossRef] [PubMed]
- Iverson, B.; Garimella, S.V. Recent advances in microscale pumping technologies: A review and evaluation. Microfluid. Nanofluidics 2008, 5, 145–174. [Google Scholar] [CrossRef]
- Tovar, A.R.; Patel, M.V.; Lee, A.P. Lateral air cavities for microfluidic pumping with the use of acoustic energy. Microfluid. Nanofluidics 2011, 10, 1269–1278. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkoyun, F.; Özçelik, A. A Battery-Powered Fluid Manipulation System Actuated by Mechanical Vibrations. Actuators 2022, 11, 116. https://doi.org/10.3390/act11050116
Akkoyun F, Özçelik A. A Battery-Powered Fluid Manipulation System Actuated by Mechanical Vibrations. Actuators. 2022; 11(5):116. https://doi.org/10.3390/act11050116
Chicago/Turabian StyleAkkoyun, Fatih, and Adem Özçelik. 2022. "A Battery-Powered Fluid Manipulation System Actuated by Mechanical Vibrations" Actuators 11, no. 5: 116. https://doi.org/10.3390/act11050116
APA StyleAkkoyun, F., & Özçelik, A. (2022). A Battery-Powered Fluid Manipulation System Actuated by Mechanical Vibrations. Actuators, 11(5), 116. https://doi.org/10.3390/act11050116





